Food and Nutrition Sciences
Vol.07 No.07(2016), Article ID:67373,11 pages
10.4236/fns.2016.77056
Effect of Drying Techniques on Antioxidant Capacity of Guava Fruit
Priyanka Patel, Rajitha Sunkara, Lloyd T. Walker, Martha Verghese
Nutritional Biochemistry Laboratory, Normal, AL, USA

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 21 April 2016; accepted 11 June 2016; published 15 June 2016
ABSTRACT
Frequent consumption of fruits and vegetables has been associated with low risk of chronic diseases. Guava (Psidium guajava Linn.) is a tropical, seasonal fruit rich in antioxidants, vitamin C and polyphenol compounds. Drying is one of the common methods to preserve and extend the shelf life of guava. The objective of this study was to determine the effect of drying techniques on the antioxidant activity of guava fruit. Guava was air dried in air dryer (45˚C), freeze dryer and by osmatic drying techniques. Fresh guava extracts (FGE), freeze dried guava extracts (FDGE), oven dried guava extracts (ODGE) and osmotic-dehydrated guava extracts (OSGE) guava extracts were prepared and analyzed for total polyphenols (TP), flavonoids, antioxidant potential by 2,2-di- phenyl-1-picrylhydrazyl (DPPH), ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC), trolox equivalent antioxidant capacity (TEAC), total antioxidant capacity (TAC) and nitric oxide radical scavenging activity (NORS). Inhibitory potential of guava extracts on enzymes α-glucosidase, α-amylase and lipase was also determined. TP in FG, FD, OD, and OS were 415.69 ± 56.95, 295.30 ± 4.11, 303.57 ± 1.41, and 182.93 ± 6.48 mg gallic acid equivalent (GAE)/100g, respectively. Flavonoids in Fresh, FD, OD, and OS were 202.01 ± 0.16, 96.93 ± 1.73, 105.07 ± 0.58, and 76.13 ± 2.74 mg catechin equivalent (CE)/100 g, respectively. FD extracts were the most effective in scavenging DPPH radical. Whereas FRAP, TEAC and TAC activities were found to be higher in FG followed by OD and FD. However, NORS activity of FD was significantly (p ≤ 0.05) lower compared to other treatments. Inhibition of α-glycosidase, α-amylase and lipase enzymes was (19% - 90%) observed at 0.4, 0.8, and 0.8 mg/ml, respectively. In conclusion, considering this in-vitro study, drying could be effectively utilized to preserve guava fruit with minimum effect on health benefits.
Keywords:
Guava, Antioxidant, Drying Methods, Enzymes

1. Introduction
Oxidative stress plays a significant role in the progression of chronic diseases, which is a result of an imbalance between oxidants and antioxidants production [1] . Overproduction of reactive oxygen species (ROS) and reactive nitrogen species (RNS) can severely cause tissue damage. On another side, these free radicals are also important in various physiological and pathophysiological response, including cell signaling, gene expression, and apoptosis [2] [3] . During various metabolic processes, NADPH oxidase, xanthine oxidase, and nitric oxide synthases are produced as a by-product of ROS and RNS. However, endogenous antioxidants counteract oxidative stress by generating antioxidants enzymes such as superoxide ions, catalase, and glutathione peroxidase [2] [4] [5] . Consumption of fruits and vegetables rich in phytochemicals and antioxidant helps to fight health related diseases in the long term.
Studies [6] [7] have also shown that phytochemicals can act as therapeutic agents in reducing or preventing chronic diseases, including diabetes, cancer, obesity and hypertension. Phytochemicals are secondary metabolites of plant and play an imperative role in both human and animal health [8] . Studies [9] - [11] have shown that polyphenol content that is present in plants helps to reduce oxidative stress by scavenging reactive oxygen species and thus prevent cell damage. Antioxidant capacity of each fruit is different depending upon their phenolic content and vitamins [12] .
Guava (Psidium guajava Linn.) is a seasonal tropical fruit and highly perishable. Studies [13] have shown that guava tree and its components had potential therapeutic effects and have been used as folk medicine in many parts of world. Guava is a rich source of ascorbic acid. Vitamin C content of guava is almost six times greater than one orange thus considered as highly nutritious fruit [14] . Drying is one of the techniques that can increase the shelf life of guava. Various drying methods have been used to preserve guava such as air oven method, osmotic dehydration and lyophilization [15] - [17] . Drying may affect quality or quantity of phytochemicals. The present study was conducted to determine the total phytochemical and total antioxidant activity of guava extracts and to determine the inhibitory effects of guava extracts on α-amylase, α-glucosidase and lipase activities.
2. Materials and Methods
2.1. Sample Preparation
Fresh mature guava fruits were obtained from a local food store, Huntsville, Alabama. The fruits were washed, diced (1 inch) or sliced (0.5 mm) and were subjected to selected drying treatments to reach a moisture level of ≤10%. Diced guavas were placed in the freeze dryer (VirTis Genesis 35 L SpScientific, Warminster, PA) for 48 hours. Sliced guavas were placed in an oven (Isotemp Oven, Fisher Marietta OH, model 6925) at 54˚C for 48 hours. For osmotic dehydration, sliced guavas were submerged in a brine solution for two hours and placed in an oven (Isotemp Oven, Fisher Marietta OH, model 6925) at 45˚C for 48 hours. Dried guavas were made into a fine powder using a Waring blender (Model no. 31BL92, New Hartford, Connecticut) before extraction.
Fresh guava extracts (FGE), freeze-dried guava extracts (FDGE), oven dried guava extracts (ODGE) and osmotic dehydrated guava extracts (ODGE) were prepared following the method developed by Nunes et al., (2016) [18] with slight modifications. A known quantity of guava powder was soaked in 80% methanol for 24 hours with continuous shaking. The extracts were centrifuged at 10,000 g for 15 min. Supernatants were filtered using a Whatman filter paper and the filtrates were evaporated to dryness at 50˚C using a rotary evaporator (Buchi Rotavapor R-215). The concentrates were stored at −20˚C until further use.
2.2. Total Phenolic Content
Total polyphenols were determined following the Folin-Ciocalteu’s method using gallic acid as standard [19] . Folin-Ciocalteu’s reagent (12.5 µl) along with 7% sodium carbonate (125 µl) was added to the guava extracts. Samples were then incubated for 90 min at room temperature. The absorbance was measured at 750 nm using microplate reader (Synergy HT, Bio Tek Instruments, Winooski, VT, USA).
2.3. Total Flavonoid Content
Flavonoid content was determined according to Marinova et al., (2005) [20] using an aluminum chloride colorimetric assay using catechin as a standard. Samples, 7.5% sodium nitrite (7.5 µL) and 15 µL of 10% aluminum chloride were added to 1 M NaOH. Absorbance was measured at 520 nm after 5 min incubation. Total flavonoid content was expressed as mg of catechin equivalent (CE) per 100 g.
2.4. Antioxidant Assays
2.4.1. DPPH (2,2-Diphenyl-1-Picrylhydrazyl) Radical Scavenging Activity
The free radical scavenging activity was determined using Brand-William method [21] . DPPH (0.1M) solution was briefly added to 40 µl of samples. The absorbance was read at 517 nm for 90 min at 30 min interval in dark room temperature. Percentage of DPPH inhibition was calculated as follows:
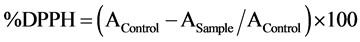
2.4.2. Ferric Reducing Antioxidative Potential (FRAP)
The FRAP of guava extracts was determined using FeSO4.7H2O standard [22] . Reagent A, 300 mM acetate buffer, pH 3.6 was prepared with 16 ml of glacial acetic acid and 3.1g of sodium acetate trihydrate. The FRAP reagent was prepared by the adding acetate buffer (30 mM), 10 mM TPTZ (2,4,6-Tris (2-pyridyl)-s-triazinein), 40 mM HCl and 20 mM FeCl3∙6H2O in 10:1:1 ratio (v/v). In a 96 well plate, 10 µl of standards and samples were added to 30 µl of FRAP reagent and distilled water. The absorbance was read at 593 nm for 4 min at 1 min intervals. The antioxidant potential of the samples was expressed as mM of FeSO4 per 100g.
2.4.3. Oxygen Radical Absorbance Capacity (ORAC)
ORAC was determined using trolox as the standard [23] . Freshly prepared 60 nm fluorescein sodium salt and 153 mM AAPH (2,2-azinobis (2-amidinopropane) dichloride) solution along with samples was incubated for 10 min at 37˚C. The absorbance of fluorescein was recorded for 60 min at excitation and emission wavelengths of 485 and 530 nm, respectively. The area under the curve (AUC) was calculated for each sample by integrating the relative fluorescence curve.
2.4.4. Trolox Equivalent Antioxidant Capacity (TEAC)
TEAC of extracts was determined using the protocol suggested by Miller et al. (1993) [24] . The ABTS 2, 2- azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (7 mM) radical cation solution was prepared by mixing concentrated ABTS radical and potassium persulfate in 1:1 ratio and the mixture was allowed to stand for 12-16 hours at dark room temperature. The ABTS cation working solution was prepared fresh by diluting with ethanol to an absorbance of (0.7 ± 0.025) at 734 nm. Diluted ABTS radical was added to samples and standards. Absorbance was read at 734 nm for 6 min at 1 min intervals. The total antioxidant capacity of samples was expressed as trolox equivalents.
2.4.5. Total Antioxidant Capacity by Phosphomolybdenum Method (TAC)
Total Anti-oxidative capacity was determined by phosphomolybdenum method using ascorbic acid as standard [25] . Guava extracts and 3 ml of reagent (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate) were mixed and incubated for 90 min at 5˚C. After the mixture was cooled, absorbance was read at 695 nm.
2.4.6. Nitric Oxide Radical Scavenging Activity (NORS)
The nitric oxide radical scavenging activity was determined using Griess Illosvoy reagent [26] . Sodium Nitroprusside (10 mM) and 1 mL of extracts or ascorbic acid standard were added in phosphate buffer (pH 7.4) and were incubated at 25˚C for 150 min. Griess reagent (1% sulphanilamide, 2% phosphoric acid and 0.1% naphthyl ethylene diamine dihydrochloride) was added to the reaction mixture (1:1). The ability of antioxidants to inhibit nitric oxide formation was determined based on the color change at 546 nm.
2.5. In-Vitro Enzymatic Assays
2.5.1. α-Glucosidase Inhibition Activity
α-glucosidase inhibition by guava extracts was determined using protocol suggested by Apostolidis et al. (2007) [27] . Phosphate buffer (50 mM; pH 6.8), enzyme and guava extracts were added. Sodium carbonate (0.1 M) and 1 mM pNPG was added to the samples after incubating for 30 min at 37˚C and absorbance was read at 405 nm. α-glucosidase inhibition was calculated as follows:
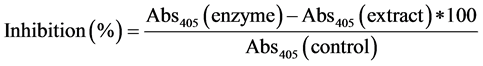
2.5.2. α-Amylase Inhibition Activity
α-Amylase inhibition by guava extracts was analyzed [27] . α-amylase and guava extracts were incubated for 10 min at 25˚C. Soluble starch (1%) and dinitrosalicylic acid was added to the samples. Samples were incubated for 10 min at 100˚C and final absorbance was read at 540 nm. α-amylase inhibition activity was calculated as follows:
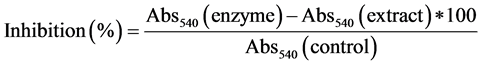
2.5.3. Lipase Activity
Inhibition of pancreatic lipase by guava extracts was determined using p-nitrophenyl butyrate (p-NPB) as a substrate [28] . Guava extracts, lipase and potassium phosphate buffer (pH 7.2) with 0.1% tween 80 was added to the samples and then incubated for 1 hour at 30˚C. After incubation, 25 mM of pNB (2,4 p-nitro phenyl butanoic acid) was added and incubated again for 5 min at 30˚C and absorbance was read at 405 nm. The inhibitory activity of lipase was calculated as follows:
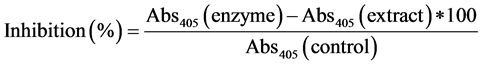
3. Results
3.1. Total Phenolic and Flavonoid Content
The polyphenols and flavonoid content in fresh guava extracts (FGE), freeze dried guava extracts (FDGE), oven dried guava extracts (ODGE) and osmotic dehydrated guava extracts (OSGE) were measured, and the results are shown in Table 1. Total polyphenols and flavonoids were significantly (p ≤ 0.05) affected by the different drying treatments. Phenolic content was significantly (p ≤ 0.05) higher in FGE followed by ODGE, FDGE and OSGE. Similarly, FGE had significantly (p ≤ 0.05) higher flavonoid content compared to ODGE, FDGE, and OSGE. Overall, phenolic and flavonoid content of guava extracts were highly affected by the drying techniques.
3.2. Antioxidant Activity of Guava Extracts
DPPH radical scavenging activity of guava extracts varied from 31.65% to 92.57% (0 - 1.2 mg), which represents the variation among different drying treatments of guava in radical scavenging (Figure 1). DPPH radical scavenging activity increased with an increase in the concentrations of FGE, FDGE and ODGE. DPPH radical scavenging activity was higher in FGE followed by the FDGE, ODGE, and OSGE. IC50 of FGE, FDGE,
Table 1. Total polyphenols and flavonoids in guava extracts.
Values (n = 3) are means ± SEM; Means in a column with superscripts (abcd) without a common letter differ p ≤ 0.05. Abbreviations: FGE: fresh guava extract, FDGE: freeze dried guava extract, ODGE: oven dried guava extract, and OSGE: osmotic dehydrated guava extract, GAE: gallic acid equivalents, CE: catechin equivalents.
Figure 1. Percent DPPH inhibition of methanol guava extracts. Values (n = 3) are means ± SEM. Abbreviations: FGE: fresh guava extract, FDGE: freeze dried guava extract, ODGE: oven dried guava extract, OSGE: osmotic dehydrated guava extract, DPPH: 2,2-diphenyl-1-picrylhydrazyl.
and ODGE was observed at 0.3, 0.5, and 0.5 mg, respectively. IC50 was not observed by OSGE; with only a 30% inhibition was observed at the 0.4 mg concentration.
Ferric reducing antioxidant power (FRAP), oxygen radical antioxidant capacity (ORAC), trolox equivalence antioxidant capacity (TEAC), total antioxidant capacity (TAC) and nitric oxide radical scavenging ability (NORS) of FGE, FDGE, ODGE and OSGE is shown in Figure 2. FRAP activity of FGE and ODGE were significantly (p ≤ 0.05) higher compared to FDGE and OSGE. However, no significant (p ≤ 0.05) differences were observed between FGE and ODGE. ORAC activity was seen significantly (p ≤ 0.05) higher in FGE compared to ODGE, FDGE and OSGE. There were significant (p ≤ 0.05) differences in ORAC among all the treatments. Trolox equivalence of FGE was significantly (p ≤ 0.05) higher compared to FDGE, ODGE and OSGE. However, there was no significant (p ≤ 0.05) difference seen between FDGE and ODGE. Drying treatments significantly (p ≤ 0.05) affected the TAC of guava extracts. TAC was significantly (p ≤ 0.05) higher in FGE compared to FDGE, ODGE and OSGE. However, no significant (p ≤ 0.05) differences were observed among FDGE, ODGE and OSGE. However, NORS of FDGE was significantly (p ≤ 0.05) lower compared to FGE, ODGE and OSGE. Overall, FRAP, ORAC, TEAC, and TAC activities of FGE and ODGE was significantly (p ≤ 0.05) higher compared to ODGE, FDGE and OSGE.
3.3. In-Vitro Enzymatic Assays
The percentage of α-glucosidase, α-amylase and lipase inhibitory activity of methanol guava extracts from three different drying treatments is shown in Figures 3-5. Guava extracts showed α-glucosidase inhibition from 32% to 90%, α-amylase inhibition from 17% to 53%, and lipase inhibition from 14% -19%. Increase in concentrations increased the α-glucosidase, α-amylase, and lipase inhibition.
α-glucosidase inhibition was higher in FGE compared to FDGE, ODGE, and OSGE (Figure 3). However, α-glucosidase inhibition by FGE, FDGE, ODGE and OSGE leveled off after inhibiting α-glucosidase by 90%, 84%, 84%, and 67%, respectively. For α-glucosidase, IC50 of FDGE was observed at 5 µg, whereas ODGE and OSGE inhibited at 50 µg.
α-amylase inhibition was higher in FDGE and ODGE compared to FGE and OSGE (Figure 4). At 0.8 mg FGE, FDGE, ODGE and OSGE leveled off after inhibiting α-amylase by 41%, 49%, 53% and 35%. IC50 of FGE, FDGE and ODGE was observed at 0.8 mg, whereas, OSGE only inhibited α-amylase by 35% at 0.8 mg concentration.
In pancreatic lipase activity ODGE (17%) and OSGE (19%) showed highest inhibition at a concentration of 0.6 mg compared to FDGE (16%) and FGE (14%). However, lipase inhibition by FGE, FDGE, ODGE, and ODGE leveled off at the 1.5 mg concentration. FGE, FDGE, ODGE and OSGE did not reach IC50 even at highest (2 mg) concentration (Figure 5).
4. Discussion
Diet plays a critical role in the maintenance of a healthy lifestyle. An increased consumption of fruits and vegetables is associated with a lower risk of chronic diseases [29] . Antioxidants that are present in tropical fruits such
Figure 2. Antioxidant potential of guava extracts. Abbreviations: FGE-fresh guava extract, FDGE: freeze dried extract, ODGE: oven dried guava extract, OSGE: osmotic dehydrated guava extract, FRAP: ferric reducing antioxidant potential, ORAC: oxygen radical absorbance capacity, TEAC: trolox equivalent antioxidant capacity, TAC: total antioxidant capacity, NORS: nitric oxide radical scavengers, AA: ascorbic acid. Values are means (n = 3) ± SEM; Means in a row with superscripts without a common letter differ p ≤ 0.05.
Figure 3. α-glucosidase inhibitory activity of guava extracts. Values (n = 3) are means ± SEM. Abbreviations: FGE: fresh guava extract, FDGE: freeze dried guava extract, ODGE: oven dried guava extract, and OSGE: osmotic dehydrated guava extract.
Figure 4. α-amylase inhibitory activity of guava extracts. Values (n = 3) are means ± SEM. Abbreviations: FGE: fresh guava extract, FDGE: freeze dried extract, ODGE: oven dried guava extract, and OSGE: osmotic dehydrated guava extract.
Figure 5. Lipase inhibition activity of guava extracts. Values are means (n = 3) ± SEM. Abbreviations: FGE: fresh guava extract, FDGE: freeze dried extract, ODGE: oven dried guava extract, and OSGE: osmotic dehydrated guava extract.
as tocopherol, carotenoids, ascorbic acid, polyphenols, and flavonoids are likely to provide protective effects against degenerative diseases [30] . Many studies [7] [31] [32] have reported that consumption of polyphenol rich foods exert various biological effects. These phenolic compounds may reduce the risk of chronic diseases, including diabetes [9] .
Extraction of phenolic compounds in fruits is not only dependent on the extraction methods but also on the nature of plants, temperature, and the polarity of the solvent. Methanol can extract most polyphenol constituents in plant materials due to its lower polarity compared to water [33] . In the present study, three different drying techniques were used to quantify the total phenolic and antioxidant capacity of guavas. A phenolic compound reduces the FC reagent, which is measured at 750 nm against a Gallic acid standard. Total phenolic content of FGE (415 mg/100g) was significantly (p ≤ 0.05) higher compared to the FDGE, ODGE and OSGE, which might be due to the progression of oxidation with the drying time [17] . Whereas, phenolic content of OSGE (182.93 mg/100g) was significantly (p ≤ 0.05) lower compared to FDGE and ODGE. This might be due to the breakdown of phytochemicals by wet thermal processing which affected the integrity of the cell membrane resulting in the migration of components, losses by leakage or breakdown of phenolic components by various chemical reactions [34] .
Flavonoids are large secondary metabolites that are widely found in plant species. In the present study, flavonoid content was estimated using the AlCl3 colorimetric method. AlCl3 forms stable acid complexes with the C-4 keto or hydroxyl group of the flavones and flavonols along with the ortho dihydroxyl groups in the A or B-ring of flavonoids. Flavonoid content of guava extracts showed a similar trend as total phenolic content could be because flavonoids are a major part of phenolic compounds [35] . Flavonoid content of FDGE, ODGE and OSGE were significantly (p ≤ 0.05) lower compared to FGE, and this might be due to the harsh drying conditions, particularly temperature and time exposure [36] . A previous study by Mohd Zainol et al. (2009) [37] also found degradation of flavonoid components in C. asiatica due to the different drying treatments (freeze dried, vacuum dried, oven dried).
Antioxidant capacity of fruits depends upon the total phenolic and flavonoid content that is present [38] . In this assay, reduction of the DPPH radical occurs in the presence of hydrogen donating antioxidants [39] . DPPH radical scavenging activity by FGE was higher (p ≤ 0.05) compared to FDGE, ODGE, and OSGE. This might be due to higher phenolic content present in FGE. A study by Suvarnakuta et al. (2011) [40] on drying effects on mangosteen rind at temperatures of 60˚C, 75˚C and 90˚C showed that the drying methods significantly affected degradation of xanthones in α-mangostin and its antioxidant capacity (IC50 -25.6 μg/ml).
In the presence of antioxidants, the reducing power of guava extracts was evaluated based on the transformation of ferric ion to ferrous ions. In the present study, FGE and ODGE extracts showed potent reducing power, which might be due to the concentration of polyphenols and Maillard reaction products due to the drying process [41] - [43] . However, FRAP activity was significantly (p ≤ 0.05) lower in OSGE, which might be due to drying. A study reported by Shofian et al. (2011) [44] showed that FRAP activity of fresh starfruit and mango were significantly (p ≤ 0.05) higher compared to the dried samples.
The ABTS radical is generated by potassium persulfate, to determine the antioxidant activity of hydrogen- donating antioxidants. Higher TEAC value of FGE might be due to the ability to donate hydrogen and terminate the oxidation process by converting free radicals into their stable form [45] . However, OSGE showed significantly (p ≤ 0.05) lower TEAC value and this loss might be due to the decomposition of natural antioxidants through losses of phenolic compounds by thermal processing. Kalt et al. (2000) [46] and Larrauri et al. (1997) [47] reported similar results in the reduction of antioxidant potential after drying (50%) and canning (65%) of red grape pomace and blueberries.
The phosphomolybdenum method was used to evaluate the total antioxidant activity of the extracts. At an acidic pH, antioxidants have the ability to reduce molybdenum (VI) to molybdenum (V) and the green color phosphate molybdenum (V) complex. TAC value in FGE compared to FDGE, ODGE and OSGE may have been higher due to the higher concentration of polyphenols and flavonoids. A study by Bag & Devi (2015) [48] on methanolic rhizome extracts observed that methanolic rhizome extract of H. rubrum showed the highest total antioxidant activity among all the three Hedychium species may be due to higher total flavonoid content.
The ORAC method estimates the antioxidant capacity of a sample by taking the oxidation reaction induced by peroxyl radical to completion through H atom transfer [49] . ORAC activity of FGE was higher compared to FDGE and OSGE. This loss might be due to the drying effect on the soluble phenolic content present in the guava extracts [6] .
The antioxidant properties of phenolic compounds are based on their redox reaction potential, which plays a crucial role in neutralization of free radicals [50] . Sodium Nitroprusside generates the nitric oxide radical with an optimum pH, which further interacts with oxygen to produce nitrite ions. Nitric oxide scavengers compete with the oxygen, resulting in lower production of nitrite ions [51] . In this study, FDGE showed significantly (p ≤ 0.05) lower NO activity compared to the FGE, ODGE, and OSGE. A study by Noda et al. (2002) [52] on freeze- dried pomegranate extracts showed similar results.
The inhibition of carbohydrate metabolizing enzymes is very critical in lowering blood glucose level, and this may be achieved by consuming fruits and vegetables, which have the ability to slow the digestion of carbohydrates. Several plants have been studied for their potential in inhibition of carbohydrate hydrolyzing enzymes and lowering blood glucose level [53] [54] . The current study showed higher inhibition of α-glucosidase activity by FGE compared to FDGE, ODGE, and OSGE. Phytochemicals that are present in fresh guava might have increased the α-glucosidase inhibition activity. However, α-amylase and pancreatic lipase activities were enhanced by the drying treatments when the phenolic content of FDGE, ODGE and OSGE was significantly (p ≤ 0.05) lower compared to FGE. Phytochemicals in guava might inhibit activity of enzymes involved in digestibility of carbohydrates and lipids [55] . Moreno et al., (2003) [56] reported similar results that grape seed extracts at a concentration of 1 mg/mL resulted in the inhibition of 80% of enzyme activity due to the presence of anthocyanin compounds.
5. Conclusion
Drying techniques differ in phytochemical content of guava extracts and its antioxidant capacity. Applying drying to the highly perishable fruit not only preserves nutritive value of fruit but also retains the antioxidants properties.
Cite this paper
Priyanka Patel,Rajitha Sunkara,Lloyd T. Walker,Martha Verghese, (2016) Effect of Drying Techniques on Antioxidant Capacity of Guava Fruit. Food and Nutrition Sciences,07,544-554. doi: 10.4236/fns.2016.77056
References
- 1. Dröge, W. (2002) Free Radicals in the Physiological Control of Cell Function. Physiological Reviews, 82, 47-95.
http://dx.doi.org/10.1152/physrev.00018.2001 - 2. Dowling, D.K. and Simmons, L.W. (2009) Reactive Oxygen Species as Universal Constraints in Life-History Evolution. Proceedings of the Royal Society of London B: Biological Sciences, rspb-2008.
http://dx.doi.org/10.1098/rspb.2008.1791 - 3. Scherz-Shouval, R. and Elazar, Z. (2011) Regulation of Autophagy by ROS: Physiology and Pathology. Trends in Biochemical Sciences, 36, 30-38.
http://dx.doi.org/10.1016/j.tibs.2010.07.007 - 4. Grek, C.L. and Tew, K.D. (2010) Redox Metabolism and Malignancy. Current Opinion in Pharmacology, 10, 362-368.
http://dx.doi.org/10.1016/j.coph.2010.05.003 - 5. Nathan, C. and Ding, A. (2010) SnapShot: Reactive Oxygen Intermediates (ROI). Cell, 140, 951-951.
http://dx.doi.org/10.1016/j.cell.2010.03.008 - 6. Kwon, Y.I.I., Vattem, D.A. and Shetty, K. (2006) Evaluation of Clonal Herbs of Lamiaceae Species for Management of Diabetes and Hypertension. Asia Pacific Journal of Clinical Nutrition, 15, 107-118.
http://apjcn.org/update%5Cpdf%5C2006%5C1%5C107%5C107.pdf - 7. Winston, C. and Beck, L. (1999) Phytochemicals: Health Protective Effects. Canadian Journal of Dietetic Practice and Research, 60, 78.
http://crawl.prod.proquest.com.s3.amazonaws.com/fpcache/79a1a691ed09746ac52d46cc9255934f.pdf?AWSAccessKeyId=AKIAJF7V7KNV2KKY2NUQ&Expires=1460569239&Signature=%2FKu6JZd0OTbbmjCqIzhVwKivMLo%3D - 8. Kim, D.O., Jeong, S.W. and Lee, C.Y. (2003) Antioxidant Capacity of Phenolic Phytochemicals from Various Cultivars of Plums. Food Chemistry, 81, 321-326.
http://dx.doi.org/10.1016/S0308-8146(02)00423-5 - 9. Sabu, M.C., Smitha, K. and Kuttan, R. (2002) Anti-Diabetic Activity of Green Tea Polyphenols and Their Role in Reducing Oxidative Stress in Experimental Diabetes. Journal of Ethnopharmacology, 83, 109-116.
http://dx.doi.org/10.1016/S1360-1385(02)02312-9 - 10. Lean, M.E., Noroozi, M., Kelly, I., Burns, J., Talwar, D., Sattar, N. and Crozier, A. (1999) Dietary Flavonols Protect Diabetic Human Lymphocytes against Oxidative Damage to DNA. Diabetes, 48, 176-181.
http://diabetes.diabetesjournals.org/content/48/1/176.full.pdf+html
http://dx.doi.org/10.2337/diabetes.48.1.176 - 11. Mittler, R. (2002) Oxidative Stress, Antioxidants and Stress Tolerance. Trends in Plant Science, 7, 405-410.
- 12. Saura-Calixto, F. and Goñi, I. (2006) Antioxidant Capacity of the Spanish Mediterranean Diet. Food Chemistry, 94, 442-447.
http://dx.doi.org/10.1016/j.foodchem.2004.11.033 - 13. Dhiman, A., Nanda, A., Ahmad, S. and Narasimhan, B. (2011) In Vitro Antimicrobial Activity of Methanolic Leaf Extract of Psidium guajava L. Journal of Pharmacy and Bioallied Sciences, 3, 226-229.
http://www.jpbsonline.org/text.asp?2011/3/2/226/80776
http://dx.doi.org/10.4103/0975-7406.80776 - 14. McCook-Russell, K.P., Nair, M.G., Facey, P.C. and Bowen-Forbes, C.S. (2012) Nutritional and Nutraceutical Comparison of Jamaican Psidium cattleianum (Strawberry Guava) and Psidium guajava (Common Guava) Fruits. Food Chemistry, 134, 1069-1073.
http://dx.doi.org/10.1016/j.foodchem.2012.03.018 - 15. Duangmal, K. and Khachonsakmetee, S. (2009) Osmotic Dehydration of Guava: Influence of Replacing Sodium Metabisulphite with Honey on Quality. International Journal of Food Science & Technology, 44, 1887-1894.
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.2008.01906.x/full
http://dx.doi.org/10.1111/j.1365-2621.2008.01906.x - 16. Osorio, C., Carriazo, J.G. and Barbosa, H. (2011) Thermal and Structural Study of Guava (Psidium guajava L) Powders Obtained by Two Dehydration Methods. Química Nova, 34, 636-640.
http://dx.doi.org/10.1590/S0100-40422011000400016 - 17. Siow, L.F. and Hui, Y.W. (2013) Comparison on the Antioxidant Properties of Fresh and Convection Oven-Dried Guava (Psidium guajava L.). International Food Research Journal, 20, 639-644.
http://www.ifrj.upm.edu.my/20%20(02)%202013/20%20IFRJ%2020%20(02)%202013%20Lee%20(282)pdf - 18. Nunes, J.C., Lago, M.G., Castelo-Branco, V.N., Oliveira, F.R., Torres, A.G., Perrone, D. and Monteiro, M. (2016) Effect of Drying Method on Volatile Compounds, Phenolic Profile and Antioxidant Capacity of Guava Powders. Food Chemistry, 197, 881-890.
http://dx.doi.org/10.1016/j.foodchem.2015.11.050 - 19. Singleton, V.L., Orthofer, R. and Lamuela-Raventos, R.M. (1999) Analysis of Total Phenols and Other Oxidation Sub-Strates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods in Enzymology, 299, 152-178.
http://dx.doi.org/10.1016/S0076-6879(99)99017-1 - 20. Marinova, D., Ribarova, F. and Atanassova, M. (2005) Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. Journal of the University of Chemical Technology and Metallurgy, 40, 255-260.
https://www.researchgate.net/profile/Maria_Atanassova/publication/258769164_Total_phenolics _and_flavonoids_in_Bulgarian_fruits_and_vegetables/links/00463528f0a28 e54a6000000.pdf - 21. Brand-Williams, W., Cuvelier, M.E. and Berset, C.L.W.T. (1995) Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Science and Technology, 28, 25-30.
http://dx.doi.org/10.1016/S0023-6438(95)80008-5 - 22. Benzie, I.F. and Strain, J.J. (1998) Ferric Reducing/Antioxidant Power Assay: Direct Measure of Total Antioxidant Activity of Biological Fluids and Modified Version for Simultaneous Measurement of Total Antioxidant Power and Ascorbic Acid Concentration. Methods in Enzymology, 299, 15-27.
http://dx.doi.org/10.1016/S0076-6879(99)99005-5 - 23. Huang, D., Ou, B., Hampsch-Woodill, M., Flanagan, J.A. and Prior, R.L. (2002) High-Throughput Assay of Oxygen Radical Absorbance Capacity (ORAC) Using a Multichannel Liquid Handling System Coupled with a Microplate Fluorescence Reader in 96-Well Format. Journal of Agricultural and Food Chemistry, 50, 4437-4444.
http://pubs.acs.org/doi/abs/10.1021/jf0201529
http://dx.doi.org/10.1021/jf0201529 - 24. Miller, N.J., Rice-Evans, C., Davies, M.J., Gopinathan, V. and Milner, A. (1993) A Novel Method for Measuring Antioxidant Capacity and Its Application to Monitoring the Antioxidant Status in Premature Neonates. Clinical Science (London, England: 1979), 84, 407-412.
http://www.clinsci.org/content/ppclinsci/84/4/407.full.pdf - 25. Prieto, P., Pineda, M. and Aguilar, M. (1999) Spectrophotometric Quantitation of Antioxidant Capacity through the Formation of a Phosphomolybdenum Complex: Specific Application to the Determination of Vitamin E. Analytical Biochemistry, 269, 337-341.
http://dx.doi.org/10.1006/abio.1999.4019 - 26. Sakat, S.S. and Juvekar, A.R. (2010) Comparative Study of Erythrina indica Lam. (Febaceae) Leaves Extracts for Antioxidant Activity. Journal of Young Pharmacists, 2, 63-67.
http://dx.doi.org/10.4103/0975-1483.62216 - 27. Apostolidis, E., Kwon, Y.I. and Shetty, K. (2007) Inhibitory Potential of Herb, Fruit, and Fungal-Enriched Cheese against Key Enzymes Linked to Type 2 Diabetes and Hypertension. Innovative Food Science & Emerging Technologies, 8, 46-54.
http://dx.doi.org/10.1016/j.ifset.2006.06.001 - 28. Mosmuller, E.W.J., Van Heemst, J.D.H., Van Delden, C.V., Franssen, M.C.R. and Engbersen, J.F.J. (1992) A New Spectrophotometric Method for the Detection of Lipase Activity Using 2, 4-Dinitrophenyl Butyrate as a Substrate. Biocatalysis, 5, 279-287.
http://dx.doi.org/10.3109/10242429209014874 - 29. Van Dam, R.M., Rimm, E.B., Willett, W.C., Stampfer, M.J. and Hu, F.B. (2002) Dietary Patterns and Risk for Type 2 Diabetes Mellitus in US Men. Annals of Internal Medicine, 136, 201-209.
http://www.ncbi.nlm.nih.gov/pubmed/11827496
http://dx.doi.org/10.7326/0003-4819-136-3-200202050-00008 - 30. Morton, J.F. (1987) Fruits of Warm Climates. Morton, Miami.
http://library.wur.nl/WebQuery/clc/539040 - 31. Arts, I.C. and Hollman, P.C. (2005) Polyphenols and Disease Risk in Epidemiologic Studies. The American Journal of Clinical Nutrition, 81, 317S-325S.
http://ajcn.nutrition.org/content/81/1/317S.full.pdf+html - 32. Srinivasan, K. (2005) Plant Foods in the Management of Diabetes Mellitus: Spices as Beneficial Antidiabetic Food Adjuncts. International Journal of Food Sciences and Nutrition, 56, 399-414.
http://www.tandfonline.com/doi/citedby/10.1080/09637480500512872
http://dx.doi.org/10.1080/09637480500512872 - 33. Cai, Y., Luo, Q., Sun, M. and Corke, H. (2004) Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sciences, 74, 2157-2184.
http://dx.doi.org/10.1016/j.lfs.2003.09.047 - 34. Davey, M.W., Montagu, M.V., Inzé, D., Sanmartin, M., Kanellis, A., Smirnoff, N. and Fletcher, J. (2000) Plant L-Ascorbic Acid: Chemistry, Function, Metabolism, Bioavailability and Effects of Processing. Journal of the Science of Food and Agriculture, 80, 825-860.
http://onlinelibrary.wiley.com/doi/10.1002/(SICI)> 1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6/epdf http://dx.doi.org/10.1002/(SICI)1097-0010(20000515)80:7<825::AID-JSFA598>3.0.CO;2-6 - 35. Ismail, H.I., Chan, K.W., Mariod, A.A. and Ismail, M. (2010) Phenolic Content and Antioxidant Activity of Cantaloupe (Cucumis melo) Methanolic Extracts. Food Chemistry, 119, 643-647.
http://dx.doi.org/10.1016/j.foodchem.2009.07.023 - 36. Schieber, A., Keller, P. and Carle, R. (2001) Determination of Phenolic Acids and Flavonoids of Apple and Pear by High-Performance Liquid Chromatography. Journal of Chromatography A, 910, 265-273.
http://dx.doi.org/10.1016/S0021-9673(00)01217-6 - 37. Zainol, M., Khairi, M., Abdul Hamid, A., Abu Bakar, F. and Pak Dek, M.S. (2009) Effect of Different Drying Methods on the Degradation of Selected Flavonoids in Centella asiatica. International Food Research Journal, 16, 531-537.
http://www.ifrj.upm.edu.my/16%20%284%29%202009/09%20IFRJ-2009-108%20Azizah%20Malaysia%202nd%20proof.pdf - 38. Mai, T.T., Thu, N.N., Tien, P.G. and CHUYEN, N.V. (2007) Alpha-Glucosidase Inhibitory and Antioxidant Activities of Vietnamese Edible Plants and Their Relationships with Polyphenol Contents. Journal of Nutritional Science and Vitaminology, 53, 267-276.
https://www.jstage.jst.go.jp/article/jnsv/53/3/53_3_267/_pdf - 39. Blois, M.S. (1958) Antioxidant Determinations by the Use of a Stable Free Radical. Nature, 181, 1199-1200.
http://www.nature.com/nature/journal/v181/n4617/pdf/1811199a0.pdf
http://dx.doi.org/10.1038/1811199a0 - 40. Suvarnakuta, P., Chaweerungrat, C. and Devahastin, S. (2011) Effects of Drying Methods on Assay and Antioxidant Activity of Xanthones in Mangosteen Rind. Food Chemistry, 125, 240-247.
http://dx.doi.org/10.1016/j.foodchem.2010.09.015 - 41. He, Q. and Venant, N. (2004) Antioxidant Power of Phytochemicals from Psidium guajava Leaf. Journal of Zhejiang University Science, 5, 676-683.
http://link.springer.com/article/10.1007/BF02840979
http://dx.doi.org/10.1631/jzus.2004.0676 - 42. Al-Farsi, M., Alasalvar, C., Morris, A., Baron, M. and Shahidi, F. (2005) Comparison of Antioxidant Activity, Anthocyanins, Carotenoids, and Phenolics of Three Native Fresh and Sun-Dried Date (Phoenix dactylifera L.) Varieties Grown in Oman. Journal of Agricultural and Food Chemistry, 53, 7592-7599.
http://pubs.acs.org/doi/abs/10.1021/jf050579q
http://dx.doi.org/10.1021/jf050579q - 43. Leong, L.P. and Shui, G. (2002) An Investigation of Antioxidant Capacity of Fruits in Singapore Markets. Food Chemistry, 76, 69-75.
http://dx.doi.org/10.1016/S0308-8146(01)00251-5 - 44. Shofian, N.M., Hamid, A.A., Osman, A., Saari, N., Anwar, F., Pak Dek, M.S. and Hairuddin, M.R. (2011) Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. International Journal of Molecular Sciences, 12, 4678-4692.
http://www.mdpi.com/1422-0067/12/7/4678/htm
http://dx.doi.org/10.3390/ijms12074678 - 45. Tachakittirungrod, S., Okonogi, S. and Chowwanapoonpohn, S. (2007) Study on Antioxidant Activity of Certain Plants in Thailand: Mechanism of Antioxidant Action of Guava Leaf Extract. Food Chemistry, 103, 381-388.
http://dx.doi.org/10.1016/j.foodchem.2006.07.034 - 46. Kalt, W., McDonald, J.E. and Donner, H. (2000) Anthocyanins, Phenolics, and Antioxidant Capacity of Processed Lowbush Blueberry Products. Journal of Food Science, 65, 390-393.
http://onlinelibrary.wiley.com/doi/10.1111/j.1365-2621.2000.tb16013
http://dx.doi.org/10.1111/j.1365-2621.2000.tb16013.x - 47. Larrauri, J.A., Rupérez, P. and Saura-Calixto, F. (1997) Effect of Drying Temperature on the Stability of Polyphenols and Antioxidant Activity of Red Grape Pomace Peels. Journal of Agricultural and Food Chemistry, 45, 1390-1393.
http://dx.doi.org/10.1021/jf960282f - 48. Bag, G.C. and Devi, P.G. (2015) Assessment of Total Flavonoid Content and Antioxidant Activity of Methanolic Rhizome Extract of Three Hedychium Species of Manipur Valley . International Journal of Pharmaceutical Sciences Review and Research, 30, 154-159.
http://globalresearchonline.net/journalcontents/v30-1/28.pdf - 49. Ou, B., Hampsch-Woodill, M. and Prior, R.L. (2001) Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. Journal of Agricultural and Food Chemistry, 49, 4619-4626.
http://pubs.acs.org/doi/pdf/10.1021/jf010586o
http://dx.doi.org/10.1021/jf010586o - 50. Bhaskar, H. and Balakrishnan, N. (2009) In Vitro Antioxidant Property of Laticiferous Plant Species from Western Ghats Tamilnadu, India. International Journal of Health Research, 2, 163-170.
http://www.ajol.info/index.php/ijhr/article/view/55413/43883 - 51. Ebrahimzadeh, M.A., Nabavi, S.M., Nabavi, S.F., Bahramian, F. and Bekhradnia, A.R. (2010) Antioxidant and Free Radical Scavenging Activity of H. Officinalis, L. Var. Angustifolius, V. Odorata, B. Hyrcana and C. Speciosum. Pakistan Journal of Pharmaceutical Sciences, 23, 29-34.
https://www.researchgate.net/profile/Mohammad_Ebrahimzadeh/publication /41000537_Antioxidant_and_free_radical_scavenging_activity_of_H._ officinalis_L._Var._angustifolius_V._odorata_B. _hyrcana_and_C._speciosum/links/0fcfd5080719b66ebd000000.pdf - 52. Noda, Y., Kaneyuki, T., Mori, A. and Packer, L. (2002) Antioxidant Activities of Pomegranate Fruit Extract and Its Anthocyanidins: Delphinidin, Cyanidin, and Pelargonidin. Journal of Agricultural and Food Chemistry, 50, 166-171.
http://dx.doi.org/10.1021/jf0108765 - 53. Bailey, C. J. and Day, C. (1989) Traditional Plant Medicines as Treatments for Diabetes. Diabetes Care, 12, 553-564.
http://care.diabetesjournals.org/content/12/8/553.full.pdf+html
http://dx.doi.org/10.2337/diacare.12.8.553 - 54. Ernst, E. (1997) Plants with Hypoglycemic Activity in Humans. Phytomedicine, 4, 73-78.
http://dx.doi.org/10.1016/S0944-7113(97)80031-1 - 55. Nakai, M., Fukui, Y., Asami, S., Toyoda-Ono, Y., Iwashita, T., Shibata, H. and Kiso, Y. (2005) Inhibitory Effects of Oolong Tea Polyphenols on Pancreatic Lipase in Vitro. Journal of Agricultural and Food Chemistry, 53, 4593-4598.
http://dx.doi.org/10.1021/jf047814+ - 56. Moreno, D.A., Ilic, N., Poulev, A., Brasaemle, D.L., Fried, S.K. and Raskin, I. (2003) Inhibitory Effects of Grape Seed Extract on Lipases. Nutrition, 19, 876-879.
http://dx.doi.org/10.1016/S0899-9007(03)00167-9











