Advances in Parkinson's Disease
Vol.2 No.2(2013), Article ID:31591,6 pages DOI:10.4236/apd.2013.22012
Wireless accelerometer configuration for monitoring Parkinson’s disease hand tremor
![]()
1Independent, Running Springs, USA; *Corresponding Author: rlemoyne07@gmail.com
2Independent, Pittsburgh, USA
3Department of Bioengineering, University of California Los Angeles, Los Angeles, USA
Copyright © 2013 Robert LeMoyne et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 28 November 2012; revised 4 January 2013; accepted 16 January 2013
Keywords: Parkinson’s disease; wireless accelerometer; Parkinson’s disease tremor Quantification
ABSTRACT
Parkinson’s disease is neurodegenerative in nature and associated with characteristic movement disorders, such as hand tremor. Wireless accelerometer applications may advance the quality of care for Parkinson’s disease patients. The acceleration waveform of the respective hand tremor can be recorded and stored for postprocessing and progressive status tracking. A wireless accelerometer configuration for monitoring Parkinson’s disease hand tremor is presented. The proposed configuration is envisioned to be conducted with the assistance of a caregiver. For initial engineering proof of concept simulated Parkinson’s disease tremor is recorded through a wireless accelerometer node and contrasted to a statically positioned and tandem activated wireless accelerometer node. Statistical significance is acquired regarding the quantification of the simulated Parkinson’s disease tremor acceleration waveform and statically positioned acceleration waveform, while demonstrating a considerable degree of accuracy, consistency, and reliability.
1. INTRODUCTION
Wireless systems that are effectively wearable constitute the potential to ameliorate progressive strain on limited medical resources. Wireless accelerometer applications have been advocated for their ability to quantify Parkinson’s disease tremor [1,2]. Wireless accelerometers systems can provide valuable information regarding the patient status. Quantified data of patient response to medication prescription can be used in optimizing therapy and therefore improving the quality of life of the patient [3].
A wireless application, which is temporarily wearable, should be uniquely designed to satisfy the goals of the patient. A full 24-hour monitoring system may be too intrusive regarding the privacy of the patient. Other advanced applications may be too complex for operation or require new technical acumen, exceeding the capability of the patient and potential caregiver.
An alternative application involves a wireless accelerometer node mounted to the dorsum aspect of the hand through a glove worn by the Parkinson’s patient. The patient can periodically mount the glove attached to the wireless accelerometer node for evaluation and then remove the device until the following periodic evaluation. Such a strategy could mitigate intrusion on the patient’s perception of personal autonomy and privacy.
The proposed configuration advocates a tandem patient and caregiver operated system. The caregiver would record a sample of the Parkinson’s disease patient’s tremor score through the operation of a wireless accelerometer node. The wireless accelerometer node conveys the accelerometer signal through wireless transmission to a local PC operated by the caregiver. The tremor acceleration signal could be stored for later post-processing. Preliminary engineering proof of concept is demonstrated through the use of a wireless accelerometer node mounted to the dorsum of the hand and secured by a glove to quantify simulated Parkinson’s disease hand tremor contrasted to a tandem activated static positioned wireless accelerometer node as a control condition.
2. BACKGROUND
2.1. General Features of Parkinson’s Disease
Parkinson’s disease is a neurodegenerative disease that is comprised of characteristic movement disorder. The general incidence for Parkinson’s disease predominantly occurs for people of 55 years age and older [4]. Logically for a population that is shifting to an older age range, Parkinson’s disease is becoming a rampant medical concern. In the United States of America roughly one million people are affected by Parkinson’s disease [5]. Especially in a nation, such as the United States of America, with a considerable sample of the population living in rural areas, a functionally wearable and wireless accelerometer application can extend the service range of specialists in urban settings [3].
The neurological origins of Parkinson’s disease are attributed to a degeneration of the dopaminergic neurons from the substantia nigra [5]. The impairment to the function of the substantia nigra results in diminishing dopamine synthesis capacity for the caudate and putamen. As a cumulative result, Parkinson’s disease symptoms, such as distinct movement disorders manifest [6].
Parkinson’s disease is correlated with distinct movement disorder characteristics. Four predominate features are:
• Shuffling of gait
• Impaired balance
• Rigidity as a result of increased muscle tone
• Resting tremor [5]
Resting tremor in some cases can diminish with voluntary movement [7]. The characteristic tremor rate occurs at roughly four to five per second [5,7].
There are three conventional approaches to ameliorate Parkinson’s disease:
• Drug therapy
• Pallidotomy [5,8]
• Deep brain stimulation [9]
The prescription of L-dopa is a standard aspect of drug therapy to treat Parkinson’s disease [5]. The use of wireless accelerometer systems has been proposed for the titration/optimization of drug therapy. The attributes of the accelerometer signal for the respective patient with Parkinson’s disease could provide specialized medical experts quantified and objective feedback regarding drug therapy efficacy [3,10].
The pallidotomy is generally considered a last resort. All other available therapy strategies have been deemed not effective. The pallidotomy involves brain surgery that produces a lesion for the internal aspect of the globus pallidus [5,8].
A relatively novel alternative involves the subthalamic, thalamic, or pallidal stimulation of the brain. The deep brain stimulator consists of multiple parameters: amplitude, frequency, pulse width, and electrode polarity. Parameter setting combinations on the scale of a thousand exist. The movement disorder specialist is tasked with optimizing the deep brain stimulation parameter settings [9]. Wireless accelerometer applications have been proposed to provide objective quantified feedback for deriving the optimal deep brain stimulation parameter settings [10].
2.2. Accelerometers for Quantifying Parkinson’s Disease Characteristics
The implementation of accelerometers and eventually wireless accelerometers has been advocated for the objective quantification of Parkinson’s disease movement disorder attributes, such as resting tremor. Wireless and accelerometer sensor technologies have evolved. Wireless accelerometer applications have achieved the threshold of being demonstrated as viable for evaluating the status of Parkinson’s disease patients [1,3,10].
Preliminary accelerometer systems have been demonstrated in the autonomous environment of the Parkinson’s disease subject. Based on the respective accelerometer signal data, the ability to classify Parkinson’s disease “on” and “off” states has been demonstrated. Further evolution of accelerometer devices can lead to individually optimized drug therapy strategies [11].
Accelerometer applications have been applied to objectively evaluate adverse side effects of drug therapy. For example, the severity of levodopa-induced dyskinesia has been quantified using accelerometers [12]. The quantitative feedback of the accelerometer signal can enhance diagnostic acuity. A drug therapy strategy that can enhance cognitive function but has the drawback of amplification of movement disorder, such as tremor, can be elucidated in an objectively quantified manner [13].
The feedback derived from the accelerometer signal can be coupled with numerical methods. Spectral analysis of the accelerometer signal has contributed to ascertain the medication efficacy [14]. Wearable, but not wireless, accelerometer applications have been applied for elucidating gait features of Parkinson’s disease patients [15]. Accelerometers have been also proposed as integral for brain machine interface systems incorporating wireless telemetry for augmenting the architecture of neural stimulator technology [16]. The feedback from accelerometer systems has been applied to discern the efficacy of deep brain stimulation [17,18].
2.3. Wireless Accelerometers for Quantified Assessment of Parkinson’s Disease Characteristics
During 2009 LeMoyne et al. preliminarily demonstrated and advocated the use of wireless accelerometer nodes for the quantification of Parkinson’s disease hand tremor features [1,19]. Giuffrida et al. evaluated KinesiaTM consisting of integrated motion sensors, such as accelerometers, for automated assessment of Parkinson’s disease tremor. KinesiaTM conveys the motion sensor data by wire to a command module mounted to the wrist for eventual wireless transmission [20]. However, the connecting wire may be accidentally dislodged, impairing the function of the device. Wearable and wireless accelerometer architectures have been proposed for quantifying the features of standard daily living activities, such as gait quality, for Parkinson’s disease subjects [21- 23]. Although such a prolonged continuous monitoring system may provide valuable insight for patient unique Parkinson’s disease, a patient’s willingness to be continuously monitored is a debatable topic.
During 2010 LeMoyne et al. pioneered the use of an iPhone for quantifying Parkinson’s disease hand tremor through using an application for measuring and emailing a recorded sample of the accelerometer waveform. The iPhone application requires mounting the iPhone to the dorsum of the hand, and then recording the acceleration waveform of the Parkinson’s disease hand tremor. The proper operation of the iPhone application for measuring Parkinson’s disease hand tremor requires understanding of the smartphone technology space [10]. Later in 2011 Kostikis et al. utilized a smartphone for evaluating Parkinson’s disease characteristics [24].
The proposed wireless accelerometer architecture promotes the use of a G-Link®Wireless Accelerometer Node for evaluating Parkinson’s disease tremor of the hand. The architecture utilizes a simple graphic user interface for activating the recording of the acceleration waveform. The wireless accelerometer node is secured to the dorsum of the hand through a glove. The proposed architecture should be easy to apply with a generally skilled caregiver.
Figure 1 illustrates the wireless accelerometer architecture. The compact nature of the G-Link®Wireless Accelerometer Node readily enables mounting to the dorsum of the hand through a glove. The G-Link®Wireless Accelerometer Node has a mass of 46 grams, and has multiple sampling rates, such as 512 Hz. The node can temporarily store multiple samples of the acceleration waveform that are later transmitted wireless to a local PC [25].
For preliminary engineering proof of concept, two G-Link®Wireless Accelerometer Nodes are incorporated in the experiment. One wireless accelerometer node is placed in a static position to serve as a control. The second wireless accelerometer node representing the experimental is mounted to the dorsum of the hand by a glove, and Parkinson’s disease hand tremor is simulated.
3. EXPERIMENTAL PROTOCOL
The preliminary implementation of the G-Link®Wireless Accelerometer Node for quantifying Parkinson’s disease hand tremor involved the evaluation of simulated Parkinson’s disease tremor as an experimental contrasted to a tandem activated static control. The apparatus for the experiment is illustrated in Figure 1. The sampling rate for both the experimental and control wireless accelerometer nodes was 512 Hz. 20 trials were conducted based on the following protocol:
1) Mount the experimental wireless accelerometer node to the dorsum of the hand, securing the wireless accelerometer node with a glove;
2) Place a tandem activated control wireless accelerometer node in a static position;
3) Activate in tandem the data-logging mode for both wireless accelerometers (simulated tremor experimental and static control);
4) Initiate simulated tremor;
5) Continue Steps 1-4 for 20 trials;
6) Download acceleration waveform signals from wireless accelerometer nodes by wireless transmission to the local PC.
4. RESULTS AND DISCUSION
4.1. Results
The resulting data for 20 trials were wirelessly conveyed to a local PC for post-processing. The data consisted of Microsoft Excel Comma-Separated-Value files representing each trial. Each Comma-Separated-Value file was comprised of four column vectors representing time, X-acceleration, Y-acceleration, and Z-acceleration. Based on the three orthogonal acceleration components, the magnitude of the acceleration vector was calculated to represent the acceleration waveform for the temporal domain.
In order to minimize transient effects, the acceleration waveform of the 5-second duration between 2.5 seconds

Figure 1. Wireless accelerometer architecture for quantifying Parkinson’s disease hand tremor using G-Link®Wireless Accelerometer Nodes; control (left) and glove mounted experimental (right).
and 7.5 seconds of the 10-second acceleration waveform sample was analyzed. The time-averaged acceleration using a trapezoid rule was acquired for each respective acceleration waveform sample of both the simulated Parkinson’s disease tremor and static condition. LeMoyne et al. has previously advocated the time-averaged acceleration technique for quantifying the acceleration waveform regarding Parkinson’s disease [1,10,19].
The time averaged acceleration data acquired between 2.5 and 7.5 seconds for the 20 trials of the statically positioned control and experimental simulated Parkinson’s disease hand tremor demonstrated a considerable degree of accuracy, consistency, and reliability while establishing statistical significance. Based on the 20 trial sample, the time averaged acceleration for both the statically positioned control and experimental simulated Parkinson’s disease hand tremor were bound with a 98% confidence level respective of a 2% margin of error about the mean. Comparison of the time averaged acceleration for the simulated Parkinson’s disease hand tremor wireless accelerometer node to the static positioned wireless accelerometer node exhibited statistical significance based on a one-way ANOVA with alpha <0.05.
Table 1 features the mean, standard deviation, and coefficient of variation regarding the 20 trials for the tandem activated wireless accelerometer nodes. The acceleration waveform of the experimental wireless accelerometer node that represents simulated Parkinson’s disease tremor is presented in Figure 2. Note the cyclical nature of the acceleration waveform in Figure 2, which is representative of simulated Parkinson’s disease hand tremor. The control wireless accelerometer node, which is placed in a static position, is illustrated in Figure 3. Note the acceleration waveform for Figure 3 is effectively constant.
4.2. Discussion
The wireless accelerometer application presented in Figure 1 represents a wireless and wearable system for evaluating Parkinson’s disease hand tremor. The apparatus consists of wireless accelerometer nodes, associated software, a base station for receiving wireless transmission with USB connector, and a local PC. The experi-

Table 1. Simulated Parkinson’s disease hand tremor contrasted to static position for wireless accelerometers (20 trials).
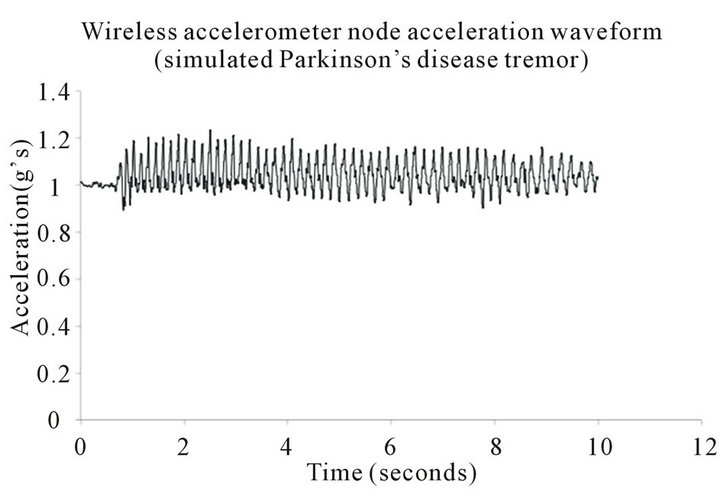
Figure 2. Acceleration waveform for simulated Parkinson’s disease tremor, incorporating glove mounted wireless accelerometer node.
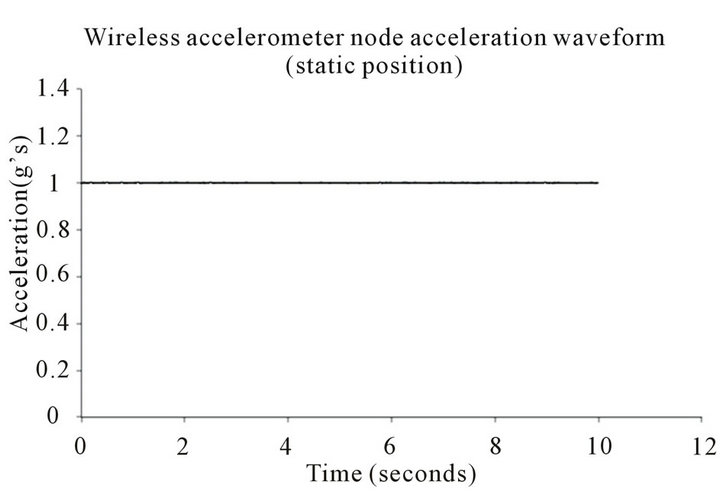
Figure 3. Acceleration waveform for the statically positioned wireless accelerometer node.
mental wireless accelerometer node is mounted to the dorsum of the hand and comfortably secured by a glove. The tandem activated control wireless accelerometer is placed in a static position. The primary components of the Parkinson’s disease evaluation system communicate by wireless transmission.
The wireless accelerometer data collected demonstrated a considerable degree of accuracy, consistency, and reliability. The acuity of the presented wireless accelerometer configuration for monitoring Parkinson’s disease hand tremor should facilitate the quality of evaluating patients with Parkinson’s disease. The quantified feedback regarding tremor may augment the titration of drug therapy and consideration of patient-unique therapy strategies.
The proposed wireless accelerometer configuration is envisioned to be operated by a generally skilled caregiver. The Parkinson’s disease patient would have their health status periodically evaluated. During an evaluation the patient wears a glove securing the wireless accelerometer node to the dorsum of the hand in a manner similar to the application illustrated in Figure 1. A series of acceleration waveforms quantifying Parkinson’s disease hand tremor are recorded and wirelessly transmitted to a local PC. The recorded acceleration waveforms can be postprocessed on the local PC using a software algorithm. Another strategy involves conveying the acceleration waveform data by email for remote post-processing as demonstrated by LeMoyne et al. [10].
5. CONCLUSIONS
A configuration incorporating a wireless accelerometer application has demonstrated a considerable degree of accuracy, consistency, and reliability for quantifying the acceleration waveform of simulated Parkinson’s disease tremor. The experiment consisted of two wireless accelerometer nodes that were tandem activated. One wireless accelerometer representing the control was placed in a static position. The other tandem activated wireless accelerometer representing the experimental was secured by a glove to the dorsum of a hand that simulated Parkinson’s disease hand tremor. 20 trials encompassing the control and experimental wireless accelerometers were conducted, and post-processing quantified the time-averaged acceleration of the respective acceleration waveforms.
Regarding the 20 trials, the time-averaged acceleration for the statically placed control and simulated Parkinson’s disease hand tremor experimental were bound by a 98% confidence level based on a 2% margin of error about the mean. Statistical significance using a one-way ANOVA with alpha <0.05 for the time-averaged acceleration of the statically placed control and simulated Parkinson’s disease hand tremor experimental was determined.
Based on the successful initial proof of concept regarding the wireless accelerometer application, an expanded investigation quantifying hand tremor for Parkinson’s disease patients may be warranted. The proposed wireless accelerometer configuration is envisioned to be operated by a general skilled caregiver for periodically quantifying the features of patients with Parkinson’s disease hand tremor. Future evolutions of the configuration involve the development of expanded software techniques for automation of feature acquisition and database storage for establishing progressive patient unique trends and therapy feedback.
REFERENCES
- LeMoyne, R., Coroian, C., Mastroianni, T., Opalinski, P., Cozza, M. and Grundfest, W. (2009) The merits of artificial proprioception, with applications in biofeedback gait rehabilitation concepts and movement disorder characterization. In: Barros de Mello, C.A., Biomedical Engineering, Intech, Vienna, 165-198.
- LeMoyne, R., Coroian, C., Mastroianni, T. and Grundfest, W. (2008) Accelerometers for quantification of gait and movement disorders: A perspective review. Journal of Mechanics in Medicine and Biology, 8, 137-152. doi:10.1142/S0219519408002656
- Patel, S., Park, H., Bonato, P., Chan, L. and Rodgers, M. (2012) A review of wearable sensors and systems with application in rehabilitation. Journal of NeuroEngineering and Rehabilitation, 9, 1-17. doi:10.1186/1743-0003-9-21
- Seeley, R.R., Stephens, T.D. and Tate, P. (2003) Anatomy and physiology. McGraw-Hill, New York.
- Kandel, E.R., Schwartz, J.H. and Jessell, T.M. (2000) Principles of neural science. McGraw-Hill, New York.
- Diamond, M.C., Scheibel, A.B. and Elson, L.M. (1985) The human brain coloring book. Harper Perennial, New York.
- Bickley, L.S. and Szilagyi, P.G. (2003) Bates’ guide to physical examination and history taking. Lippincott Williams and Wilkins, Philadelphia.
- Nolte, J. and Sundsten, J.W. (2002) The human brain: An introduction to its functional anatomy. Mosby, St. Louis.
- Volkmann, J., Moro, E. and Pahwa, R. (2006) Basic algorithms for the programming of deep brain stimulation in Parkinson’s disease. Movement Disorders, 21, S284-S289. doi:10.1002/mds.20961
- LeMoyne, R., Mastroianni, T., Cozza, M., Coroian, C. and Grundfest, W. (2010) Implementation of an iPhone for characterizing Parkinson’s disease tremor through a wireless accelerometer application. Proceeding of the 32nd Annual International Conference of the IEEE EMBS, Buenos Aires, 31 August-4 September 2010, 4954-4958.
- Keijsers, N.L., Horstink, M.W. and Gielen, S.C. (2006) Ambulatory motor assessment in Parkinson’s disease. Movement Disorders, 21, 34-44. doi:10.1002/mds.20633
- Keijsers, N.L., Horstink, M.W., van Hilten, J.J., Hoff, J.I. and Gielen, C.C. (2000) Detection and assessment of the severity of levodopa-induced dyskinesia in patients with Parkinson’s disease by neural networks. Movement Disorders, 15, 1104-1111. doi:10.1002/1531-8257(200011)15:6<1104::AID-MDS1007>3.0.CO;2-E
- Gurevich, T.Y., Shabtai, H., Korczyn, A.D., Simon, E.S. and Giladi, N. (2006) Effect of rivastigmine on tremor in patients with Parkinson’s disease and dementia. Movement Disorders, 21, 1663-1666. doi:10.1002/mds.20971
- Schrag, A., Schelosky, L., Scholz, U. and Poewe W. (1999) Reduction of Parkinsonian signs in patients with Parkinson’s disease by dopaminergic versus anticholinergic single-dose challenges. Movement Disorders, 14, 252-255. doi:10.1002/1531-8257(199903)14:2<252::AID-MDS1009>3.0.CO;2-N
- Weiss, A., Sharifi, S., Plotnik, M., van Vugt, J.P., Giladi, N. and Hausdorff, J.M. (2011) Toward automated, athome assessment of mobility among patients with Parkinson disease, using a body-worn accelerometer. Neurorehabilitation and Neural Repair, 25, 810-818. doi:10.1177/1545968311424869
- Rouse, A.G., Stanslaski, S.R., Cong, P., Jensen, R.M., Afshar, P., Ullestad, D., Gupta, R., Molnar, G.F., Moran, D.W. and Denison, T.J. (2011) A chronic generalized bidirectional brain-machine interface. Journal of Neural Engineering, 8, 1-36. doi:10.1088/1741-2560/8/3/036018
- Obwegeser, A.A., Uitti, R.J., Witte, R.J., Lucas, J.A., Turk, M.F. and Wharen Jr., R.E. (2001) Quantitative and qualitative outcome measures after thalamic deep brain stimulation to treat disabling tremors. Neurosurgery. 48, 274-281.
- Kumru, H., Summerfield, C., Valldeoriola, F. and VallsSolé, J. (2004) Effects of subthalamic nucleus stimulation on characteristics of EMG activity underlying reaction time in Parkinson’s disease. Movement Disorders, 19, 94- 100. doi:10.1002/mds.10638
- LeMoyne, R., Coroian, C. and Mastroianni, T. (2009) Quantification of Parkinson’s disease characteristics using wireless accelerometers. Proceeding of the International Conference on Complex Medical Engineering (CME-2009) of the IEEE/ICME, Tempe, 9-11 April 2009, 1-5.
- Giuffrida, J.P., Riley, D.E, Maddux B.N. and Heldman, D.A. (2009) Clinically deployable Kinesia technology for automated tremor assessment. Movement Disorders, 24, 723- 730. doi:10.1002/mds.22445
- Cancela, J., Pansera, M., Arredondo, M.T., Estrada, J.J., Pastorino, M., Pastor-Sanz, L. and Villalar, J.L. (2010) A comprehensive motor symptom monitoring and management system: The bradykinesia case. Proceeding of the 32nd Annual International Conference of the IEEE EMBS, Buenos Aires, 31 August-4 September 2010, 1008-1011.
- Pastorino, M., Cancela, J., Arredondo, M.T., Pansera, M., Pastor-Sanz, L., Villagra, F., Pastor, M.A. and Martin, J.A. (2011) Assessment of bradykinesia in Parkinson’s disease patients through a multi-parametric system. Proceeding of the 33rd Annual International Conference of the IEEE EMBS, Boston, 30 August-3 September 2011, 1810-1813.
- Cancela, J., Pastorino, M., Arredondo, M.T., Pansera, M., Pastor-Sanz, L., Villagra, F., Pastor, M.A. and Gonzalez, A.P. (2011) Gait assessment in Parkinson’s disease patients through a network of wearable accelerometers in unsupervised environments. Proceeding of the 33rd Annual International Conference of the IEEE EMBS, Boston, 30 August-3 September 2011, 2233-2236.
- Kostikis, N., Hristu-Varsakelis, D., Arnaoutoglou, M., Kotsavasiloglou, C. and Baloyiannis, S. (2011) Towards remote evaluation of movement disorders via smartphones. Proceeding of the 33rd Annual International Conference of the IEEE EMBS, Boston, 30 August-3 September 2011, 5240-5243.
- 2013. http://www.microstrain.com/g-link.aspx

