Food and Nutrition Sciences
Vol. 3 No. 8 (2012) , Article ID: 21572 , 8 pages DOI:10.4236/fns.2012.38141
Alteration in T-Cell Cytokine Production by Vitamin A and Zinc Supplementation in Mice
![]()
1Department of Nutrition, Faculty of Health and Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran; 2Department of Food Science and Technology, Faculty of Health and Nutrition, Tabriz University of Medical Sciences, Tabriz, Iran.
Email: mdalizadeh@tbzmed.ac.ir
Received May 10th, 2012; revised June 18th, 2012; accepted June 25th, 2012
Keywords: Zinc; Vitamin A; IgA; IL-5; IL-2; IFN-γ
ABSTRACT
Simultaneous zinc and vitamin A deficiency are common health problems in developing countries. The objective of this study was to assess effect of supplementation of high zinc or vitamin A on immune function. After three months of feeding with a zinc and vitamin A deficient diet, mice were assigned into four groups which, for additional two months, received a normal or high zinc along with vitamin A deficient diet and a normal or high vitamin A along with zinc deficient diet. Serum and intestinal mucosa immunoglobulin A (IgA) were determined and supernatants of splenocytes were used to assess interlukin (IL)-2, IL-5, IFN-γ. Mice maintained on zinc deficient diet with normal or high vitamin A resulted in significantly lower production of IFN-γ. Also, supplementation of high dose vitamin A augmented production of the cytokine as compared to normal intake of the vitamin. Supplementation of either normal or high zinc along with low vitamin A diet significantly led to higher production of IFN-γ as compared to those receiving zinc limited but adequate vitamin A. High intake of zinc along with vitamin A deficient diet significantly enhanced secretion of IL-2. Levels of serum and mucosal IgA and IL-5 were not be significantly modulated. Moreover, animals fed with high doses of zinc showed increased IL-2 production than those that had normal intake of zinc. Results indicated that zinc and vitamin A supplementation up-regulates production of T-cell cytokines, IFN-γ and IL-2.
1. Introduction
Effective function of the immune system as the most important contributor to maintenance of good health and defense against infections is strongly affected by nutriational status. In recent years, the effect of nutrition, in particular micronutrients on various aspects of immune function has become increasingly apparent [1,2].
Zinc is among the most important micronutrients involved in modulation of immune function. It is recognized to be present in almost every cell, known to support the healthy immune system and needed for wound healing [3]. It also regulates several functions of lymphocytes, such as mitogenesis [4], antibody synthesis [5], activation of T cells and natural killer cells, and more specifically cellular immunity [6]. In persons sufferings from zinc deficiency (ZD), clinical signs of depressed immunity such as frequent infections and bullous pustular dermatitis are abundant [7-9]. Furthermore, ZD has been shown to increase the susceptibility of infants and children to gastrointestinal infections due to its adverse effects on gastrointestinal tract structure and function [10, 11]. The intestine is one of the tissues most sensitive to ZD. Flattening of villi, decreased numbers of crypts, inflammatory cell infiltration of the lamina propria and lesions of intestinal mucosa have been reported in both experimental animals and humans suffering from ZD [12-14].
Alternatively, vitamin A and its metabolites have essential role in modulating a broad range of immune processes, such as lymphocyte activation and proliferation [15,16], immunoglobulin production [17], T-Helper-Cell differentiation and development of T-Helper-2-Cell [18], cell growth, differentiation and the maintenance of epithelium [19] and regulation of the immune response [20, 21]. Vitamin A deficient infants and preschool children are more susceptible to respiratory tract infections as well as disseminated virus infections such as measles [22]. The mortality rate and incidence of diseases in these children are decreased by vitamin A supplementation, suggesting that host defense is affected by the vitamin A deficiency [23-25].
It is well known that simultaneous VAD and ZD are common in many developing countries and strong interaction exists in absorption, transport and metabolism of the two nutrients [26]. However, to our best knowledge, there is no report to address concurrent effects of this two nutrients on any aspects of specific T-Helper-1 (Th1) and Th2-driven immune responses.
To simulate current nutritional status of the two trace elements in many developing parts of the world, animals were first maintained on a vitamin A and zinc limited diet and effect of varying doses of vitamin A or zinc supplementation on immune responses in the animals were examined.
2. Materials and Methods
2.1. Animals and Husbandry
A controlled 12 h light-dark cycle, temperature (20˚C - 22˚C) and relative humidity of 50% - 60% were maintained in the animal room. All animals had free access to casein based semi-purified experimental diet (Oriental Yeast Co., Japan) and distilled water, throughout. The food was prepared every day and food intake was determined daily. Body weights were recorded twice per week and total body weight gain was determined.
2.2. Experimental Design and Diets
Young adult, male, pathogen free, ddY mice (N = 22, average weight 27 - 29 g) and aged 6 weeks were fed with a low vitamin A and zinc (550 IU vitamin A/kg, 5.2 mg zinc/kg) diet for twelve weeks and then randomly assigned into four groups with two of groups supplemented only with adequate vitamin A (group B; 5000 IU vitamin A/kg, 5.2 mg zinc/kg) or high vitamin A (group C; 105 IU vitamin A/kg, 5.2 mg zinc/kg) and two other groups supplemented only with normal zinc (group D; 60.2 mg zinc/kg, 550 IU vitamin A/kg) or high zinc (group E; 500 mg zinc/kg, 550 IU vitamin A/kg) for an additional eight weeks. Mice in group A, as a control group, (n = 6) were fed a normal diet containing sufficient retinyl acetate, as vitamin A, and zinc (5000 IU vitamin A/kg, 60.2 mg zinc/kg) for whole the period. A detailed formula of the diets is provided in Table 1.
2.3. Blood Collection
At the end of experiment mice were killed under deep anesthesia with Nembatul (Dainippon Pharmaceutical Co., Ltd., Japan,). The blood was collected via main abdominal vein and immediately centrifuged and the sera stored in −20˚C until used.
2.4. Preparation of Intestinal Mucosa Extracts
Segments of small intestine were carefully released from the nearly 2 cm after stomach to the ileocecal valve. Mucosal piles were immediately weighed and maintained
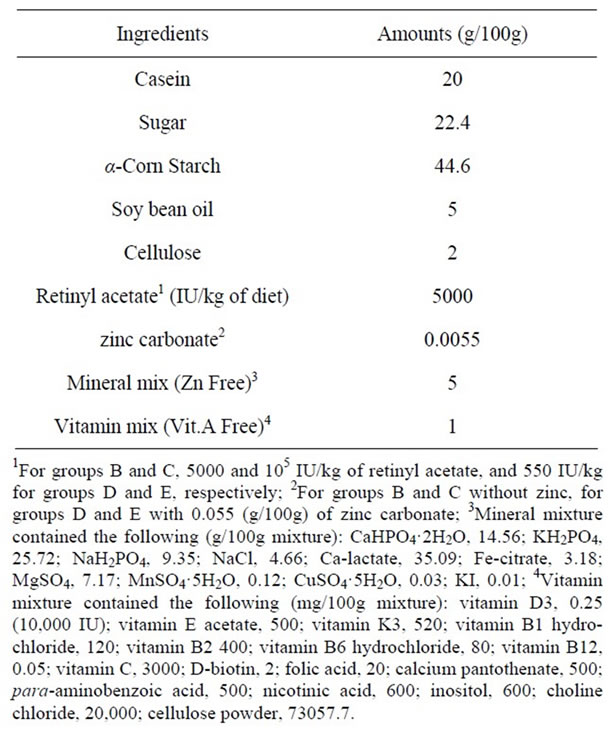
Table 1. Composition of normal diet.
in 1 ml of cold phosphate buffer saline (PBS) and homogenized with digital Teflon homogenizer (AS ONE, model: AN, Japan) for 1 minute at 4000 rpm. After centrifugation for 20 minutes at 4˚C with the speed of 13,500 rpm, supernatants were used for Immunoglobulin A (IgA) quantification.
2.5. Quantification of Serum and Intestinal Mucosa Total IgA
Serum and intestinal mucosa total IgA were determined by enzyme-linked immunosorbent assay (ELISA) method using a mouse IgA ELISA quantitation kit (system number E90-103 from Bethyl laboratories, Inc., TX, USA) according to the manufacturer-s instructions. In brief, goat anti-mouse IgA-affinity purified antibody were dispensed in each well and incubated for an hour at room temperature. They were then washed with a wash solution containing 50 mM Tris, 0.14 M NaCl, 0.05% Tween 20 for three times. Non specific binding sites were blocked with blocking solution and incubated for 30 minutes at room temperature. After washing for three times, standards and samples were added and incubated for 60 minutes at room temperature. HRP conjugated Goat anti-mouse IgA were added to each well and incubated for 60 min at room temperature. Finally tetramethylbenzidine (TMB) was used as substrate and absorbance was read with ELISA reader (Corona Electric, MTP-32, Japan) at 450 nm.
2.6. Cytokine Production against Anti-CD3
The spleens were isolated and used for preparation of cell suspensions under aseptic conditions. Each spleen was put into a Petri dish containing 10 ml of RPMI 1640 media (Sigma, USA) and scratched by coarse glass slides under sterile conditions. The cell suspensions were passed through a nylon mesh and then centrifuged for 10 minutes in 2000 rpm at 4˚C. To lyse the red blood cells, 1 ml of a solution of 0.83% NH4Cl in Tris-HCl (pH 7.4) buffer was added into the cell pellet. Then, the samples were incubated at 37˚C for 10 minutes and mixed with 10 ml of the media and centrifuged. An additional washing was done using complete RPMI 1640 medium containing 100 U/ml penicillin, 100 µg/ml streptomycin and 5 × 10−5 M of 2-mercaptoethanol and 10% heat inactivated fetal bovine with subsequent centrifugation. The supernatants were removed and 1 ml of the complete media was added. To test viability of the cells, 100 µl of the resultant solution was mixed with equal volume of a solution of 0.4% trypan blue. Culture plates were treated with filtered anti-mouse CD3 hamster monoclonal antibody (R & D system Inc., USA) and incubated overnight at 4˚C. On the next day, the contents of the wells were aseptically aspirated and washed with complete media. Finally, 500 μl of the media and 500 μl of cell suspensions were added into each well to provide final concentration of 5 × 106 cell/ml.
2.7. Measurement of Interleukin IL-5
The level of IL-5 in supernatants was determined using a commercially available mouse IL-5 ELISA kit (PIERSE ENDOGEN, Rockford, USA). Briefly, mouse IL-5 standard was reconstituted in RPMI 1640 and diluted serially at range of 0 to 320 pg/ml in the medium. Then, 50 µl of plate reagent containing 0.1% sodium azide was added into each well of anti-mouse IL-5 pre-coated micro plates with 50 µl of the standards and samples. The plates were then sealed and incubated at 37˚C for 2 hours. After 5 times washing, 100 µl/well of anti-mouse IL-5 Peroxidase Conjugate Reagent was added and incubated at 37˚C for 1 hour. Then, the wells were washed five times with the wash buffer and treated with 100 µl of TMBZ substrate and incubated for 30 minutes. The reaction was stopped by solution containing 0.18 M H2SO4 and absorbance was read using the ELISA reader at 450 nm.
2.8. IL-2 and Interferon IFN-γ Assay
Concentrations of IL-2 and IFN-γ in culture supernatants of splenocytes stimulated with anti-CD3 were determined using commercially available kits (eBioscience mouse ELISA kits) following manufacturer’s instructions. Briefly, capture antibodies of the two cytokines were diluted in coating buffer and added into each well of micro plates (100 µl/ well) and incubated overnight at 4˚C. The plates were washed three times with PBS buffer containing 0.05% Tween-20 (PBS-T). They were then treated with 200 µl of assay diluent and incubated at room temperature for 1 hour. 100 µl/wells of standards or samples were added into wells and incubated at room temperature for 2 hours. After washing 5 times, detection monoclonal antibody was added and incubated at room temperature for an hour. Then, the wells were washed and 100 µl/well of avidin-HRP was added. Finally, after washing 7 times, TMBZ was added and absorbance was read using the ELISA reader at 450 nm.
2.9. Statistics
The Data were analyzed with a SPSS for windows statistical package (version 10). Statistical analysis was done using one-way analysis of variance (ANOVA).
For post Hoc comparisons, least significant (LSD) test was used. The data were expressed as mean ± SD and considered significant at P < 0.05.
3. Results
3.1. Animal Growth and Food Intake
As shown in Table 2, there were no significant differences in final body weights and weight gains among all experimental groups. Animals kept on a high vitamin A with low zinc diet (group C) had significantly more (P < 0.05) food intake as compared with the others, whereas, this group had negative weight gain and less feed efficiency as compared with the other groups.
3.2. Serum and Mucosal IgA
As shown in Table 3, serum and mucosal level of IgA could not be modulated with experimental diet and no difference was observed among experimental groups.
However, remarkable reduction of IgA was shown in group fed with high zinc but low vitamin A diet (group E) for both serum and mucosal IgA.
3.3. Production of IL-5, IFN-γ and IL-2
As shown in Figure 1, in the animals depleted for both zinc and vitamin A, supplementation of either zinc or vitamin A did not show any modulator effect on production of IL-5 by splenocytes stimulated with anti-CD3. The highest production of IL-5 was found in group C receiving high level of dietary vitamin A. However, the difference was not statistically significant.
As shown in Figure 1, mice maintained on zinc deficient diet with adequate vitamin A (group B) and high amount of the nutrient (group C) had significantly lower
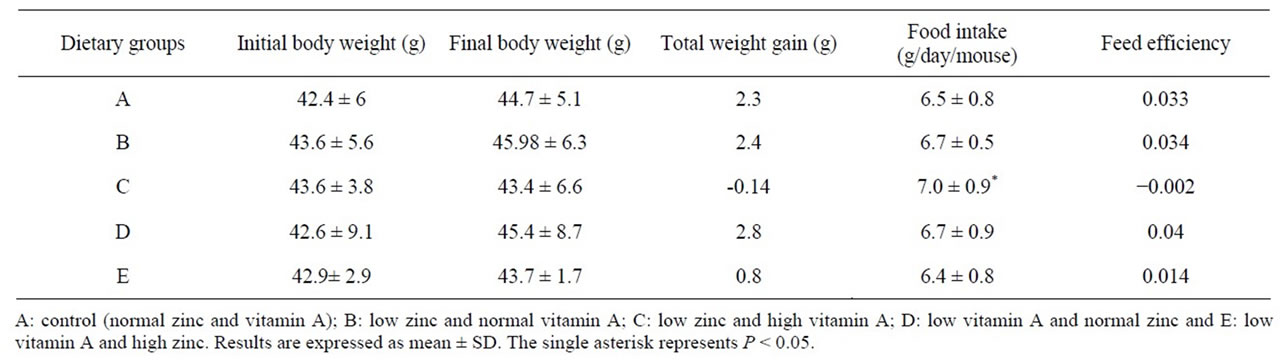
Table 2. Body weight and food consumption.
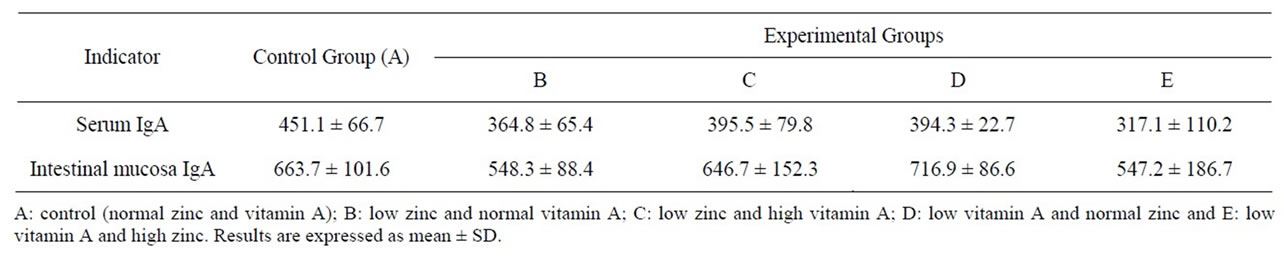
Table 3. Concentration of serum and mucosa IgA (μg/ml).
production of IFN-γ as compared to the control group (P < 0.001, P < 0.05, respectively). Also, supplementation of high vitamin A along with low zinc (group C) significantly augmented (P < 0.05) production of the cytokine as compared to the group maintained on recommended level of vitamin A (group B). Animals supplemented with either normal (group D) or high zinc (group E) along with low vitamin A had significantly higher production of IFN-γ as compared to group B, receiving a diet with limited zinc and adequate vitamin A (P < 0.05, P < 0.01, respectively).
As shown in Figure 1, lack of vitamin A in the animals supplemented with sufficient level of zinc (group D) and Also lack of zinc in the animals supplemented with sufficient level of vitamin A (group B) did not affect production of IL-2 in splenocytes stimulated with antiCD3 as compared to the control group.
High intake of vitamin A along with low zinc (group C) remarkably augmented (P = 0.06) production of IL-2 as compared to group maintained on recommended level of vitamin A (group B). The highest titer of IL-2 was detected in group kept on high dosage of zinc along with vitamin A free diet (group E). High zinc, low vitamin A diet (group E) significantly enhanced secretion of IL-2 by splenocytes in comparison to the control group (P < 0.01). Moreover, animals fed with high dosage of zinc (group E) showed increased IL-2 production than those which had normal intake of zinc (group D) (P < 0.05). Finally, animals fed with a high zinc, low vitamin A diet (group E) showed a significantly higher production of IL-2 as compared to group B kept on low zinc, and normal vitamin A diet (P < 0.01).
4. Discussion
Zinc and vitamin A status influences several aspects of metabolism, including absorption, transport, and utilizetion of the two nutrients. Fluctuation in the status of one or both micronutrients may reasonably alter the metabolism of the other, with functional consequences on the health of the individual [26]. We observed a higher food intake with negative weight gain and a decreased feed efficiency in mice receiving low zinc, high vitamin A diet. These finding is attributable to lack of zinc or high intake of vitamin A. Earlier investigations have defined anorexia as an important signs for zinc deficiency [27,28] and reported less food intake, less weight gain for zinc deficient animals [28,29]. Therefore, less weight gain in zinc deficient animals is a consequence of anorexia and subsequent low food intake. Hence, we speculate that observed high food intake beside less weight gain might be due to high intake of vitamin A. The observation is similar to reports that high dose supplementation of vitamin A results in high food intake and less weight gain in mice [30,31]. From the other side, in the present study, animals kept on a low zinc with enough vitamin A diet (group B) did not show the same trend, which can strongly support that high food intake is consequence of high consumption of vitamin A and is not related to zinc deficiency.
The secretary IgA production system is the principal arm of the mucosal immune response that protects the
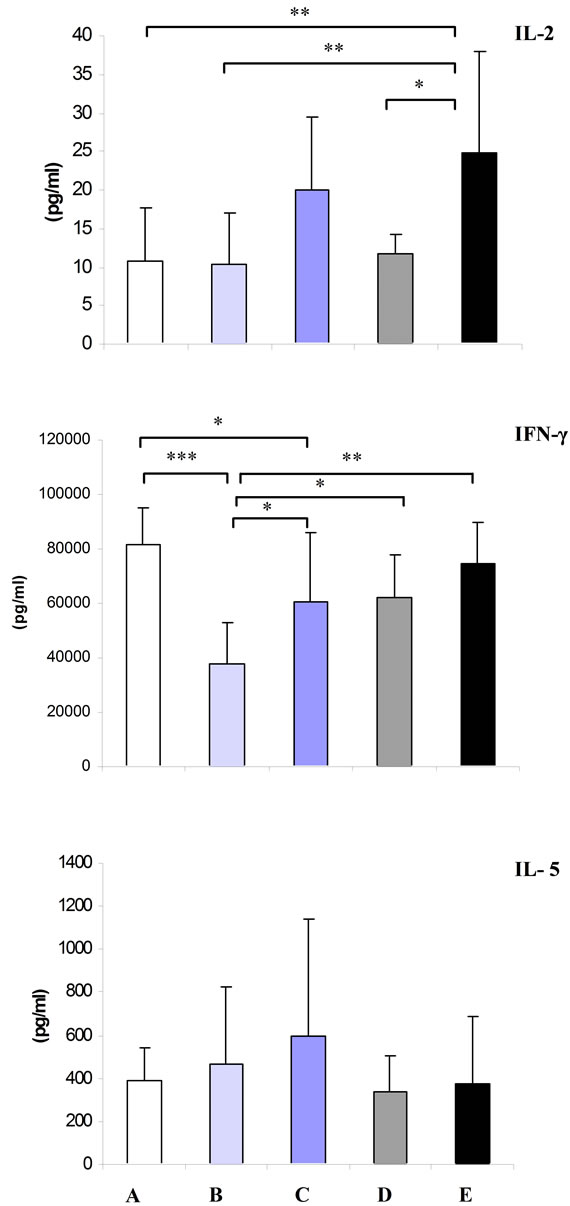
Figure 1. Mean ± SD of IL-5, IFN-γ and IL-2 secreted by splenocytes stimulated with anti-CD3 in vitamin A and zinc treatment groups. A: control (normal zinc and vitamin A); B: low zinc and normal vitamin A; C: low zinc and high vitamin A; D: low vitamin A and normal zinc and E: low vitamin A and high zinc. Single, double and treble asterisk represents P < 0.05, P < 0.01 and P < 0.001, respectively.
upper respiratory and enteric tract against pathogenic organisms. Modulation of IgA production by varying dietary levels was reported in previous reports [32-34]. In the current study, remarkable reduction of serum and intestinal mucosa IgA in mice on a diet with high zinc, low vitamin A (group E) compared to mice in control and normal zinc, low vitamin A groups suggest that IgA reduction correlate with high intake of zinc. The finding is in consistent with a previous report [35]. We speculate that a higher zinc supplementation suppresses IgA production and is associated with shifting of immune responses to Th1 dominance. This was well supported by enhanced IFN-γ and IL-2 production (Th1 cytokines) in the same group.
Several in vitro investigations suggested that dietary vitamin A regulated the function of Th2 cells. For example it has been reported that retinoic acid can induce IL-5 synthesis by splenocytes [36,37]. The modest augmentation of IL-5 level by high supplementation of dietary vitamin A in zinc restricted animals (group C) supported the findings. In addition, zinc restriction in the same animals did not affect production of IL-5 that was in concordant with previously reported studies indicating that ZD did not modulate production of Th2 cytokines [38,39]. IFN-γ and IL-2 are key cytokines to shift balance of immune system toward Th1. There are lots of studies indicating down-regulation of Th1 cytokines with vitamin A supplementation [40-43]. By contrast, the others reported positive effect of the nutrient on IFN-γ [44]. Zinc deficiency is reported to reduce IFN-γ production [38]. So, in the current study we observed a significant reduction of IFN-γ in the animals supplemented with vitamin A during ZD (groups B and C) in comparison to the mice in control group. But, an augmentation in production of IFN-γ in animals fed with high doses of vitamin A (group C) than those fed with sufficient doses of the vitamin (group B) clearly supported up-regulation of IFN-γ production by vitamin A. Further investigations might be of help to better clarify the relationship between IFN-γ production and vitamin A with or without co-existing ZD.
IL-2, produced by Th1 cells, is a T cell growth factor. It triggers peripheral T lymphocytes to enter the S phase of the cell cycle for cell division and enhances natural killer cells for host resistance to tumor development and growth and bacterial infection. The current report indicated that high dose supplementation vitamin A regulated positively production of IL-2. This was in good agreement with recent reports explained inhibitory effect of retinoic acid on spontaneous apoptosis of activated T lymphocytes through a retinoic acid receptor dependent increase in IL-2 production [45,46]. All-trans-retinoic acid is considered as stimulus of cell cycle machinery and proliferation of normal human T cells by increasing IL-2 secretion through mechanisms involving RARs [47]. In harmony with the mentioned reports, we have been able to show augmented levels of IL-2 in zinc deficient animals with high intake of vitamin A (group C) than adequate intake of vitamin A. This might be implying that vitamin A functions as a Th1 modulator.
Furthermore, the important role of zinc as an essential trace element for cell mediated immunity has already been well established [48,49]. It is demonstrated that zinc mediates positively the gene expression of IL-2 and IFN-γ in the Th1 cell lines [50]. Moreover, ZD induced by an experimental diet in human subjects decreased the level of IFN-γ, and zinc supplementation to these zincdeficient subjects reversed the level of IFN-γ [51]. In the study, zinc-deficient cells exhibited decreased levels of IFN-γ mRNA and cytokine compared with zinc-sufficient cells, which suggesting that zinc may mediate the gene expression of IFN-γ in Th0 and Th1 cells [51]. Our findings confirmed the reports. We observed that in the animals with vitamin A limitation, high dose utilization of zinc (group E) up regulated production of the both IFN-γ and IL-2 as compared to low or normal intake of zinc (groups B and D). So, it is concluded that the findings can further support the efficacy of zinc on cellular immunity.
In conclusion, these findings indicated that supplementation of zinc augments production of IL-2 and IFN- γ, the two key T cell cytokines. Also, supplementation of vitamin A in zinc deficient animals enhances production of the same cytokines. Therefore, this study was emphasized that examining synergistic effect of the two elements is very important in better clarifying the effect of zinc or vitamin A on many aspects of immune function.
5. Acknowledgements
The authors thank Prof. Shigeru Yamamoto former chairman of Department of international public health nutrition at The University of Tokushima, Japan for his enthusiastic support while conducting this research and generous providing of reagents. We also thank Dr. Kayoko Uezu for her valuable advice and technical help.
REFERENCES
- C. P. Wong, Y. Song, V. D. Elias, K. R. Magnusson and E. Ho, “Zinc Supplementation Increases Zinc Status and Thymopoiesis in Aged Mice,” The Journal of Nutrition, Vol. 139, No. 7, 2009, pp. 1393-1397. doi:10.3945/jn.109.106021
- L. Alappat, M. Valerio and A. B. Awad, “Effect of Vitamin D and β-Sitosterol on Immune Function of Macrophages,” International Immunopharmacology, Vol. 10, No. 11, 2010, pp. 1390-1396. doi:10.1016/j.intimp.2010.08.003
- T. Treadwell, “Commentary: Enhanced Healing of Surgical Wounds of the Lower Leg Using Weekly Zinc Oxide Compression Dressings,” Dermatologic Surgery, Vol. 37, No. 2, 2011, pp. 166-167.
- M. D. Lastra, R. Pastelin, A. Camacho, B. Monroy and A. E. Aguilar, “Zinc Intervention on Macrophages and Lymphocytes Response,” Journal of Trace Elements in Medicine and Biology, Vol. 15, No. 1, 2001, pp. 5-10. doi:10.1016/S0946-672X(01)80019-5
- R. A. Winchurch, J. Togo and W. H. Adler, “Supplemental Zinc Restores Antibody Formation in Cultures of Aged Spleen Cells. III. Impairment of II-2-Mediated Responses,” Clinical Immunology and Immunopathology, Vol. 49, No. 2, 1988, pp. 215-222.
- B. Bao, A. S. Prasad, F. W. Beck and M. Godmere, “Zinc Modulates mRNA Levels of Cytokines,” American Journal of Physiology—Endocrinology and Metabolism, Vol. 285, No. 5, 2003, pp. E1095-E1102.
- S. L. Jensen, C. McCuaig, A. Zembowicz and M. A. Hurt, “Bullous Lesions in Acrodermatitis Enteropathica Delaying Diagnosis of Zinc Deficiency: A Report of Two Cases and Review of the Literature,” Journal of Cutaneous Pathology, Vol. 35, Suppl. 1, 2008, pp. 1-13. doi:10.1111/j.1600-0560.2008.00981.x
- C. T. Walsh, H. H. Sandstead, A. S. Prasad, P. M. Newberne and P. J. Fraker, “Zinc Health Effects and Research Priorities for the 1990’s,” Environmental Health Perspectives, Vol. 102, Suppl. 2, 1994, pp. 5-46. doi:10.2307/3431820
- P. D. Zalewski, “Zinc and Immunity: Implications for Growth, Survival and Function of Lymphoid Cells,” Journal of Nutritional Immunology, Vol. 4, No. 3, 1996, pp. 39-101.
- Z. Karakas, N. Demirel, M. Tarakcioglu and N. Mete, “Serum Zinc and Copper Levels in Southeastern Turkish Children with Giardiasis or Amebiasis,” Biological Trace Element Research, Vol. 84, No. 1-3, 2001, pp. 11-18. doi:10.1385/BTER:84:1-3:011
- M. E. Penny, J. M. Peerson, R. M. Marin, A. Duran, C. F. Lanara, B. Lonnerdal, R. E. Black and R. H. Brown, “Randomized, Community-Based Trial of the Effect of Zinc Supplementation, with and without Other Micronutrients, on the Duration of Persistent Childhood Diarrhea in Lima. Peru,” Journal of Pediatric, Vol. 135, No. 2, 1999, pp. 208-217. doi:10.1016/S0022-3476(99)70024-7
- W. Zhong, C. J. McClain, M. Cave, Y. J. Kang and Z. Zhou, “The Role of Zinc Deficiency in Alcohol-Induced Intestinal Barrier Dysfunction,” American Journal of Physiology—Endocrinology and Metabolism, Vol. 298, No. 5, 2010, pp. G625-G633. doi:10.1152/ajpgi.00350.2009
- S. Southon, G. Livesey, J. M. Gee and I. T. Johnson, “Intestinal Cellular Proliferation and Protein Synthesis in Zinc-Deficient Rats,” British Journal of Nutrition, Vol. 53, No. 1, 1985, pp. 595-603. doi:10.1079/BJN19850013
- B. Vallee and K. H. Falchuk, “The Biochemical Basis of Zinc,” Physiological Reviews, Vol. 73, No. 1, 1993, pp. 79-118.
- A. Ertesvag, H. C. Aasheim, S. Naderi and H. K. Blomhoff, “Vitamin A Potentiates CpG-Mediated Memory B-Cell Proliferation and Differentiation: Involvement of Early Activation of p38MAPK,” Blood, Vol. 109, No. 9, 2007, pp. 3865-3872. doi:10.1182/blood-2006-09-046748
- J. A. Hall, J. L. Cannons, J. R. Grainger, L. M. Dos Santos, T. W. Hand, S. Naik, E. A. Wohlfert, D. B. Chou, G. Oldenhove, M. Robinson, M. E. Grigg, R. Kastenmayer, P. L. Schwartzberg and Y. Belkaid, “Essential Role for Retinoic Acid in the Promotion of CD4(+) T Cell Effector Responses via Retinoic Acid Receptor Alpha,” Immunity, Vol. 34, No. 3, 2011, pp. 435-447. doi:10.1016/j.immuni.2011.03.003
- P. Aukrust, F. Muller, T. Ueland, et al., “Decreased Vitamin A Levels in Common Variable Immunodeficiency: Vitamin A Supplementation in Vivo Enhances Immunoglobulin Production and Down Regulates Inflammatory Responses,” European Journal of Clinical Investigation, Vol. 30, No. 3, 2000, pp. 252-259. doi:10.1046/j.1365-2362.2000.00619.x
- K. A. Hoag, F. E. Nashold, J. Goverman and C. E. Hayes, “Retinoic Acid Enhances the T Helper 2 Cell Development That Is Essential for Robust Antibody Responses through Its Action on Antigen-Presenting Cells,” The Journal of Nutrition, Vol. 132, No. 12, 2002, pp. 3736- 3739.
- C. K. Park, Y. Ishimi, M. Ohmura, M. Yamaguchi and S. Ikegami, “Vitamin A and Carotenoids Stimulate Differentiation of Mouse Osteoblastic Cells,” Journal of Nutritional Science and Vitaminology, Vol. 43, No. 3, 1997, pp. 281-296. doi:10.3177/jnsv.43.281
- H. K. Blomhoff and E. B. Smeland, “Role of Retinoids in Normal Hematopoiesis and in the Immune System,” In: R. Blomhoff, Ed., Vitamin A in Health and Disease, Marcel Dekker, New York, 1994, p. 451.
- Q. Chen and A. C. Ross, “Vitamin A and Immune Function: Retinoic Acid Modulates Population Dynamics in Antigen Receptor and CD38-Stimulated Splenic B Cells,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 102, No. 40, 2005, pp. 14142-14149. doi:10.1073/pnas.0505018102
- C. Cameron, F. Dallaire, C. Vézina, G. Muckle, S. Bruneau, P. Ayotte and E. Dewailly, “Neonatal Vitamin A Deficiency and Its Impact on Acute Respiratory Infections among Preschool Inuit Children,” Canadian Journal of Public Health, Vol. 99, No. 2, 2008, pp. 102-106.
- A. Imdad, M. Y. Yakoob, C. Sudfeld, B. A. Haider, R. E. Black and Z. A. Bhutta, “Impact of Vitamin A Supplementation on Infant and Childhood Mortality,” BMC Public Health, Vol. 11, Suppl. 3, 2011, p. S20. doi:10.1186/1471-2458-11-S3-S20
- P. Donnen, A. Sylla, M. Dramaix, G. Sall, N. Kuakuvi and P. Hennart, “Effect of Daily Low Dose of Vitamin A Compared with Single High Dose on Morbidity and Mortality of Hospitalized Mainly Malnourished Children in Senegal: A Randomized Controlled Clinical Trial,” European Journal of Clinical Nutrition, Vol. 61, No. 12, 2007, pp. 1393-1399. doi:10.1038/sj.ejcn.1602671
- A. Imdad, K. Herzer, E. Mayo-Wilson, M. Y. Yakoob and Z. A. Bhutta, “Vitamin A Supplementation for Preventing Morbidity and Mortality in Children from 6 Months to 5 Years of Age,” Cochrane Database of Systematic Reviews, Vol. 12, 2010, Article ID: CD008524.
- P. Christian and K. P. West Jr., “Interactions between Zinc and Vitamin A: An Update,” The American Journal of Clinical Nutrition, Vol. 68, Suppl. 2, 1998, pp. 435- 441.
- C. W. Levenson, “Zinc Regulation of Food Intake: New Insights on the Role of Neuropeptide Y,” Nutrition Reviews, Vol. 61, No. 7, 2003, pp. 247-249. doi:10.1301/nr.2003.jul.247-249
- L. M. Gaetke, R. C. Frederich, H. S. Oz and C. J. McClain, “Decreased Food Intake Rather than Zinc Deficiency Is Associated with Changes in Plasma Leptin, Metabolic Rate, and Activity Levels in Zinc Deficient Rats (Small Star, Filled),” The Journal of Nutritional Biochemistry, Vol. 13, No. 4, 2002, pp. 237-244. doi:10.1016/S0955-2863(01)00220-0
- I. S. Kwun, Y. E. Cho, R. A. Lomeda, S. T. Kwon, Y. Kim and J. H. Beattie, “Marginal Zinc Deficiency in Rats Decreases Leptin Expression Independently of Food Intake and Corticotrophin-Releasing Hormone in Relation to Food Intake,” The British Journal of Nutrition, Vol. 98, No. 3, 2007, pp. 485-489. doi:10.1017/S0007114507730763
- S. Moriguchi, L. Werner and R. R. Watson, “High Dietary Vitamin A (Retinyl Palmitate) and Cellular Immune Functions in Mice,” Immunology, Vol. 56, No. 1, 1985, pp. 169-177.
- R. Albers, M. Bol, R. Bleumink, A. A. Willems and R. H. Pieters, “Effects of Supplementation with Vitamins A, C, and E, Selenium, and Zinc on Immune Function in a Murine Sensitization Model,” Nutrition, Vol. 19, No. 11-12, 2003, pp. 940-946. doi:10.1016/S0899-9007(03)00178-3
- Y. Yang, Y. Yuan, Y. Tao and W. Wang, “Effects of Vitamin A Deficiency on Mucosal Immunity and Response to Intestinal Infection in Rats,” Nutrition, Vol. 27, No. 2, 2011, pp. 227-232. doi:10.1016/j.nut.2009.11.024
- C. B. Stephensen, Z. Moldoveanu and N. N. Gangopadhyay, “Vitamin A Deficiency Diminishes the Salivary Immunoglobulin A Response and Enhances the Serum Immunoglobulin G Response to Influenza A Virus Infection in BALB/c Mice,” The Journal of Nutrition, Vol. 126, No. 1, 1996, pp. 94-102.
- U. Wiedermann, L. A. Hanson, J. Holmgren, H. Kahu and U. I. Dahlgren, “Impaired Mucosal Antibody Response to Cholera Toxin in Vitamin A-Deficient Rats Immunized with Oral Cholera Vaccine,” Infection and Immunity, Vol. 61, No. 9, 1993, pp. 3952-3957.
- D. K. Chattopadhyay, C. R. Maity and D. J. Nag, “Effect of Zinc Supplementation on Mycospecific Immunoglobulins in Tuberculosis Patients,” Journal of the Indian Medical Association, Vol. 108, No. 2, 2010, pp. 92-93.
- T. Nikawa, K. Odahara, H. Koizumi, Y. Kido, S. Teshima, K. Rokutan and K. Kishi, “Vitamin A Prevents the Decline in Immunoglobulin A and Th2 Cytokine Levels in Small Intestinal Mucosa of Protein-Malnourished Mice,” The Journal of Nutrition, Vol. 129, No. 5, 1999, pp. 934- 941.
- C. B. Stephensen, R. Rasooly, X. Jiang, M. A. Ceddia, C. T. Weaver, R. A. Chandraratna and R. P. Bucy, “Vitamin A Enhances in Vitro Th2 Development via Retinoid X Receptor Pathway,” The Journal of Immunology, Vol. 168, No. 9, 2002, pp. 4495-4503.
- A. S. Prasad, “Effects of Zinc Deficiency on Th1 and Th2 Cytokine Shifts,” The Journal of Infectious Diseases, Vol. 182, Suppl. 1, 2000, pp. 62-68.
- A. S. Prasad, F. W. Beck, S. M. Grabowski, J. Kaplan and R. H. Mathog, “Zinc Deficiency: Changes in Cytokine Production and T-Cell Subpopulations in Patients with Head and Neck Cancer and in Noncancer Subjects,” Proceedings of the Association of American Physicians, Vol. 109, No. 1, 1997, pp. 68-77.
- Y. H. Tao and Y. Yang, “Effects of Vitamin A on the Differentiation, Maturation and Functions of Dendritic Cells from Cord Blood,” Chinese Journal of Pediatrics, Vol. 42, No. 5, 2004, pp. 340-343.
- M. Iwata, Y. Eshima and H. Kagechika, “Retinoic Acids Exert Direct Effects on T Ells to Suppress Th1 Development and Enhance Th2 Development via Retinoic Acid Receptors,” International Immunology, Vol. 15, No. 8, 2003, pp. 1017-1025. doi:10.1093/intimm/dxg101
- X. Wu, X. Liu and J. Tang, “The Effect of Vitamin A on Secretion of IFN-Gamma and IL-4 in A549 Cells Induced by Mycoplasma Pneumoniae,” Journal of Huazhong University of Science and Technology-Medical Sciences, Vol. 28, No. 6, 2008, pp. 649-652. doi:10.1007/s11596-008-0607-6
- D. Cui, Z. Moldoveanu and C. B. Stephensen, “HighLevel Dietary Vitamin A Enhances T-Helper Type 2 Cytokine Production and Secretory Immunoglobulin A Response to Influenza A Virus Infection in BALB/c Mice,” The Journal of Nutrition, Vol. 130, No. 5, 2000, pp. 1132-1139.
- L. M. Allende, A. Corell, A. Madrono, R. Gongora, C. Rodriguez-Gallego, A. Lopez-Goyanes, M. Rosal and A. Arnaiz-Villena, “Retinol (Vitamin A) Is a Cofactor in CD3-Induced Human T-Lymphocyte Activation,” Immunology, Vol. 90, No. 3, 1997, pp. 388-396. doi:10.1111/j.1365-2567.1997.00388.x
- N. Engedal, A. Ertesvag and H. K. Blomhoff, “Survival of Activated Human T Lymphocytes Is Promoted by Retinoic Acid via Induction of IL-2,” International Immunology, Vol. 16, No. 3, 2004, pp. 443-453. doi:10.1093/intimm/dxh048
- H. K. Blomhoff, “Vitamin A Regulates Proliferation and Apoptosis of Human Tand B-Cells,” Biochemical Society Transactions, Vol. 32, No. 6, 2004, pp. 982-984.
- A. Ertesvag, N. Engedal, S. Naderi and H. K. Blomhoff, “Retinoic Acid Stimulates the Cell Cycle Machinery in Normal T Cells: Involvement of Retinoic Acid Receptor-Mediated IL-2 Secretion,” The Journal of Immunology, Vol. 169, No. 10, 2002, pp. 5555-5563.
- A. K. Baltaci, R. Mogulkoc, C. S. Bediz and A. Pekel, “Effects of Zinc Deficiency and Pinealectomy on Cellular Immunity in Rats Infected with Toxoplasma Gondii,” Biological Trace Element Research, Vol. 104, No. 1, 2005, pp. 47-56. doi:10.1385/BTER:104:1:047
- A. K. Baltaci, C. S. Bediz, R. Mogulkoc, E. Kurtoglu and A. Pekel, “Effect of Zinc and Melatonin Supplementation on Cellular Immunity in Rats with Toxoplasmosis,” Biological Trace Element Research, Vol. 96, No. 1-3, 2003, pp. 237-245. doi:10.1385/BTER:96:1-3:237
- A. S. Prasad, B. Bao, F. W. Beck and F. H. Sarkar, “Zinc Enhances the Expression of Interleukin-2 and Interleukin-2 Receptors in HUT-78 Cells by Way of NFKappaB Activation,” Journal of Laboratory and Clinical Medicine, Vol. 140, No. 4, 2002, pp. 272-289. doi:10.1067/mlc.2002.127908
- F. W. Beck, A. S. Prasad, J. Kaplan, J. T. Fitzgerald and G. J. Brewer, “Changes in Cytokine Production and T Cell Subpopulations in Experimentally Induced ZincDeficient Humans,” American Journal of Physiology, Vol. 272, No. 6, 1997, pp. E1002-E1007.

