Journal of Minerals and Materials Characterization and Engineering
Vol. 1 No. 6 (2013) , Article ID: 40042 , 5 pages DOI:10.4236/jmmce.2013.16048
Evaluation of the Refractory Properties of Nigerian Ozanagogo Clay Deposit
1National Metallurgical Development Centre, Jos, Nigeria
2Ahmadu Bello University, Zaria, Nigeria
Email: *alsanja@yahoo.com
Copyright © 2013 Alexander Asanja Jock et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received September 2, 2013; revised October 18, 2013; accepted October 29, 2013
Keywords: Ozanagogo; Clay; Porosity; Refractory
ABSTRACT
In this paper, the refractory properties of clay from Ozanagogo had been studied for possible utilization in refractory production. The clay had a specific gravity of 2.57, linear firing shrinkage of 1.01%, 2.14 g/cm3 bulk density and porosity of 20.4%. A cold crushing strength of 17.48 MN/m2 was obtained with modulus of rupture of 8.28 MN/m2. The thermal shock resistance exceeded 30 cycles and the refractoriness was 1750˚C. The sample was analysed for its chemical composition, and it was revealed that it contained 38.07% alumina (Al2O3), 46.00% silica (SiO2) and iron impurities (Fe2O3) of 0.78%. The results generally showed that Ozanagogo clay could be used as a refractory material.
1. Introduction
Clays differ very considerably among themselves in structure, workability, plasticity, particle-size distribution, and mineralogical composition. These differences lead to such terms as flint clays, plastic clays, fireclays, kaolins, ball clays and clay minerals are grouped into kaolinite, montmorillonite and the illite. Clays have variety of applications such as in refractory making, as catalysts, absorbents, binders and fillers, and the useful characteristics of the most versatile material are being appreciated. The production of special grogs or aggregates, the purification, bleaching and organic modifications of clays are all acquiring new emphasis at present.
Refractories are materials capable of withstanding very high temperature without an undue deformation, softening or change in composition [1]. They include aluminosilicates, silica, magnesite, chrome, carbon, carbides, nitrides etc., and they are classified as acid, basic and neutral refractories. Refractories are employed for the construction of furnaces, kilns, crucibles, flues used in high temperature operations. Refractory clays are broadly grouped into fireclay and kaolins, both are based on the mineral kaolinite (Al2O3·2SiO2·2H2O). Fireclays are the most widely used refractory materials and about 70% of refractories are fireclay produced mainly from clays with alumina content ranging from 25% to 45% [2]. Impurities in fireclay are pyrites, quartz, calcites, ferrous, carbonates and some organic compounds. The organic impurities impart plasticity to the clay while impurities like quartz and iron reduce their refractoriness.
Clay deposits are widely distributed in Nigeria [3-6]. In spite of the extensive use and the demand for clay in industrial processes, Ozanagogo clay deposit in Delta State is used mainly for local paint and building bricks, and investigation of its refractory properties is being conducted so as to reveal its other potentials as a refractory material. The clay is chosen for evaluation mainly because of its bulk availability and proximity to Ajaokuta Steel Complex [3].
2. Materials and Methods
2.1. Sample Collection and Preparation
The clay sample was randomly collected in lumps form from the deposit site at Ozanagogo in Delta State, South Nigeria. The lumps were sun dried for a week to reduce moisture content and enhance grinding. The clay lumps were then crushed, ground and sieved.
2.2. Chemical Composition Analysis
The ground clay sample was dried and the chemical composition in wt% of Al2O3, SiO2, Fe2O3, TiO2, CaO, Na2O and K2O was determined using Energy Dispersive X-Ray Fluorescence Spectrometer (ED-XRF) model PW1660, XRA. The loss on ignition (LOI) of the clay (Mainly volatile matters) was determined by measuring the weight loss of a known mass of the sample after firing in furnace at 1000˚C for 1 hour 30 minutes. Loss on ignition was calculated using this relation:
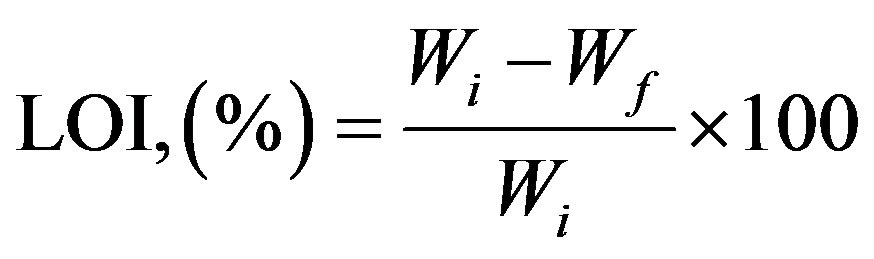 (1)
(1)
where, Wi and Wf are initial and final weight respectively.
2.3. Specific Gravity
The specific gravity test is usually conducted on materials that do not dissolve in or get attacked by water. The test piece was cut from within the core of a refractory shape moulded from the clay and crushed to a size not exceeding 3 mm [7]. The crushed material was then mixed and reduced to a 50 g sample by cone and quartering method. The sample material obtained was dried at 110˚C to a constant weight, and then 10 g sample was weighed using a glass stoppered weighing bottle. A pyconometer with stopper was dried at 110˚C, cooled in a desiccator and its weight (Wp) was noted. The pyconometer was filled with distilled water at room temperature; with its stopper put in place, the weight (W1) was noted. The test refractory sample was put in dry pyconometer, covered with the stopper and weighed (W). The stopper was removed and distilled water was added to the sample to fill the pyconometer to its half capacity. This was gently boiled for 10 minutes to avoid loss of sample due to popping. The weight (W2) of the pyconometer containing the test sample and the water was recorded. The specific gravity was calculated from the following:
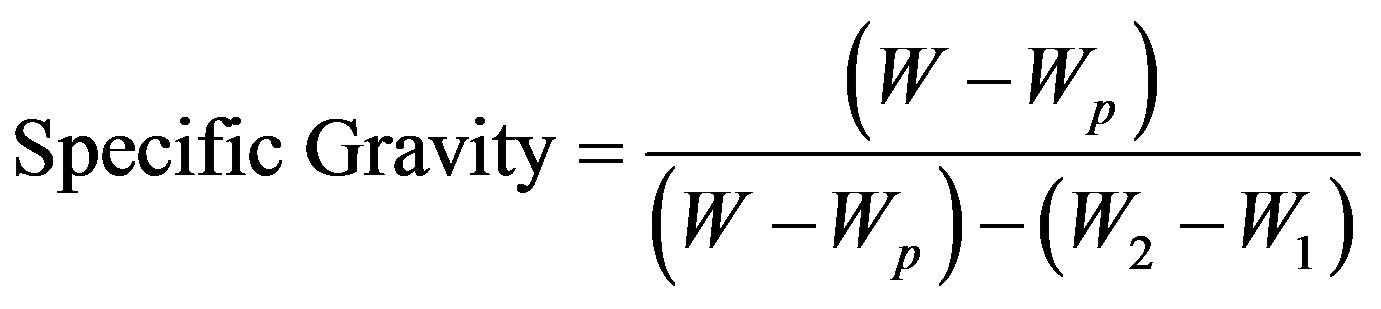 (2)
(2)
where, W is the weight of test sample and dry pyconometer with stopper, Wp is the weight of dry pyconometer with stopper, W1 and W2 are the weights of pyconometer with stopper filled with distilled water and pyconometer with stopper containing the test sample and distilled water respectively.
2.4. Brick Production
The sequence adopted in making refractory bricks is crushing, grinding, sizing, mixing and forming, drying, firing, and cooling [8,9].
The clay sample was dried and calcined by firing to 1200˚C for 8 hours in a muffle furnace (RHF 16/15 model), making the clay lose its plasticity by forming grog (firesand). The grog was then crushed, ground and screen-ed through sieves size of 2000 µm, 710 µm and 212 µm to represent the coarse, medium and fine fractions respectively, necessary to improve the manufacture of high packing density products. The grog aggregates were blended with 10% unfired clay which was capable of developing desire plasticity when mixed with 5% water. The resulting mixture was compressed with a hydraulic press in a cylindrical mould of dimensions 100 mm × 50 mm × 25 mm. The pressed brick was left overnight to air-dry and then oven dried at 110˚C for 8 hours before firing in a muffle furnace upto 1300˚C. Firing of the brick sample was done gradually at the rate of 5˚C/minute.The sample was soaked at 1300˚C for 2 hours and allowed to cool gradually in the furnace overnight.
2.5. Firing Shrinkage
The firing shrinkage is found to be the most useful and relevant property in the production of refractory bricks. It is determine by measuring the dimensional changes between the dried and fired bricks. In this work, the distance between the two ends of the sample was measured with a venier caliper after the drying and firing processes. The firing shrinkage was calculated thus:
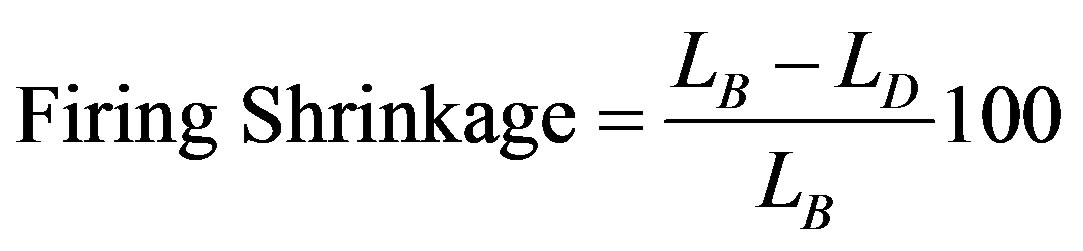 (3)
(3)
where, LB = Dry dimension; LD = fired dimension.
2.6. Bulk Density
The bulk density of a refractory body is represented by the weight per unit volume including pore space. The method used in determining the bulk density of the refractory sample was boiling method. A moulded fired brick specimen measuring 30 mm by 30 mm by 20 mm was prepared. The brick was air dried for 24 hours and oven dried at 110˚C to a constant weight (D). After which it was transferred to a beaker and boiled with distilled water for 2 hours to assist in releasing trapped air. It was then allowed to soak and the saturated weight free of excess water (W) was taken. The specimen was then suspended in water using a beaker and the suspended weight (S) was taken. The bulk density was then calculated using the relationship [1],
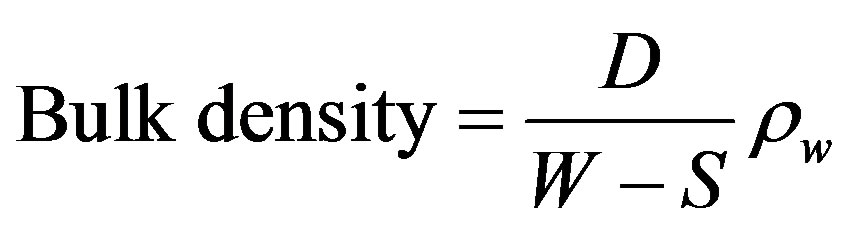 (4)
(4)
where, D = Dried weight; W = Saturated weight; S = suspended weight; ρw = Density of water.
2.7. Apparent Porosity
Porosity is the percentage relationship between the volume of the pore space and the total volume of the sample. Using boiling method, the test specimen measuring 30 mm × 30 mm × 20 mm was cut from the fired refractory brick and then dried in an oven at 110˚C to a constant weight (D). The dried specimen was suspended freely in distilled water and boiled for 2 hours, cooled to room temperature and its weight (S) noted. The specimen was removed and the soaked or saturated weight in air (W) was recorded. The apparent porosity was calculated using,
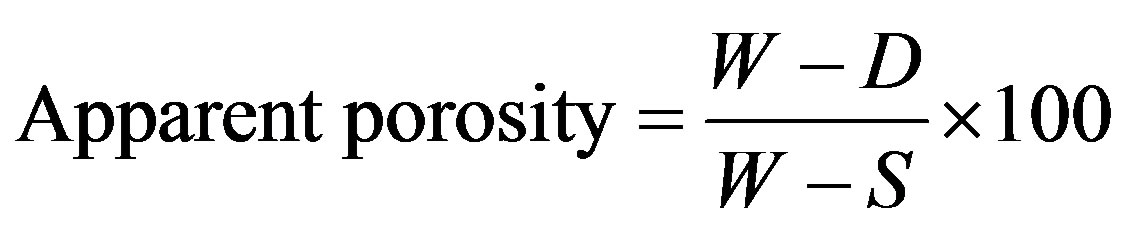 (5)
(5)
where, W = soaked or saturated weight; D = Dried weight; S = suspended weight.
2.8. Cold Crushing Strength
This is the load at which cracks appear in the specimen. The test piece was cut from the fired brick in the form of cubes of about 25 mm size. The test piece was marked to indicate the direction in which forming pressure was applied and the two faces normal to this direction were prepared as bearing faces. Cardboard was placed between the platens of the press and the bearing faces of the test piece. The load was applied at a uniform rate until the test piece failed to support the load. The maximum recorded load was taken as the crushing load and the area obtained from the size of the test piece before the application of load was calculated and recorded. The cold crush-ing strength was determined using the formula,
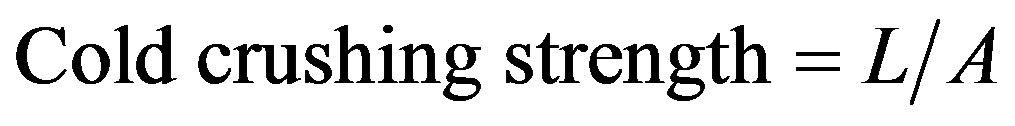 (6)
(6)
where, L = Maximum load (KN); A = Cross sectional area.
2.9. Modulus of Rupture (MOR)
This is a measure of the fracture strength (by bending) of a refractory product against the forces of breakage or crack due to application of certain amount of pressure. The test piece at room temperature was cut from the fired brick into 25 mm × 25 mm × 100 mm box. The test piece was supported near its ends and loaded at the centre (three-point-load) until failure occurs. The breaking load (maximum load) was obtained using the motorized processing Monsanto Tensometer, type “W” and the MOR was calculated by,
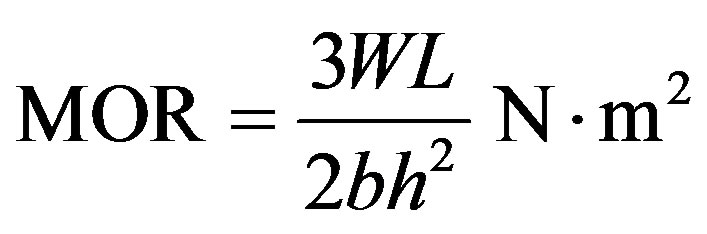 (7)
(7)
where, W = load at which the specimen failed (N); b = width of specimen (m); h = height of specimen (m); L = distance between the centre of the two supports (m).
2.10. Thermal Shock Resistance
Thermal shock resistance is the number of heating and cooling (cycles) needed to cause conspicuous crack on the sample [10]. This test was performed by heating the specimen in a muffle furnace preset at 1200˚C for 10 minutes, after which, it was air cooled for another 10 minutes and observed for cracks. This process was repeated up till 30 cycles without crack being observed.
2.11. Refractoriness
The refractoriness is a measure of fusibility of material. It indicates the temperature at which the material softens. The Pyrometric Cone equivalent (PCE) method was used to determine the refractoriness on the ground Ozanagogo clay sample [1,10]. The clay sample was dried and ground to fines, and watered to make a plastic mass. The test piece was then formed into a suitable mould of pyramid shape with 12.7 mm × 12.7 mm base and 38 mm height.
British standard and test piece cones were fixed with cement at the centre of a refractory plaque. Both cones were placed in the furnace. The heating rate over the last 200˚C below the estimated fusion temperature was carefully controlled at 5˚C per minute and was observed by the use of an optical pyrometer. The heating continued till the tip of the test cone bent touching the refractory plaque. The plaque bearing the test cone was removed from the furnace and the test cone examined when cold. Refractoriness of the test cone was determined by the temperature equivalent on the standard cone that bent to a large extent similar to the test cone.
3. Results and Discussion
3.1. Results
The results of chemical analysis, physical properties of Ozanagogo clay and comparison of the properties with established standard are as shown in Tables 1-3 respectively.
3.2. Discussion of Results
The chemical composition of Ozanagogo clay presented in Table 1 showed that the alumina (Al2O3) content was 38.07 wt%, silica (SiO2) 46.00 wt% and iron (Fe2O3) content was 0.78 wt%. The alumina in the clay was within the range of 25% - 45% required for fireclay refractories (the higher the alumina content in clay, the higher the refractoriness) [10]. The iron oxide (0.78 wt%) is within the acceptable range of 0.5% - 2.4% for refractory clays. Similarly the low values of the fluxing oxides such as CaO and MgO further confirmed that the clay could be used for refractory application since high amount of these oxides are expected to cause adverse effects on the refractory properties such as lowering the melting point of the clay [1]. The loss on ignition (LOI) is 13.70% and is also within the range of 9% - 14% required in a fireclay

Table 1. Chemical analysis of Ozanagogo clay.
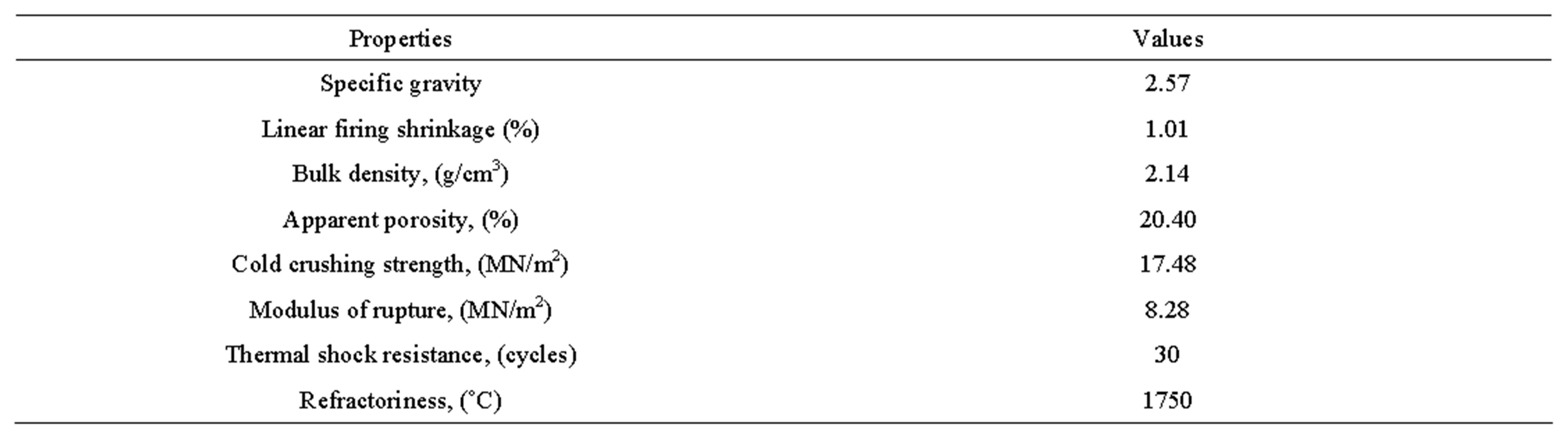
Table 2. Physical properties of Ozanagogo clay.
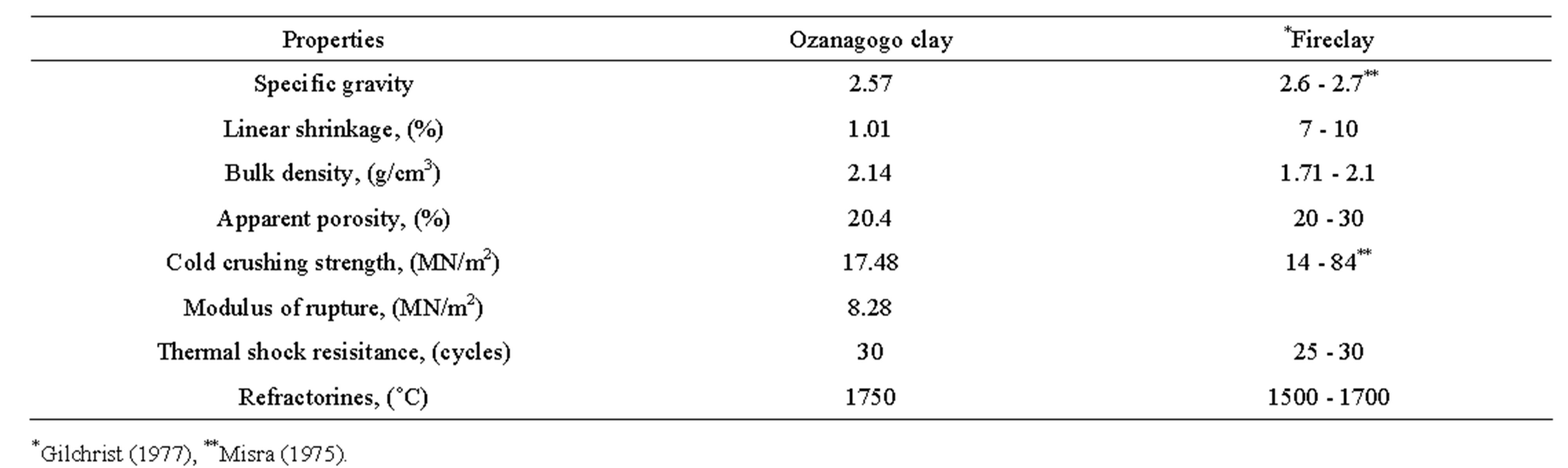
Table 3. Comparison of the determined properties of Ozanagogo clay with some established refractory standard.
[11]. The loss on ignition (LOI) is the organic or combustible or volatile matter loss on heating and this affect the values of shrinkage and porosity to some extent.
3.3. Specific Gravity
The specific gravity result of Ozanagogo clay obtained was 2.57 and this was closed to range of 2.6 - 2.7 required for high heat duty fireclay refractory as shown in Table 3.
3.4. Linear Firing Shrinkage
The result of the linear firing shrinkage of the clay determined was 1.01% and less than the recommended range standard of 7% - 10% for refractory clays (Table 3). However, the low value obtained in this study could be due to the fact that 90% of the brick composition was made of grog, which is thermally stable and also serves as anti-shrinkage agent [10].
3.5. Bulk Density
The bulk density of the clay was 2.14 g/cm3 and this value was slightly greater than the typical bulk density for fireclay refractories which was 1.91 g/cm3 as suggested by Gilchrist [11]. The high bulk density obtained that was above the typical one could be explained by the level of grittiness or coarse size of the grog fractions used.
3.6. Apparent Porosity
Ozanagogo clay gave an apparent porosity of 20.40%, which was within the range of 20% - 30% required for firebrick clay as reported by Gilchrist [11] in Table 3.
3.7. Cold Crushing Strength
The cold crushing strength of the clay determined is 17.48 MN/m2, and is above the minimum recommended value of 14 MN/m2 for dense fired brick reported as by Misra [10]. This value, however, shows that Ozanagogo clay can comfortably withstand impacts at low temperatures as the cold crushing strength is an indicator of the effect of firing on ceramic bond.
3.8. Modulus of Rupture
The modulus of rupture of the clay was 8.28 MN/m2. This was quite good as fireclay refractories whose strength ranged between 2 MN/m2 and 4 MN/m2 were believed to have performed well [7].
3.9. Thermal Shock Resistance
The result of the thermal shock resistance displayed in Table 2 shows that the number of cycles without failure is 30. This value falls within the 20 - 30 number of cycles recommended for fireclay refractories reported as by Gilchrist [11].
3.10. Refractoriness
The result in Table 2 showed that the refractoriness of the sample occurred at a temperature of 1750˚C. This high temperature might have been due to the appreciable amount of the alumina content (38.07%) in the clay. The alumina in the clay was a strong indicator of its refractoriness and the higher the alumina, the higher the refractoriness [1].
4. Conclusion
Ozanagogo clay has been investigated in this work for its suitability as a refractory material. It was revealed that on the basis of physio-chemical characteristics of this kaolinitic deposit, it could be processed for use as refractory material. The investigated properties gave excellent results for refractory application. Ozanagogo clay is found to be a good substitute for imported refractories used for lining of furnaces, kilns, crucibles ladles, soaking pits and flues. The deposit site also has an advantage of close proximity to Ajaokuta Steel Complex in Nigeria. This work also suggested that further work should be carried out to determine other important properties such as refractoriness under load (RUL) and slag resistance. The clay should be exploited not only for making refractory bricks but also for chemical and paper industries.
REFERENCES
- S. B. Hassan, “Modern Refractories: Production, Properties, Testing and Application,” Timo Commercial Printers Samaru, Zaria, 2005, pp. 13-22, 28-40.
- A. R. Chesti “Refractories: Manufacture, Properties and Applications,” Prentice-Hall of India private Ltd., New Delhi, 1986, pp. 55-63.
- A. Adekanmbi, “The Nigerian Steel Industry: Evaluation and Raw Materials Development, a Historical Excursion (Emphasis on Ajaokuta Steel Project),” XcessAmonqe Press, Ibadan, 2005, pp. 172-185.
- J. O. Borode, O. O. Onyemaobi and J. A. Omotoyinbo, “Suitability of some Nigerian Clays as Refractory Raw Materials,” Nigerian Journal of Engineering Management, Vol. 1, 2000, pp. 14-18.
- S. B. Hassan and J. O. Adewara, “Refractory Properties of Some Nigerian Clays,” Nigerian Society of Engineers Technical Transaction, Vol. 29, No. 3, 1994, pp. 13-19.
- P. S. Irabor, “Physical and Chemical Investigation on Some Nigerian Kaolinite Clays for Use in the Ceramics and Allied Industries,” Nigerian Journal of Engineering Research and Development, Vol. 1, No. 1, 2002, pp. 54- 59.
- ASTM Standards Part 17, “Refractories, Glass, Ceramic Materials, Carbon and Graphite Products,” ASTM, Philadelphia, 2005, pp. 7-9, 51-61.
- J. S. Reed, “Introduction to the Principles of Ceramic Processing,” John Wiley and Sons, New York, 1986, pp. 32, 154, 330.
- J. H. Chesters, “Refractories: Production and Properties,” The Iron and Steel Institute, London, 1973, pp. 3-13, 296- 312.
- M. I. Misra, “Refractories: Their manufacture, Properties and Uses,” 4th Edition, SMT, Lakshmi Misra, Krishna Colony, Ghamapur, 1975, pp. 8-13, 22-42.
- J. P. Gilchrist, “Fuel, Furnaces and Refractories,” Pergamon Press, Oxford, 1977, pp 35-70.
NOTES
*Corresponding author.

