Food and Nutrition Sciences
Vol.08 No.02(2017), Article ID:74320,10 pages
10.4236/fns.2017.82017
Influence of Nano-Spray Dried Sodium Chloride on the Physicochemical Characteristics of Surface-Salted Cheese Crackers
Marvin L. Moncada1, Carlos E. Astete2, Cristina M. Sabliov2, Douglas W. Olson1, Charles A. Boeneke1, Kayanush J. Aryana1*
1School of Nutrition and Food Sciences, Louisiana State University Agricultural Center, Baton Rouge, LA, USA
2Department of Biological and Agricultural Engineering, Louisiana State University Agricultural Center, Baton Rouge, LA, USA

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: January 21, 2017; Accepted: February 21, 2017; Published: February 24, 2017
ABSTRACT
Particle size reduction of salt to submicron range increases its surface area resulting in increased saltiness perception. The objective was to evaluate the incorporation of nano-spray dried sodium chloride on the physicochemical characteristics of surface-salted cheese crackers. The sodium chloride solution (3% w/w) was sprayed through a 4-μm nozzle with 125 L/min air flow, 38 kPa pressure, 95˚C head temperature, and 90% spray to form the smallest submicrosalt particles. The cheese cracker treatments consisted of 3 different salt sizes (regular, microsalt and nano-spray dried salt) and 3 different concentrations (2%, 1.5% and 1%). The 9 (3 sizes × 3 concentrations) cheese cracker treatments were tested for salt concentration and sodium content at week 1. Water activity ( ), texture-fracturability, and color were determined at week 1 and 4 months of storage. The
), texture-fracturability, and color were determined at week 1 and 4 months of storage. The  and the
and the ,
,  ,
,  and
and  values in all treatments increased from 1 week to 4 months. The use of nano-spray dried salt on surface-salted cheese crackers allowed for a reduction of 25% - 50% of salt content without affecting the physicochemical attributes.
values in all treatments increased from 1 week to 4 months. The use of nano-spray dried salt on surface-salted cheese crackers allowed for a reduction of 25% - 50% of salt content without affecting the physicochemical attributes.
Keywords:
Nano-Spray Drying, Salt, Colour, Texture, Water Activity

1. Introduction
Salt (NaCl) is important from both a healthy and sensory standpoint. It is necessary for normal physiological function [1] . Saltiness is one of the five basic taste sensations (sweet, salty, sour, bitter, and umami). Humans and all animals possess an inherent appetite for salt [2] . High intakes of processed foods, such as prepared meals, pizza, and savory snacks, have resulted in an excessive consumption of dietary salt [3] , leading to health problems including hypertension and increased risk for heart disease and stroke [4] . However, salt reduction can lead to changes in the physicochemical characteristics of food and sensory attributes. Therefore, research and development activities have been performed to reduce the salt content of foods without adversely affecting the sensory and physicochemical properties of the food.
Food color can be considered the most important product-intrinsic sensory attribute governing the sensory and hedonic expectations that influences consumer buying decision and affects their perception of the freshness of the product. Therefore, color is often measured, usually by using 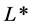 on a scale from 0 (absolute blackness) to 100 (representing a perfect reflecting diffuser (absolute whiteness)),
on a scale from 0 (absolute blackness) to 100 (representing a perfect reflecting diffuser (absolute whiteness)), 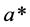 (positive values indicating degree of redness and negative values indicating degree of greenness) and
(positive values indicating degree of redness and negative values indicating degree of greenness) and 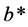 (positive values representing extent of yellowness and negative values representing extent of blueness).
(positive values representing extent of yellowness and negative values representing extent of blueness).
The presence of salt reduces the 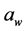 in foods, meaning that there is less water available for hydration of materials [5] . Foods with high
in foods, meaning that there is less water available for hydration of materials [5] . Foods with high 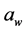 have a texture that is described as moist, juicy, tender, and chewy. Low
have a texture that is described as moist, juicy, tender, and chewy. Low 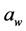 products normally have texture attributes described as crisp and crunchy (chips and crackers) [5] .
products normally have texture attributes described as crisp and crunchy (chips and crackers) [5] .
Malovany [6] reported that cheese cracker sales in the United States for 2012 were $817.1 million and they are among the most popular snack products in North America. To reduce the sodium content of cheese crackers, Moncada et al. [7] manufactured cheese crackers that incorporated nano-spray dried salt. It was shown that these cheese crackers with nano-spray dried salt incorporated on their surfaces that resulted in a 25% and 50% reduction in salt content were accepted by consumers. These salt nanoparticles were observed by scanning electron micrographs, and 80% of these salt particles were between 500 to 1900 nm when analyzed by laser diffraction particle size analysis. It is not known whether 25% and 50% reduction in salt, applied in the form of nanoparticles instead of normal-sized salt particles, would alter , texture-fracturability and color of surface-salted cheese crackers. The objective of this present study was to evaluate the effect of incorporation of nano-spray dried salt on surfaces of cheese crackers on the resulting physicochemical characteristics (
, texture-fracturability and color of surface-salted cheese crackers. The objective of this present study was to evaluate the effect of incorporation of nano-spray dried salt on surfaces of cheese crackers on the resulting physicochemical characteristics (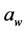 , texture-fracturability, and color) of these crackers.
, texture-fracturability, and color) of these crackers.
2. Material and Methods
2.1. Experimental Design
The sodium chloride nano-spray dried particles were used in the sodium reduced cheese cracker treatments. The treatments consisted of 3 different salt sizes (regular, microsalt and nano-spray dried salt) and 3 different concentrations (2%, 1.5% and 1%). The 9 treatments (3 sizes × 3 concentrations) were tested for salt concentration and sodium content at week 1 and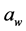 , texture-fracturability, and color at week 1 and 4 months of storage. The experimental design for
, texture-fracturability, and color at week 1 and 4 months of storage. The experimental design for 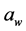 texture-fracturability, and color was a CRD with repeated measures.
texture-fracturability, and color was a CRD with repeated measures.
2.2. Manufacture of Nano-Spray Dried Sodium Chloride Particles
A solution of salt in deionized water (3% w/w) was prepared, completely dissolved and filtered through Whatman Number 2 filter paper (Clifton, NJ, USA) and subsequently was processed by nano-spray drying (Nanospray dryer B-90, BÜCHI Labortechnik AG, Flawil, Switzerland). The sodium chloride solutions were sprayed through the 4 μm nozzle. The air flow (125 l/min), pressure (3.8 kPa), head temperature (95˚C) and spray percentage (90%) were kept constant in all treatments.
2.3. Cheese Cracker Manufacture
The cheese cracker production, salt addition and packaging were performed according to Moncada et al. [7] . Briefly, the dough was rolled into sheets of 3 mm thickness and then trimmed into 25.4 mm rectangles, and a hole was incorporated at the center of each rectangle for release of water vapor to prevent puffing during baking. Baking was conducted at 177˚C for 25 min. The surface was very lightly sprayed with fine droplets of Canola oil. Crackers were weighed, salted at 1%, 1.5% and 2% by weight to the surface, and subsequently packaged in modified atmosphere packaging using KOCH UltraVac (Kansas City, MO, USA) packaging machine and BOPPT/VMCPP-Biaxially-Oriented Polypropylene- Plastics technology/Cast Polypropylene bags, (Uline, Houston, TX, USA) and stored at 22˚C until analysis.
2.4. Salt Concentration Determination
The salt concentration of the cheese crackers was determined by the Mohr method [8] with slight modification; titration with silver nitrate. The cheese cracker samples were evaluated at week 1. A 25 g sample of cheese crackers was weighed and transferred to a 500 mL Erlenmeyer flask with 250 mL deionized water. The solution was mixed for 1 - 3 min using a magnetic stirrer and subsequently 10 mL of the solution were pipetted into a 250 mL flask with 25 mL of deionized water. Potassium chromate (7 drops) was added to the solution. The solution was then titrated with 0.1 N silver nitrate until the solution changed color (bright red). The percentage of salt was determined using the equation provided by Sheen and Kahler [8] .
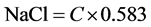
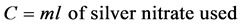
2.5. Sodium Content Determination
Sodium content was determined by the mathematical calculation from the salt content at week 1. The percentage of salt was multiplied by the number of grams of sample used; subsequently that result was multiplied by the sodium percentage in the NaCl molecule and then multiplied by 1000 (mg/g) to get sodium content in mg/g.
2.6. Water Activity (
The 



2.7. Texture-Fracturability Analysis
The texture-fracturability analysis was performed with a Stable Micro Systems model TA.XTPlus Texture Analyzer (Texture Technologies Corp., Hamilton, MA, USA) using an HDP/BS blade. The weight calibration was performed daily with a 2000 g weight standard and the height calibration was set at 15 mm. The set up used in the texture profile analysis (TPA) included: pretest speed of 1 mm/s, test speed of 5 mm/s and posttest speed of 2 mm/s, the trigger system using “force”, and defining 2.4 mm of distance.
The cheese cracker samples had a uniform surface. On the day of the analysis, the samples were removed from the modified atmosphere packaging sealed bag. Five samples from each treatment were analyzed. Fracturability (g) was evaluated.
2.8. Color Analysis
Evaluation of color was performed on a group of cheese cracker surfaces at 1 week and 4 months of room temperature storage, using a HunterLab MiniScan XE Plus, portable color spectrophotometer (Hunter Associates Laboratory Inc., Reston, VA, USA). The instrument was calibrated using the black and white standard tiles that came with the instrument. The operating conditions were 10˚ observer, D65 illuminant and 45/0 sensor. An average of five values was taken per sample. An optical aperture of 1.7 cm was used.








2.9. Statistical Analysis
The cheese cracker data from the physical and chemical analysis were analyzed using Proc Mixed of Statistical Analysis System (SAS®, Cary, NC, USA). Differences of least square means were used to determine significant differences at P < 0.05 for main effects (treatment and time) and their interaction effects (treatment * time). Significant differences were determined at α = 0.05. Significant differences (P < 0.05) among the main effects were analyzed using Tukey’s adjustment and Macro program to determine differences between treatments.
3. Results and Discussion
3.1. Salt and Sodium Contents
The salt and sodium contents of surface-salted cheese crackers as influenced by different salt particle sizes/concentrations are presented in Table 1. These analyses confirmed that the salt usage levels of 2%, 1.5% and 1% were precise. Nurul et al. [10] found sodium contents of fish cracker samples were in the range of 1184 - 1888 mg/100 g. This sodium in fish crackers was the result of the addition of salt and monosodium glutamate during the manufacturing process.
3.2. Water Activity (
The 



Table 1. Least square means for salt and sodium contents of surface-salted cheese cracker as influenced by treatment.
Table 2. Least square means for water activity (
abcdefghiLS Means not containing a common letter within the attribute are significantly (P < 0.05) different.
Table 3. Probability > F of different particle size treatments, time and their interaction for the water activity (
0.34 at the end of storage (4 months). Crackers containing 2%, 1.5% and 1% nano-spray dried salt had significantly (P < 0.05) lower 





3.3. Texture Fracturability
The texture fracturability analysis of surface-salted cheese crackers as influenced by different salt particle sizes/concentrations is shown in Table 2. There was a significant (P < 0.001) effect for treatment * time interaction, treatment and time (Table 3). Crackers containing 1.5% microsalt and 1% regular salt significantly (P < 0.05) decreased in fracturability from 1 week to 4 months (Table 2). Also, crackers containing 1.5% microsalt had significantly (P < 0.05) lower fracturability than crackers containing 1.5% regular salt at 4 months (Table 2). The reduction of 25% (1.5%) and 50% (1%) of salt content in surface-salted cheese crackers through use of nano-spray dried salt did not show significant differences in texture fracturability. Nakamura et al. [16] studied the quality deterioration of rice crackers over time using the one bite test with tensipresser and concluded that the hardness of cracker markedly increased during storage of 20 days at 35˚C. Its changes were attributed to the retrogradation of rice starch over time. In this study we did not use rice starch.
3.4. Color
3.4.1.
The 

Different factors affect lightness of foods. Mohamed et al. [18] concluded that the type of flour used affected the clarity (color) of the fish crackers. Another factor that could have affected the color of fish crackers was the Maillard reaction, resulting from sugar in the formulation. This reaction occurs between the free amino group of lysine and other amino acids and the carbonyl groups of reducing sugars such as glucose, fructose, lactose and maltose [19] . Additionally, Camire et al. [19] reported that the Maillard reaction occurs during high temperatures and low moisture conditions used during industrial treatments of foods.
Hempel et al. [20] evaluated wafer crackers made with ultrafiltered syrup and different types of flour and found that the color of the crackers was lighter than without use of ultrafiltered syrup. They evidently attributed the results to the reduction (isomerization) of reducing sugar monomers in the syrup. Opposite results were found by Nurul et al. [10] who evaluated the effect of different ratios of fish meat to tapioca flour in fish cracker and found a decrease in lightness (
3.4.2.
The 


3.4.3.
The 



3.4.4.
The 





3.4.5.
The 

4. Conclusion
The effect of different salt concentrations (2%, 1.5%, and 1%) and particle sizes (nano-spray dried salt, microsalt, and regular salt) at the surface of cheese crackers on physicochemical characteristics of the crackers was evaluated. The 
Acknowledgements
This study was funded by USDA Hatch funds and Louisiana State University Agricultural Center, Baton Rouge, LA.
Conflicts of Interests
The authors declare no conflicts of interest.
Cite this paper
Moncada, M.L., Astete, C.E., Sabliov, C.M., Olson, D.W., Boeneke, C.A. and Aryana, K.J. (2017) Influence of Nano-Spray Dried Sodium Chloride on the Physicochemical Characteristics of Surface-Salted Cheese Crackers. Food and Nutrition Sciences, 8, 267-276. https://doi.org/10.4236/fns.2017.82017
References
- 1. Salt Institute (2016) Health Overview.
http://www.saltinstitute.org/health/overview/ - 2. Salt Institute (2016) Salt 101.
http://www.saltinstitute.org/salt-101/ - 3. He, J., Ogden, L.G., Vupputuri, S., Bazzano, L.A., Loria, C. and Whelton, P.K. (1999) Dietary Sodium Intake and Subsequent Risk of Cardiovascular Disease in Overweight Adults. The Journal of the American Medical Association, 282, 2027-2034.
https://doi.org/10.1001/jama.282.21.2027 - 4. Centers for Disease Control and Prevention (2016) Salt.
http://www.cdc.gov/salt/index.htm - 5. Aqualab (2015) Water Activity for Product Safety and Quality.
http://www.aqualab.com/education/water-activity-for-product-safety-and-quality/ - 6. Malovany, S. (2013) State of the Industry Report: Top Cracker Brands. Snack World, 26.
- 7. Moncada, M., Sabliov, C., Astete, C., Olson, D., Boeneke, C. and Aryana, K. (2015) Nano Spray-Dried Sodium Chloride and Its Effects on the Microbiological and Sensory Characteristics of Surface-Salted Cheese Crackers. Journal of Dairy Science, 98, 5946-5954.
https://doi.org/10.3168/jds.2015-9658 - 8. Sheen, R.T. and Kahler, H.L. (1938) Effects of Ions on Mohr Method for Chloride Determination. Industrial and Engineering Chemistry, Analytical Edition, 10, 628-629.
https://doi.org/10.1021/ac50127a004 - 9. Hunter Associates Laboratory (2003) MiniScan XE Plus User’s Guide.
- 10. Nurul, H., Boni, I. and Noryati, I. (2009) The Effect of Different Ratios of Dory Fish to Tapioca Flour on the Linear Expansion, Oil Absorption, Colour and Hardness of Fish Crackers. International Food Research Journal, 16, 159-165.
- 11. Hozova, B., Buchtova, V., Dodok, L. and Zemanovic, J. (1997) Microbiological, Nutritional and Sensory Aspects of Stored Amaranth Biscuits and Amaranth Crackers. Nahrung, 41, 151-158.
- 12. Weather Underground (2013) Baton Rouge, LA.
https://www.wunderground.com/history/airport/KBTR/2013/4/22/DailyHistory.html?req_city=&req_state=&req_statename=&reqdb.zip=&reqdb.magic=&reqdb.wmo - 13. Barbosa-Cánovas, G., Fontana, A.J. and Schmidt, S. (2008) Water Activity in Foods: Fundamentals and Applications. IFT Series, Vol. 13, John Wiley & Sons, Hoboken.
http://books.google.com/books - 14. Uline (2013) Packaging Materials.
http://www.uline.com - 15. Adams, M. and Moss, M. (2000) Food Microbiology. 2nd Edition, The Royal Society of Chemistry, London.
- 16. Nakamura, S., Suzuki, D., Kitadume, R. and Ohtsubo, K. (2012) Quality Evaluation of Rice Crackers Based on Physicochemical Measurements. Bioscience, Biotechnology, and Biochemistry, 76, 794-804.
https://doi.org/10.1271/bbb.110931 - 17. Malchev, E., Loncheva, N., Tanchev, S. and Kalpakchieva, K. (1982) Quantitative Changes in Carotenoids during the Storage of Dried Red Pepper. Nahrung, 26, 415-420.
https://doi.org/10.1002/food.19820260503 - 18. Mohamed, S., Abdullah, N. and Muthu, M.K. (1989) Physical Properties of Fried Crisps in Relation to the Amylopectin Content of the Starch Flours. Journal of the Science of Food and Agriculture, 49, 369-377.
https://doi.org/10.1002/jsfa.2740490312 - 19. Camire, M.E., Camire, A. and Krumhar, K. (1990) Chemical and Nutritional Changes in Foods during Extrusion. Critical Reviews in Food Science and Nutrition, 29, 35-57.
https://doi.org/10.1080/10408399009527513 - 20. Hempel, S., Jacob, A. and Rohn, H. (2007) Influence of Inulin Modification and Flour Type on Sensory Quality of Prebiotic Wafer Crackers. European Food Research and Technology, 224, 335-341.
https://doi.org/10.1007/s00217-006-0326-9 - 21. Bosset, J., Sieber, O.R. and Gallmann, P.U. (1995) Light Transmittance: Influence on the Shelf Life of Milk and Milk Products. Bulletin of the International Dairy Foundation, 300, 19-39.
- 22. Kristensen, D., Orlien, V., Mortensen, G., Brockhoff, P. and Skibsted, L.H. (2000) Light-Induced Oxidation in Sliced Havarti Cheese Packaged in Modified Atmosphere. International Dairy Journal, 10, 95-103.
https://doi.org/10.1016/S0958-6946(00)00028-5








