Food and Nutrition Sciences
Vol.4 No.8(2013), Article ID:35187,8 pages DOI:10.4236/fns.2013.48103
Gender May Affect the Dose-Dependent Action of Capsaicinoids on Plasma and Hepatic Cholesterol Levels of Rats*#
![]()
College of Food Science, Southwest University, Chongqing, China.
Email: †liuxiong503@yahoo.cn
Copyright © 2013 Guoshan Fang et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received May 21st, 2013; revised June 21st, 2013; accepted June 30th, 2013
Keywords: Capsaicinoids; Cholesterol; Bile Acids; Dose-Dependent; Sex Difference
ABSTRACT
Sex differences on the effect of dose-dependent capsaicinoids on lipid metabolism were studied in rats. 24 rats of each sex were administered orally 0 mg/kg, 2.5 mg/kg, 5 mg/kg or 7.5 mg/kg capsaicinoids daily for 28 days. In male rats, body weight gained, and the levels of total lipids, total cholesterol and triglycerides in the liver were significantly decreased as the dose of capsaicinoids increased. On the other hand, plasma total cholesterol (TC), triglycerides (TG), HDLand LDL-cholesterol concentration and liver weight were not affected by capsaicinoids. While in female rats, plasma TC, TG, HDL-C and LDL-C concentration, liver total lipids, TC and TG concentration were significantly decreased as the dose of capsaicinoids increased. The mRNA level of hepatic TRPV1, ileac ASBT and IBABP were increased as the dose of capsaicinoids increased in all rats groups. The mRNA level of hepatic HMG-CoA reductase, CYP7A1 and FXR were significantly decreased in female rats groups. These results show that the hypocholesterolemic effect of capsaicinoids in dose-dependent manner in rats was mediated by inhibited synthesis of endogenous cholesterol, female rats were more sensitive than male rats on hypolipidemic effect of capsaicinoids.
1. Introduction
Chili is the major source of nature capsaicinoids, which consists of capsaicin, dihydrocapsaicin, nordihydrocapsaicin, homodihydrocapsaicin, and homocapsaicin, etc. Snitker S. et al. [1] had reported that treatment with 6 mg/d capsinoids orally appeared to be safe and was associated with abdominal fat loss. Recently studies have shown that protective effect of dietary capsaicinoids on gastric mucosa may relate to the increase of gastric mucosal blood flow (GMBF) [2], promote gastric motility [3], regulate gastric acid and stimulate the secretion of prostate E1 [4,5]. Furthermore, a host of beneficial physiological effects have been brought to the fore by extensive animal studies, such as anti-inflammatory potential [6], antioxidant property [7], anti-carcinogenic [8,9] and regulation of blood pressure [10]. Capsaicinoids have also been proposed for use in strategies for weight loss and weight maintenance [11].
Cholesterol lowering through the consumption of capsaicinoids draws much attention lately. Much evidence indicates that the decrease of triglyceride concentration in plasma and liver by capsaicinoids may relate to body fat oxidation increasing [12-14]. However, little is known about the effect of capsaicinoids on cholesterol metabolism. Srinivasan K et al. [15] reported that the activity of hepatic cholesterol-7 alpha-hydroxylase (CYP7A1), the rate-limiting enzyme of bile acid biosynthesis, were significantly elevated by capsaicin, bile acid biosynthesis increasing; the concentration of cholesterol in plasma and liver increased also. It may due to capsaicin that increased the cholesterol biosynthesis in liver and/or intestinal absorption of cholesterol in rats. Manjunatha and Srinivasan [12] had demonstrated that administered capsaicin significantly lower the hepatic cholesterol concentration in rats fed cholesterol-free diet, but no effect in those fed high cholesterol diet. They [16] had also reported that administration of capsaicin slightly lowered the concentration of cholesterol in plasma and liver in rats fed high fat diet containing 30% fat. However, some studies had shown a significant decrease in plasma total cholesterol (TC) concentration in rats fed 0.015% capsaicin, but little effect in rats fed high cholesterol diet [17,18]. Taken together, capsaicin has significant effect on enhancing fat oxidation and lowering cholesterol concentration in plasma, but it is still unclear for the effect of capsaicin on cholesterol metabolism. The liver plays a major role in cholesterol metabolism, including cholesterol synthesis, bile salt homeostasis, plasma protein synthesis, hormone production, and detoxification. Miller et al. [19] had reported that dihydrocapsaicin conjugated liver microsomes protein both in vitro and in vivo studies, the nonreversible conjugation may influence the activity of metabolic enzyme in the liver and the function of detoxication. Abdel et al. [20] had demonstrated that capsaicin could be detected in bile and administration of capsaicin reduces bile flow and biliary proteins in rats. Capsaicin also increased the amount of cholesterol in bile acids and the ratio of cholesterol/phospholipid [21]. All in above have shown that the potential effect of capsaicinoids on cholesterol metabolism, but it is still unclear the mechanisms of cholesterol lowering effect of capsaicinoids.
In the trial of lipid-lowering, there were significant differences in effect of garlic oil between men and women for HDL cholesterol (HDL-C) and TC/HDL-C. Women showed favorable effects in terms of coronary heart disease (CHD) risk factors, whereas men had small adverse effects [22]. Furthermore, many studies have also shown sex differences in the experimental capsaicin model for trigeminal sensitization, pain perception, anxiety and hyperalgesia, and female more sensitive than male [23- 26]. Summarize, we guess there were sex differences in the action of capsaicinoids on cholesterol levels of rats.
Therefore, the aim of the present study was to evaluate the effect and mechanism of capsaicinoids on cholesterol metabolism in different sex rats. We used a cholesterolfree diet to eliminate the possibility of related diet effects on cholesterol metabolism in rats.
2. Methods and Materials
2.1. Test Materials
Capsaicinoids, containing 355.42 g/kg capsaicin and 592.74 g/kg dihydrocapsaicin, was purchased from (Henan Bis-biotech Co., Ltd, Henan, China). All the commercial solid diets were purchased from Chongqing Tengxin Inc. (Chongqing, China).
2.2. Animals and Diets
This study was approved by the Laboratory Animal Care Committee of Southwest Univ. Rats were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of Southwest Univ.
Four-week-old male and female Sprague-Dawley [Crl:CD(SD)] rats (Chongqing Tengxin Inc., Chongqing, China) were used in this experiment. The rats were housed individually in screen-bottomed, stainless steel cages in a room maintained at 25˚C ± 1˚C with a 12-h light/12-h dark cycle (light, 0800 to 2000 h). Rats were acclimatized by feeding a commercial solid diet (Chongqing Tengxin Inc., Chongqing, China) (Table 1) for 7 d. After acclimation, 24 rats of each sex were selected and randomized to control and test groups (n = 6/group) based on animal body weight gain. Groups of four animals were daily treated by gavage (1 ml/100g/d) with capsaicinoids at 0, 2.5 mg/kg, 5 mg/kg or 7.5 mg/kg respectively at 15:00. The animals in the control group received equivalence rapeseed oil instead. Rats were given free access to food and water over 28 d.
2.3. Sampling and Analytical Procedures
Food intake was recorded daily and body weight was measured every three days. On the last day of the experimental period, a blood sample was collected from the neck at night (19:00 to 23:00 h) under a light ether anesthesia. The blood was collected into a tube (Vacutainer, Liuyang City Medical Instrument Factory, Henan, China) containing heparin as an anticoagulant. Serum was separated by centrifugation at 1400 g at 4˚C for 15 min, and stored at −80˚C until analysis. The liver was then immediately perfused with cold saline (9 g NaCl/L), removed, washed with cold saline, blotted dry on filter paper, weighed, frozen in liquid N2 and stored at −80˚C until further analysis. Next the ileum was removed, the contents of the ileum were washed by flushing ice-cooled saline, blotted dry on filter paper, weighed, frozen in liquid N2 and stored at −80˚C until further analysis.
2.4. Chemical Analysis
The concentrations of TC, triglyceride (TG), LDL-C and
Table 1. Composition of the commercial solid diet1.
HDL-C in the plasma were determined enzymatically using commercial diagnostic kits (Beihai biotechnology, Shanghai, China). Liver lipids were extracted with chloroform:methanol (2:1, v/v) according to the method of Folch and others [27]. The liver total lipid content was determined gravimetrically after extraction. The concentrations of liver TC and TG were determined enzymatically (Beihai biotechnology Co. Ltd., Shanghai, China) as described elsewhere [28].
2.5. RNA Extraction from Liver and Ileum and RT-PCR Analysis of Gene Expression
Total RNA was extracted from frozen livers or ileums in accordance with the method described by Chomczynski and Sacchi [29]. RNA integrity was verified by agarose gel electrophoresis after purification of the mRNA using Oligotex-dT30 (Takara Bio, Dalian, China). One microgram of purified mRNA was used for cDNA synthesis using reverse transcriptase (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) accordance with the manufacturer’s instructions. Expression of the mRNAs for hepatic transient receptor potential vanilloid 1 (TRPV1), cholesterol 7α-hydroxylase (CYP7A1), farnesoid X receptor (FXR), 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, ileac apical sodiumdependentbile acid transporter (ASBT) and ileum bile acid binding protein (IBABP) were determined by realtime monitoring of PCR using a Light Cycler instrument (Roche Diagnostics, Mannheim, Germany). cDNA (2 μl) was amplified in a total volume of 20 μl using the SYBR Premix Ex Taq Ⅱ (TaKaRa Biotechnology (Dalian) Co., Ltd., Dalian, China) and specific primers at 0.4 μM each. The reaction mixture was incubated for an initial denaturation at 95˚C for 30 s, followed by 40 cycles at 95˚C for 5 s and 60˚C for 20 s. The sequences of the gene-specific primers (Sangon Biological Engineering, Shanghai, China) used in the study are listed in Table 2.
2.6. Statistical Analysis
The results were reported as mean ± SD. Variance analysis was established by one-way ANOVA. Significant differences between pairs of groups were calculated using Duncan’s multiple range tests with significance level reported at P ≤ 0.05, as indicated. All the statistical analyses were conducted using the SPSS for Windows 12.0 software (SPSS, Chicago, IL, USA).
3. Results
In male rats, body weight gain was significantly decreased as the gavage dose of capsaicinoids increased (Table 3), the concentration of TC, HDL-C, LDL-C and TG in plasma were not differ significantly, while in female rats, body weight gain was not affected by the capsainoids dose. Food intake was essentially similar in all groups both in male and female rats, treatment with 2.5 mg/kg·d capsainoids orally in male rats excepted. In female rats, the concentration of plasma TC and HDL-C lowered significantly without dose-dependent manner, LDL-C and TG concentration lowered significantly as the dose of capsainoids increased.
Liver weight decreased as the dose of capsainoids increased in all animals (Table 4), although the differences were not significant in male rats groups. Total lipids, total cholesterol (rmale = −0.948, Pmale = 0.052, rfemale = −0.989, Pfemale = 0.012) and triglycerides (rmale = −0.911, Pmale = 0.089; rfemale = −0.980, Pfemale = 0.020) concentrations in the liver were decreased as the dose of capsainoids increased both in male and female rats. The amount of total cholesterol (P < 0.05) and triglycerides (P < 0.01) significantly lower than control group in female rats with the dose of 7.5 mg/kg capsainoids.
The mRNA levels of hepatic TRPV1 (rmale = 0.975, Pmale = 0.025; rfemale = 0.991, Pfemale = 0.009), ileac ASBT and IBABP were increased as the dose of capsainoids increased in all animals groups (Table 5). The mRNA
Table 2. Primer sequence, product size and annealing temperature.
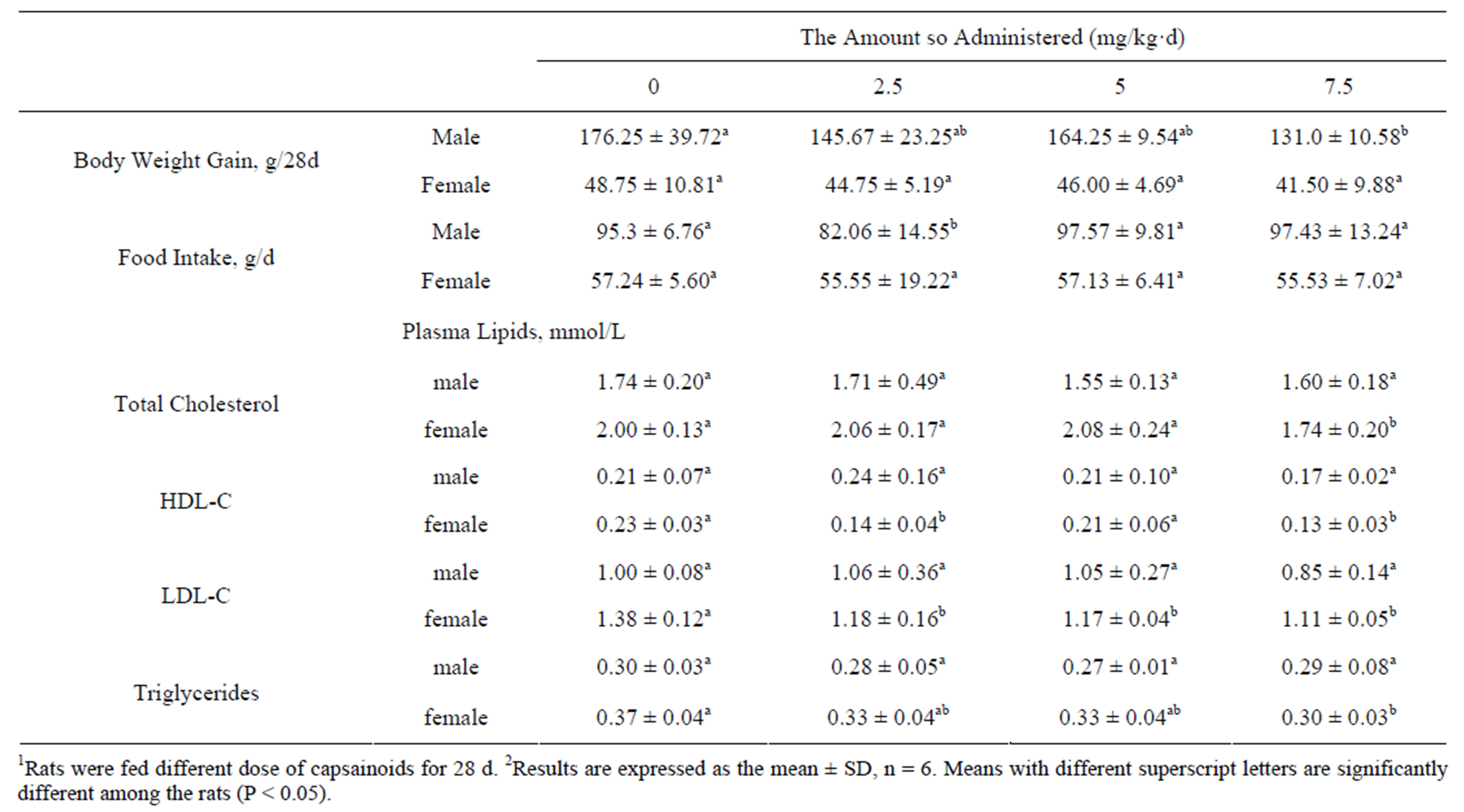
Table 3. Effects of capsainoids on body weight and plasma lipids in rats1,2.
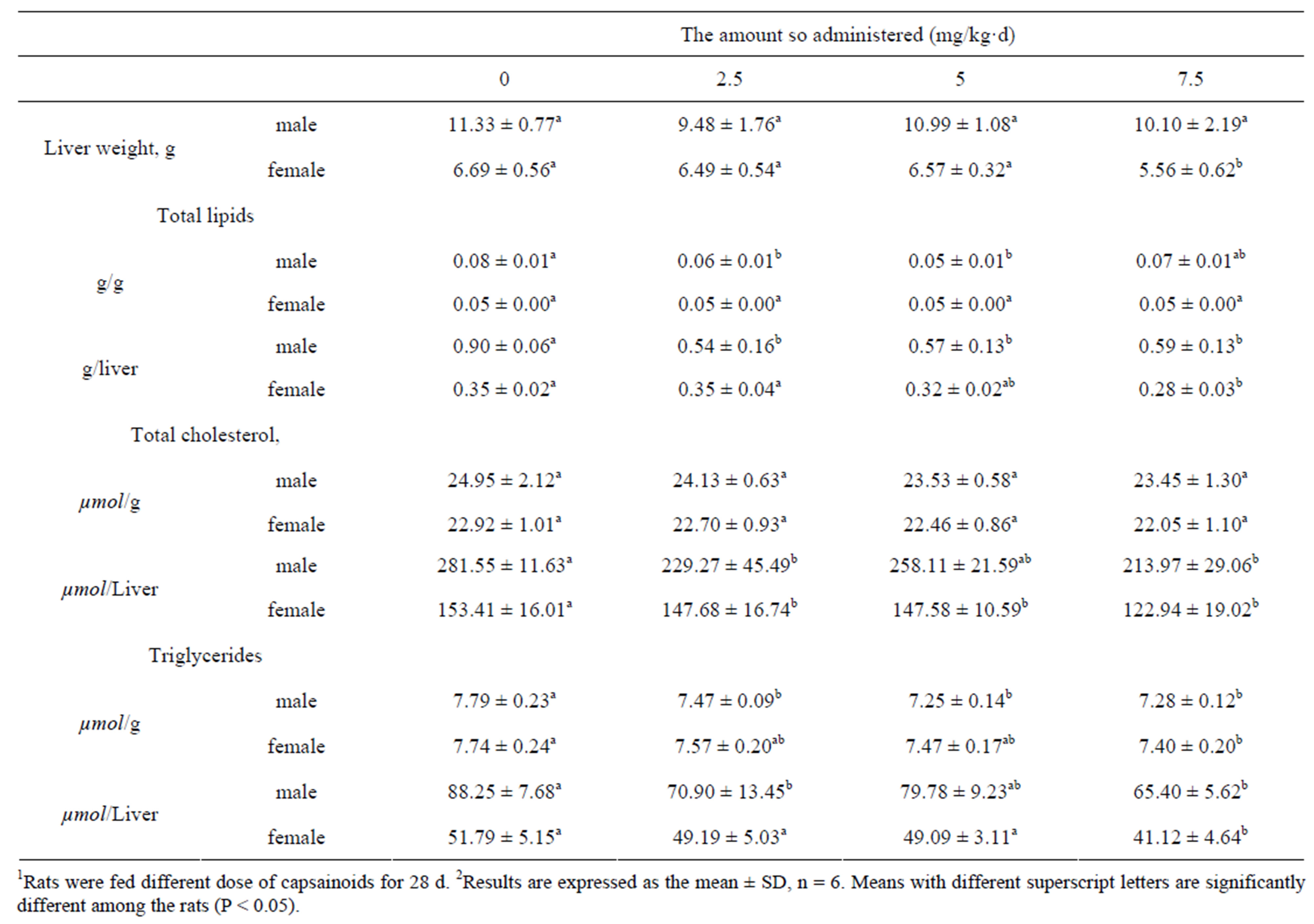
Table 4. Effects of capsainoids on liver lipids and weight in rat1,2.
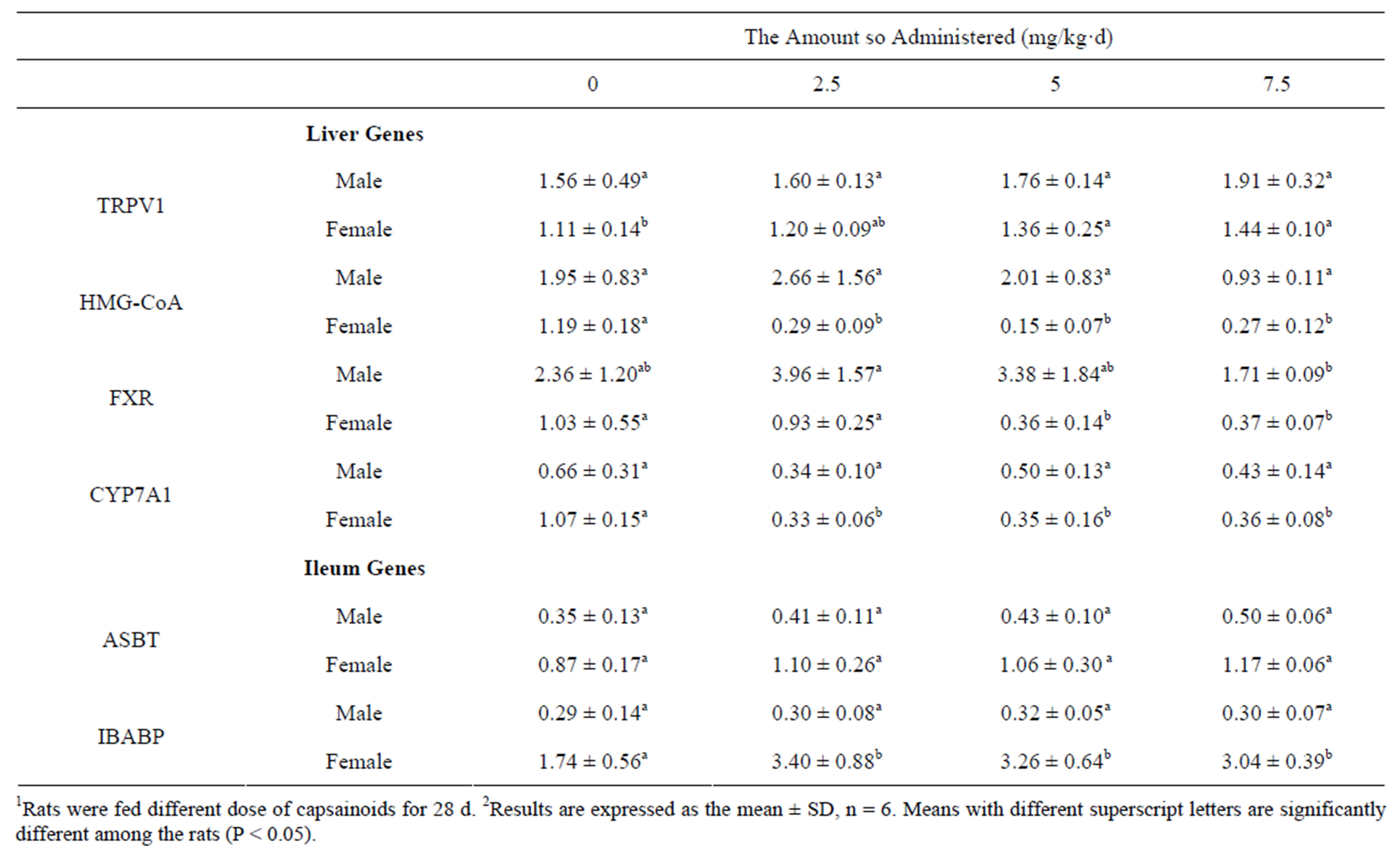
Table 5. Effects of capsainoids on mRNA levels of genes on cholesterol metabolism in rats (arbitary units)1,2.
levels of hepatic HMG-CoA reductase, CYP7A1 and FXR were significantly decreased in female rats fed capsainoids.
4. Discussion
Sex difference of dose-dependent effect of capsainoids on cholesterol and bile acid metabolism was studied in rats, males being less sensitive than females to all doses of capsainoids on hypolipidemic effect, especially the concentration of TC, HDL-C, LDL-C and TG in plsama were decreased significantly in female rats, so we mainly take female rats as examples to discuss what mechanism underline the hypolipidemic effects.
With the increase of capsainoids dose, the gain in body weight of male rats was significantly lowerd and females not, which is consistent with previously published studies that weight gain of rats decreased significantly in male rats fed with high-fat diet with capsaicin treatment [30]. It is also reported that the intake of capsaicin augment energy expenditure and enhance fat oxidation and may aid weight management [31,32]. The food intake was essentially similar in various capsainoids fed groups and control group both in male and female rats, treatment with 2.5 mg/kg·d capsainoids orally in male rats excepted. These findings stating that the consumption of capsainoids has little influence on appetite and food intake in male and female rats.
Total cholesterol concentrations in plasma and liver were lowerd after capsainoids administration compared with control group, there were significant differences in female rats. The primary change in cholesterol metabolism in the female rats may be due to its biosynthesis, degradation, absorption, excretion, distribution transport, or other mechanisms. Several animals studies have also shown that capsainoids reduces plasma and hepatic cholesterol concentration [12,33-35], the mechanism of this hypocholesterolemic effect of capsainoids remains unclear. However, in this study, this effect of capsainoids in the female rats must involve changes in endogenous sterol metabolism because test diets were not supplemented with cholesterol. Our datas indicating that the level of hepatic HMG-CoA reductase mRNA, the ratelimiting enzyme in the cholesterol synthesis, was significantly decreased in capsainoids treated female rats. Lipoprotein cholesterol synthesis would be inhibited by decreased free cholesterol concengtration in the liver, the amount of LDL-C transported to plasma decreased finally.
In rats, the classic pathway is the only pathway in which cholesterol is utilized in cholalic acid synthesis [36]. The first rate-limiting enzyme in the classic pathway is CYP7A1. CYP7A1 plays a central role in the regulation of bile acid and cholesterol metabolism, and transcription of the gene is controlled by bile acids and hormones acting through a complex interaction with a number of potential steroid-hormone-binding sites. In this study, the mRNA level of CYP7A1 is significantly lower in rats fed the capsainoids diet compared with control group. However, some studies have shown a significant increase in CYP7A1 activity in rats fed capsaicin [15]. This discrepancy may be explained by different dose of capsainoids, or the amount of lipids in diets. In current study, we used a cholesterol-free diet. There was no supernumerary free cholesterol for synthesis of bile acids in the liver when ectogenous cholesterol deficiency and synthesis of endogenous cholesterol was suppressed, so the mRNA level of CYP7A1 decreased finally.
Bile acids are the end products of cholesterol metabolism. Bile acids synthesis decreased due to total cholesterol levels in the liver reduced, and secreted via bile into the intestine decreased, new synthesis of cholesterol in bile acid pool reduced, the amount of bile acids return to the liver must be increased to complete their enterohepatic circulation. Thus, the activity of ileal ASBT and IBABP, key role of enterohepatic circulation, increased in ileum tissue, while the activity of FXR and CYP7A1 significantly decreased in the liver. Bile acid concentrations are controlled by a feedback regulatory pathway whereby activation of FXR represses transcription of both the CYP7A1 gene, encoding the rate-limiting enzyme in the classic bile acid synthesis pathway, and the CYP8B1 gene, required for synthesis of cholic acid. FXR-activating ligands induce the mRNA expression of ASBT in rat and inhibit in rabbit, but no effect on human being [37]. FXR conjunted with ligands to up-regulate ileac IBABP and BSEP transcription and stimulate the transportation of bile acids from small intestine to liver. In current study, the mRNA levels of ileac ASBT and IBABP increased in ileum tissue, while the mRNA levels of hepatic FXR and CYP7A1 significantly decreased in the liver. The current results suggest capsaicinoids increased the amounts of bile acids returning to liver for redeeming the deficiency of bile acids synthesized in liver. The increase of the amounts of bile acids in enterohepatic circumfluence would reduce the fecal excretion of bile acids. Unfortunately, bile acids and sterols in fecal excretion did not be measured in current study. Nalini et al. [21] earlier demonstrated that the levels of fecal bile acids and neutral sterols in fecal excretion were significantly decreased in capsaicin administered rats.
The capsaicin receptor, known as transient receptor potential vanilloid subfamily member 1 (TRPV1), is an important membrane receptor that has been implicated in obesity, diabetes, metabolic syndrome and cardiovascular diseases. It is reported that activation of TRPV1 by capsaicin prevents adipogenesis and lower the level of triglyceride in plasma and liver [38]. In our study, the level of hepatic TRPV1 (rmale = 0.975, Pmale = 0.025; rfemale = 0.991, Pfemale = 0.009) mRNA increase as the does of capsainoids increased, although there was no significant difference in male rats. We also found that the level of hepatic HMG-CoA reductase mRNA, FXR mRNA and CYPYA1 mRNA decreased corresponding to the increase of the level of hepatic TRPV1 mRNA in female rats. Calcium ions play a central role in many cellular processes including transmitter release, cell proliferation, gene transcription, and cell death [39,40], so we conjecture the mechanism of hypocholesteremic effect of capsainoids is that capsaicin-induced Ca2+ concentration change through TRPV1 channels may affect enzymatic activity and expression of cholesterol metabolism genes, such as HMG-CoA reductase, CYP7A1, FXR, in the liver. Nevertheless, the mechanism of capsainoids cholesterol-lowering effects need to be further explored.
5. Conclusion
In conclusion, the dose-dependent effect of capsainoids lowered plasma and liver cholesterol concentration differently in male and female rats. Female was sensitive to capsainoids. The results provided only limited support to the hypothesis that gender different in hypolipidemic of capsainoids could be explained by inhibited endogenous cholesterol synthesis and increased reflux of bile acids in female rats.
6. Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC) (No: 31071529), and the Fundamental Research Funds for the Central Unversities (XDJK2009B004). We are also indebted to Xinfeng Su, Hongjia Lu, Hongli Wen for excellent technical assistance.
REFERENCES
- S. Snitker, Y. Fujishima, H. Shen, S. Ott, X. Pi-Sunyer, Y. Furuhata, et al., “Effects of Novel Capsinoid Treatment on Fatness and Energy Metabolism in Humans: Possible Pharmacogenetic Implications,” American Journal of Clinical Nutrition, Vol. 89, No. 1, 2009, pp. 45-50. doi:10.3945/ajcn.2008.26561
- M. N. Satyanarayana, “Capsaicin and Gastric Ulcers,” Critical Reviews in Food Science and Nutrition, Vol. 46, No. 4, 2006, pp. 275-328. doi:10.1080/1040-830491379236
- P. Holzer, E. Painsipp and R. Schuligoi, “Differential Effects of Intragastric Acid and Capsaicin on Gastric Emptying and Afferent Input to the Rat Spinal Cord and Brainstem,” BMC Neuroscience, Vol. 6, 2005, pp. 60-69. doi:10.1186/1471-2202-6-60
- K. Imatake, T. Matsui and M. Moriyama, “The Effect and Mechanism of Action of Capsaicin on Gastric Acid Output,” Journal of Gastroenterology, Vol. 44, No. 5, 2009, pp. 396-404. doi:10.1007/s00535-009-0018-x
- K. Arai, T. Ohno, T. Saeki, S. Mizuguchi, K. Kamata, I. Hayashi, et al., “Endogenous Prostaglandin I2 Regulates the Neural Emergency System through Release of Calcitonin Gene Related Peptide,” Gut, Vol. 52, No. 9, 2003, pp. 1242-1249. doi:10.1136/gut.52.9.1242
- C. S. Kim, T. Kawada, B. S. Kim, I. S. Han, S. Y. Choe, T. Kurata, et al., “Capsaicin Exhibits Anti-Inflammatory Property by Inhibiting IkB-a Degradation in LPS-Stimulated Peritoneal Macrophages,” Cellular Signalling, Vol. 15, No. 3, 2003, pp. 299-306. doi:10.1016/S0898-6568(02)00086-4
- S. M. Henning, Y. J. Zhang, N. P. Seeram, R. P. Lee, P. W. Wang, S. Bowerman, et al., “Antioxidant Capacity and Phytochemical Content of Herbs and Spices in Dry, Fresh and Blended Herb Paste Form,” International Journal of Food Sciences and Nutrition, Vol. 62, No. 3, 2011, pp. 219-225. doi:10.3109/09637486.2010.530595
- R. Yu, M. A. Choi, T. Kawada, B. S. Kim, I. S. Han and H. Yoo, “Inhibitory Effect of Capsaicin against Carcinogen-Induced Oxidative Damage in Rats,” Journal of Food Science and Nutrition, Vol. 7, No. 1, 2002, pp. 67-71. doi:10.3746/jfn.2002.7.1.067
- Y. M. Baek, H. J. Hwang, S. W. Kim, H. S. Hwang, S. H. Lee, J. A. Kim, et al., “A Comparative Proteomic Analysis for Capsaicin-Induced Apoptosis between Human Hepatocarcinoma (HepG2) and Human Neuroblastoma (SK-NSH) Cells,” Proteomics, Vol. 8, No. 22, 2008, pp. 4748- 4767. doi:10.1002/pmic.200800094
- D. C. Yang, Z. D. Luo, S. T. Ma, W. T. Wong, L. J. Ma, J. Zhong, et al., “Activation of TRPV1 by Dietary Capsaicin Improves Endothelium-Dependent Vasorelaxation and Prevents Hypertension,” Cell Metabolism, Vol. 12, No. 2, 2010, pp. 130-141. doi:10.1016/j.cmet.2010.05.015
- K. Diepvens, K. R. Westerterp and M. S. WesterterpPlantenga, “Obesity and Thermogenesis Related to the Consumption of Caffeine, Ephedrine, Capsaicin, and Green Tea,” American Journal of Physiology—Regulatory, Integrative and Comparative Physiology, Vol. 292, No. 1, 2007, pp. R77-R85. doi:10.1152/ajpregu.00832.2005
- H. Manjunatha and K. Srinivasan, “Hypolipidemic and Antioxidant Effects of Dietary Curcumin and Capsaicin in Induced Hypercholesterolemic Rats,” Lipids, Vol. 42, No. 12, 2007, pp. 1133-1142. doi:10.1007/s11745-007-3120-y
- N. Inoue, Y. Matsunaga, H. Satoh and M. Takahashi, “Enhanced Energy Expenditure and Fat Oxidation in Humans with High BMI Scores by the Ingestion of Novel and Non-Pungent Capsaicin Analogues (Capsinoids),” Bioscience, Biotechnology, and Biochemistry, Vol. 71, No. 2, 2007, pp. 380-389. doi:10.1271/bbb.60341
- L. L. Zhang, D. Y. Liu, L. J. Ma, Z. D. Luo, T. B. Cao, J. Zhong, et al., “Activation of Transient Receptor Potential Vanilloid Type-1 Channel Prevents Adipogenesis and Obesity,” Circulation Research, Vol. 100, No. 7, 2007, pp. 1063-1070. doi:10.1161/01.RES.0000262653.84850.8b
- K. Srinivasan and K. Sambaiah, “The Effect of Spices on Cholesterol 7 Alpha-Hydroxylase Activity and on Serum and Hepatic Cholesterol Levels in the Rat,” International Journal for Vitamin and Nutrition Research, Vol. 61, No. 4, 1991, pp. 364-369.
- H. Manjunatha and K. Srinivasan, “Hypolipidemic and Antioxidant Effects of Curcumin and Capsaicin in HighFat-Fed Rats,” Canadian Journal of Physiology and Pharmacology, Vol. 85, No. 6, 2007, pp. 588-596. doi:10.1139/Y07-044
- R. K. Kempaiah and K. Srinivasan, “Integrity of Erythrocytes of Hypercholesterolemic Rats during Spices Treatment,” Molecular and Cellular Biochemistry, Vol. 236, No. 1-2, 2002, pp. 155-161. doi:10.1023/A:1016199000149
- R. K. Kempaiah and K. Srinivasan, “Beneficial Influence of Dietary Curcumin, Capsaicin and Garlic on Erythrocyte Integrity in High-Fat Fed Rats,” Journal of Nutritional Biochemistry, Vol. 17, No. 7, 2006, pp. 471-478. doi:10.1016/j.jnutbio.2005.09.005
- C. H. Miller, Z. Zhang and S. M. Hamilton, “Effects of Capsaicin on Liver Microsomal Metabolism of the Tobacco Specific Nitrosamine NNK,” Cancer Letters, Vol. 75, No. 1, 1993, pp. 45-52. doi:10.1016/0304-3835(93)90206-O
- O. M. E. Abdel, O. A. Heikal and S. M. El-Shenawy, “Effect of Capsaicin on Bile Secretion in the Rat,” Pharmacology, Vol. 73, No. 3, 2005, pp. 121-128. doi:10.1159/000081954
- N. Nalini, V. Manju and V. P. Menon, “Effect of Spices on Lipid Metabolism in 1, 2-Dimethylhydrazine-Induced Rat Colon Carcinogenesis,” Journal of Medical Food, Vol. 9, No. 2, 2006, pp. 237-245. doi:10.1089/jmf.2006.9.237
- X. H. Zhang, D. Lowe, P. Giles, S. Fell, M. J. Connock and D. J. Maslin, “Gender May Affect the Action of Garlic Oil on Plasma Cholesterol and Glucose Levels of Normal Subjects,” Journal of Nutrition, Vol. 131, No. 5, 2001, pp. 1471-1478.
- Y. C. Lu, C. W. Chen, S. Y. Wang and F. S. Wu, “17 β-Estradiol Mediates the Sex Difference in CapsaicinInduced Nociception in Rats,” The Journal of Pharmacology and Experimental Therapeutics, Vol. 331, No. 3, 2009, pp. 1104-1110. doi:10.1124/jpet.109.158402
- P. Gazerani, O. K. Andersen and L. Arendt-Nielsen, “A Human Experimental Capsaicin Model for Trigeminal Sensitization. Gender-Specific Differences,” Pain, Vol. 118, No. 1-2, 2005, pp. 155-163. doi:10.1016/j.pain.2005.08.009
- M. T. Jensen and K. L. Petersen, “Gender Differences in Pain and Secondary Hyperalgesia after Heat/Capsaicin Sensitization in Healthy Volunteers,” Journal of Pain, Vol. 7, No. 3, 2006, pp. 211-217. doi:10.1016/j.jpain.2005.10.013
- M. Frot, J. S. Feine and M. C. Bushnell, “Sex Differences in Pain Perception and Anxiety: A Psychophysical Study with Topical Capsaicin,” Pain, Vol. 108, No. 3, 2004, pp. 230-236. doi:10.1016/j.pain.2003.11.017
- J. Folch, M. Less and G. H. Sloane-Stanley, “A Simple Method for the Isolation and Purification of Total Lipids from Animal Tissue,” The Journal of Biological Chemistry, Vol. 226, 1957, pp. 497-509.
- T. P. Carr, C. J. Andresen and L. L. Rudel, “Enzymatic Determination of Triglyceride, Free Cholesterol, and Total Cholesterol in Tissue Lipid Extracts,” Clinical Biochemistry, Vol. 26, No. 1, 1993, pp. 39-42. doi:10.1016/0009-9120(93)90015-X
- P. Chomczynski and N. Sacchi, “Single-Step Method of RNA Isolation by Acid Guanidinium Thiocyanate-PhenolChloroform Extraction,” Analytical Biochemistry, Vol. 162, 1987, pp. 156-159. doi:10.1016/0003-2697(87)90021-2
- J. W. Choi, H. S. Hwang, D. H. Kim, J. I. Joo and J. W. Yun, “Proteomic Analysis of Liver Proteins in Rats Fed with a High-Fat Diet in Response to Capsaicin Treatments,” Biotechnology and Bioprocess Engineering, Vol. 15, No. 4, 2010, pp. 534-544. doi:10.1007/s12257-010-0029-8
- H. C. Reinbach, A. Smeets, T. Martinussen, P. Møller and M. S. Westerterp-Plantenga, “Effects of Capsaicin, Green Tea and CH-19 Sweet Pepper on Appetite and Energy Intake in Humans in Negative and Positive Energy Balance,” Clinical Nutrition, Vol. 28, No. 3, 2009, pp. 260- 265. doi:10.1016/j.clnu.2009.01.010
- M. J. Ludy, G. E. Moore and R. D. Mattes, “The Effects of Capsaicin and Capsiate on Energy Balance: Critical Review and Meta-Analyses of Studies in Humans,” Chemical Senses, Vol. 37, No. 2, 2012, pp. 103-121. doi:10.1093/chemse/bjr100
- N. S. B. M. Atapattu and U. D. Belpagodagamage, “Effect of Dietary Chilli Powder on Growth Performance and Serum Cholesterol Contents of Broiler Chicken,” Tropical Agricultural Research and Extension, Vol. 13, No. 4, 2010, pp. 106-109.
- J. F. Zhong, M. J. Xu, W. H. Qiu and H. X. He, “Influence of Capsaicin on Growth Performance, Serum Indicators and Small Intestinal Digestive Enzyme Activity of Xianghuang Chicken,” Acta Ecologiae Animalis Domastici, Vol. 30, 2009, pp. 61-65.
- T. Kawada, K. Hagihara and K. Iwai, “Effects of Capsaicin on Lipid Metabolism in Rats Fed a High Fat Diet,” Journal of Nutrition, Vol. 116, No. 7, 1986, pp. 1272- 1278.
- T. Claudel, B. Staels and F. Kuipers, “The Farnesoid X Receptor: A Molecular Link between Bile Acid and Lipid and Glucose Metabolism,” Arteriosclerosis, Thrombosis, and Vascular Biology, Vol. 25, No. 10, 2005, pp. 2020- 2030. doi:10.1161/01.ATV.0000178994.21828.a7
- H. Li, F. Chen, Q. Shang, L. X. Pan, B. L. Shneider, J. Y. L. Chiang, et al., “FXR-Activating Ligands Inhibit Rabbit ASBT Expression via FXR-SHP-FTF Cascade,” American Journal of Physiology—Gastrointestinal and Liver Physiology, Vol. 288, No. 1, 2005, pp. 60-66. doi:10.1152/ajpgi.00170.2004
- L. Q. Ma, J. Zhong, Z. G. Zhao, Z. D. Luo, S. T. Ma, J. Sun, et al., “Activation of TRPV1 Reduces Vascular Lipid Accumulation and Attenuates Atherosclerosis,” Cardiovascular Research, Vol. 92, No. 3, 2011, pp. 504-513. doi:10.1093/cvr/cvr245
- M. J. Berridge, P. Lipp and M. D. Bootman, “The Versatility and Universality of Calcium Signalling,” Nature Reviews: Molecular Cell Biology, Vol. 1, No. 1, 2000, pp. 11-21. doi:10.1038/35036035
- B. Nilius, G. Owsianik, T. Voets and J. A. Peters, “Transient Receptor Potential Cation Channels in Disease,” Physiological Reviews, Vol. 87, No. 1, 2007, pp. 165- 217. doi:10.1152/physrev.00021.2006
NOTES
*This work was supported by the National Natural Science Foundation of China (NSFC) (No: 31071529), and the Fundamental Research Funds for the Central Unversities (XDJK2009B004).
#Conflict of interest: The authors have no conflict of interest to disclose.
†Corresponding author.

