American Journal of Plant Sciences
Vol.4 No.3(2013), Article ID:28707,5 pages DOI:10.4236/ajps.2013.43066
In Vitro Propagation and Conservation of Zeyheria montana Mart: An Endangered Medicinal Plant
![]()
1UNAERP—Unidade de Biotecnologia de Plantas Medicinais, Ribeirão Preto, Brasil; 2National Center for Natural Products Research, The University of Mississippi, Oxford, USA.
Email: *apereira@unaerp.br
Received January 3rd, 2013; revised February 5th, 2013; accepted February 14th, 2013
Keywords: Cerrado; Bignoniaceae; Micropropagation; Germplasm Storage; Lapachol and Triterpenes
ABSTRACT
Roots of Zeyheria montana, a species native to the savanna (Cerrado) region of central Brazil, produce lapachol, a naphthoquinone with anticancer activity. Lapachol is also the precursor of β-lapachone, a novel drug candidate for preventive and adjuvant cancer therapies. The leaves of Z. montana are a renewable source of ursolic acid and oleanoic acid, compounds known for their anticancer, antioxidant and antimicrobial properties. The potential prophylactic use of β-lapachone, as well as the medicinal properties of ursolic acid, highlights the importance of this study on Z. montana’s germplasm conservation. Multiple shoots were induced on Woody Plant media with supplemented 0.1 mg·L−1 of thidiazuron (TDZ). Rooting was promoted on half strength WP (Woody Plant) media containing 1.0 mg·L−1 of indole-3- butyric acid (IBA). Plantlet acclimatization to ex-vitro condition was done at a 70% success rate using different substrates. It was possible to store Z. montana’s elite germplasm using in vitro cultures of media containing 2% sucrose plus 4% sorbitol for six months without subcultures.
1. Introduction
The medicinal importance of Zeyheria montana Mart. (bolsa de pastor) a plant native to Brazilian Cerrado, extends beyond the phytotherapeutic use. Its leaves produce ursolic, oleanolic and betulic acids, pentacyclictriterpenoids, with validated biological properties, such as anticancer activity [1], inhibitory effects on NO production and iNOS induction [2], anti-inflammatory [3], antifungal [4], diuretic [5]. Ethanol extract from Z. montana leaf possesses anti-nociceptive [6] and anti-inflammatory [7] activities and flavanones showed cytotoxicity against human NCI-ADR/RES and K562 cell lines [8].
Additionally, Z. montana roots are an alternative source of lapachol, a naphtoquinone with anticancer activity [9], producing 19 fold more than Tabebuia, the better known natural source of lapachol and b-lapachone [10]. In the 1970s, the clinical efficacy of lapachol and related compounds in cancer therapy was marked, but Cragg and Newman [11] reported that the National Cancer Institute discontinued the clinical trials on lapachol and related compounds due to high toxicity. Recent findings, however, indicate that b-lapachone may halt the transformation of normal cells to cancer cells, thus holding promise for a dual use, as an anticancer and anti-carcinogenic agent [12].
Only few conservation studies have been carried out so far as a relief effort to conserve Z. monatana germplasm [13-15]. Little is known on asexual propagation of elite plants to serve as propagule to achieve potential crops, and to improve quality, safety and efficacy of phytomedicine. According to Felippe and Silva [16], seed production is inconsistent, and flowering and fruit production are delayed for longer periods of time. Overcoming juvenility, Z. montana fruits are produced every other year.
In light of the therapeutic importance of Z. montana and its endangered status, the objective of this study was to establish in vitro propagation and conservation protocols to preserve the species germplasm.
2. Material and Methods
2.1. Plant Material
Plants and seeds were collected in Pedregulho County, São Paulo, Brazil (Lat. 20˚S 17'06", Long. 47˚W 26'12", Alt. 968 m) and the following studies were conducted micropropagation, in vitro conservation protocols and phytochemical analysis. A voucher specimen was deposited at the Universidade de Ribeirão Preto (UNAERP) Herbarium (HPM-063).
2.2. Micropropagation
Species belonging to Bignoniaceae have an outgrow of tissue (wings) that aids their dispersal. Seeds of Z. montana were collected. Next the wings were mechanically removed and later washed in tap water for 12 hours, and then soaked in 0.5% (w/v) benomyl solution for 24 h, constantly agitating the solution at 120 rpm. Before immersion in benomyl solution, seed wings were mechaniccally removed and finally immersed in 0.5% (w/v) calcium hypochlorite solution for 30 min. Disinfested seedswere inoculated on woody plant basal (WP) culture medium [17], supplemented with 2% (w/v) sucrose, solidified with 0.2% phytagelâ and adjusted to pH 6.0. Cultures of axenic seedlings became the source of nodal explants. The nodal segments were transferred to WP medium supplemented with various cytokinins [kinetin (KIN), 6-benzylaminopurine (BAP), 2-isopenthenyladenine (2ip)] at various concentrations ranging from 0.1, 1.0, 3.0 and 5.0 mg·L−1. A second experiment was conducted using only thidiazuron (TDZ) at 0.1, 1.0, 3.0 and 5.0 mg·L−1 or in combination with 0.1 mg·L−1 of indole- 3-acetic acid (IAA) as WP medium supplement. After a 40-day period of incubation at 25˚C ± 2˚C and 55% - 60% relative humidity under a 16-h photoperiod with 85-W cool-white GE fluorescent lamps (light intensity 40 mmol m–2·s–1), cultures were evaluated for survival, multiple shoots, height, and presence of callus at the shoot base. The experimental design was fully randomized with three replicates of 10 explants for each treatment (n = 30). The experiment was conducted in a factorial design of 4 × 4 (auxin and cytokinin), means were analyzed by ANOVA followed the mean separation using Tukey test at 5% level of significance.
For rooting, the shoots were transferred to medium containing WP and WP/2 salts and 1% (w/v) sucrose, solidified with 0.4% phytagel®, adjusted to pH 6.0 supplemented with a-naphthaleneacetic acid (NAA) and indole-3-butyric acid (IBA) at two concentrations 0.1 and 1.0 mg·L−1. After a 60-day incubation period, cultures were evaluated for survival, presence of root and root length. Plantlets were acclimatized to the soil. Rooted and un-rooted plantlets 5 cm tall were directly planted into trays containing different substrates, which consisted of coarse sand, sand and soil at 1:1 v/v, and the substrate Plantimax®. After a 90-day period, survival counting was conducted following by growth measurements such as plant height.
2.3. In Vitro Germplasm Storage
Induction of slow growth to increase subculture intervals from 4 weeks to a 6, 8 or 12 months period is a highly effective way to store healthy in vitro plantlets. To induce into slow growth, cultures were transferred to WP medium, supplemented with 2% sucrose, 4% mannitol or 4% sorbitol in the presence of 2 mg·L−1 calcium pantothenate and 2 mg·L−1spermidine in different combinations and stored at 18˚C under a 12-h photoperiod under cool-white GE fluorescent lamps with low light intensity (23 mmol·m−2·s−1). After six month in storage, cultures were then transferred to the media without the osmotic agent (sorbitol and mannitol) and analyzed for re-growth, the efficacy of the procedure for in vitro storage. The experimental design was fully randomized, repeated three times, and each treatment had 10 explants (N = 30). Data were analyzed statistically by ANOVA followed by the Tukey test, with the level of significance set at 5%.
2.4. Phytochemical Analysis
Dried and ground root samples (200 mg), harvested from in vitro cultivated plants and 2-yr old plants collected in Sao João da Aliança, a county of Goias state, were extracted with methanol (5 mL) using ultrasonic Cleaner (USC 700, Unique, frequency of 55 kHz) for 30 minutes. A volume of 4 ml was removed, filtered and placed in a speed vac to dry. The procedure was repeated three times for each sample, and methanol HPLC grade (1 mL) was added to resuspend and filter the extract. HPLC analyses were done in a liquid chromatographer Shimadzu, model SPD-M10Avp with a diode array detector and SupelcosilTM column LC18: (4.6 × 250 mm, Supelco). The mobile phase was solvent A, MeOH; solvent B, 0.1% acetic acid in H2O; flux of 1.0 mL/min. To quantify lapachol and triterpenes, the flow rate and detection limits were different. For lapachol 0 - 20 min of 40% - 85% A, 20 - 33 min of 85% A, 33 - 35 min of 85 - 40 A and 35 - 40 min 40% A detection was maintained at 280 nm, as for triterpenes, 85% de A for 25 min detected at 210 nm.
3. Results and Discussion
As the use of botanicals for primary care is rising, cultivation studies to determine good agronomic practices are an essential for production of quality products. Z. montana and others medicinal plants native to the tropical savannas have great potential to become a specialty crop for small farmers in Brazil. For establishing this in vitro propagation procedure, 2000 seeds were disinfected and inoculated on WP media.
All attempts to introduce explants from adult donor plants have failed due to microbial contamination. Nodal segments of seedlings became the source of explants for this protocol. Buds were highly sensitive to the type of cytokinin and concentration, with the highest survival and induction of multiple shoots on media containing 2ip (100%) at 5.0 mg·L−1 (Table 1). Supplementing 2ip to media, the survival rate increased from 70% to 100% survival, with a significant increase on induction of multiple shoots. With the exception of kinetin, supplementing with BA, or 2ip or TDZ at 3 mg·L−1, cultures survived better than the control (WP).
Adding TDZ at 0.1 mg·L−1 into the media, shoots grew taller than all the other treatments including the control and 2ip. With cultures growing on media containing TDZ at higher concentrations than 0.1 mg·L−1, shoots showed signs of vitrified growth. Thus, healthier and taller shoots were induced on WP media supplemented with 0.1 mg·L−1 TDZ (Table 1), producing 4.0 new buds per shoot after a 40-day culture period (Table 2) at 2.1 mg·L−1 (Figure 1). On average, each inoculated bud produced four new buds to be re-inoculated per culture cycle.
During the protocol development, many cultures were discharged due to vitrified growth symptoms and the presence of endophytic organisms. These microbes slowly appeared epiphytically on the media from time to time. These problems, however, were easily treated. For reducing vitrified growth, Dillen and Buysens [18] suggested the use of vented lids to facilitate evaporation
Table 1. The effect of type of cytokinin and concentration on the survival of the cultures.
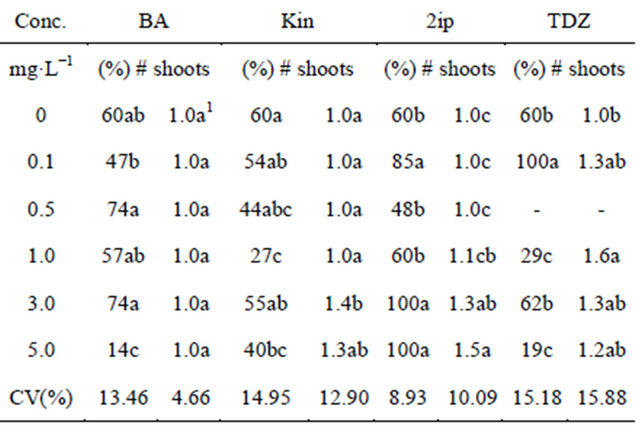
1Means followed by the same letter within the column do not differ significantly according to Tukey test (0.5%). (BA = 6-Benzylaminopurine; Kin = Kinetin; 2ip = 2-isopenthenyladenine; TDZ = Thidiazuron).
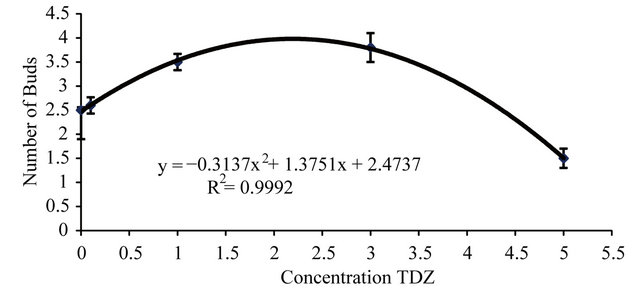
Figure 1. Effect of thidiazuron rates (mg·L−1) on number of induced buds of Z. montana.
which reduced vitrified growth. Supplementing an antibiotic to the media will also prevent endophytes growth. Only recently being investigated are the presence of endophytes and their potential role on the synthesis of secondary compounds. Strobel et al. [19] described paclitaxel being synthesized by Taxus sp and unrelated fungal endophytes including Taxomyces andreanae, also including Pestalotia, Pestalotiopsis, Fusarium, Alternaria, Pithomyces, and Monochaetia. More recently, Pugh et al. [20] reported that bacterial lipoproteins and lipopolysaccharides present in immune enhancing botanicals are the actives responsible for in vitro macrophage activation. Thus, researchers working in medicinal plants and plant tissue culture will have to search for ways to co-culture the host and beneficial endophytes.
Explants of Z. montama treated with IBA at 1 mg·L−1 produced more roots, plantlets were taller with a better survival rate than the other treatments (Table 2), plantlets cultured on IBA had a better survival rate during acclimatization to soil condition with an overall average of 70% survival after a 40-day period.
Few accessions of Z. montana were highly sensitive to in vitro culture conditions while others were able to adapt, grow and later acclimatize into soil. Of the in vitro adapted accessions, more than 800 cultures were actively growing a year later. To maintain live, healthy cultures and reduce the number of subcultures establishing the in vitro repository, slow growth was induced.
By adding sorbitol and mannitol as osmotic agents, growth of shoot cultures was reduced, and no subcultures were needed within six months (Table 3). Sorbitol containing media showed significantly better results than mannitol, and adding calcium pantothenate or spermidine as adjuvants did not improve the survival in storage. A 72% survival rate was achieved in spermidine in sorbitiol containing media; such survival was slightly better than with without spermidine (Table 3). For the savanna species, osmotic enhancement has been a successful procedure to maintain cultures under slow growth [21]. Species of temperate climate such as Podophyllum peltatum
Table 2. Effects of auxin type and concentration on rooting Z. montana shoots.
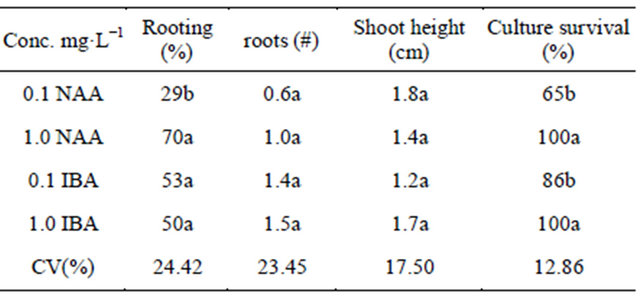
Means followed by the same letter within column do not differ significantly according to Tukey test (p < 0.05). Data collected after 40-day culture period (NAA= a-naphthaleneacetic acid; IBA = indole-3-butyric acid).
low temperature and the use of synthetic seed technology (5˚C) works as a better conservation approach for midterm in vitro storage [22].
Phytochemical analysis of in vitro Z. montana-produced plantlets revealed that leaves are sources of triterpenes while lapachol was produced in the roots. Plant aging yielded higher yields of both naphtoquinones and triterpenes (Table 4). According to Jácome et al. [10] in 100 mg of dried roots, Z. montana produced 11.0 mg of lapachol, 6.1 mg of α-lapachone and 4.3 mg of dehydro- α-lapachone. The lapachol extracted from two-year-old plants of two populations varied between 0.001 to 0.3 mg·g−1 of dry weight (data not shown) indicating that the content may vary among plants and between population. Bertoni et al. [23] reported a genetic variability of 15.97% among Z. montanapopulations; such variability could control the lapachol yield among two-year-old plants. More phytochemical analysis of lapachol in the roots and triterpenes in the leaves are needed to identify high yielding genotypes. In conclusion, our work shows that conservation of Z. montana is possible by in vitro technology, although few accessions were sensitive to in vitro conditions. After inoculation and induction of buds under in vitro growing conditions, maintenance of active growing clones, as a source of propagules for commercial field plantings is quite an effective procedure.
Table 3. Effects of osmotic agents on culture survival of Z. montana.
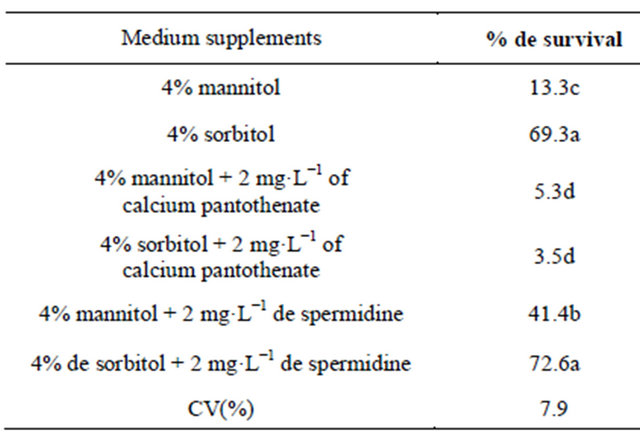
Means followed by the same letter within column do not differ significantly according to Tukey test (p < 0.05).
Table 4. The content of lapachol and triterpenes found in 2- yr old Z. montana plants and in vitro micropropagated plants.
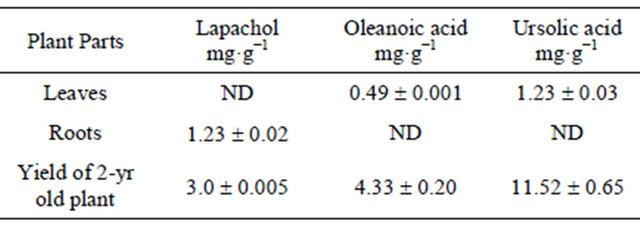
ND: Not detected.
4. Acknowledgements
Research funded by FAPESP, The State of Sao Paulo Research Foundation, Brazil. Project Number (Projetotemático—Biota 99/10610-1).
REFERENCES
- B. Y. Hwang, H. B. Chai, L. B. Kardono, S. Riswan, N. R. Farnsworth, G. A. Cordell, J. M. Pezzuto and A. D. Kinghorn, “Cytotoxic Triterpenes from the Twigs of Celtis Philippinensis,” Phytochemistry, Vol. 62, No. 2, 2003, pp. 197-201. doi:10.1016/S0031-9422(02)00520-4
- J. Tao, T. Morikawa, I. Toguchida, S. Ando, H. Matsuda and M. Yoshikawa, “Inhibitors of Nitric Oxide Production from the Bark of Myrica rubra: Structures of New Biphenyl Type Diarylheptanoid Glycosides and Taraxerane Type Triterpene,” Bioorganic & Medicinal Chemistry, Vol. 10, No. 12, 2002, pp. 4005-4012. doi:10.1016/S0968-0896(02)00314-0
- G. B. Singh, S. Singh, S. Bani, B. D. Gupta and S. K. Banerjee, “Anti-Inflammatory Activity of Oleanolic Acid in Rats and Mice,” Journal of Pharmacy and Pharmacology, Vol. 44, No. 5, 1992, pp. 456-458. doi:10.1111/j.2042-7158.1992.tb03646.x
- H. Q. Tang, J. Hu, L. Yang and R. X. Tan, “Terpenoids and Flavonoids from Artemisia Species,” Planta Medica, Vol. 66, No. 4, 2000, pp. 391-393. doi:10.1055/s-2000-8538
- M. E. Alvarez, A. O. Maria and J. R. Saad, “Diuretic Activity of Fabiana patagonica in Rats,” Phytotherapy Research, Vol. 16, No. 1, 2002, pp. 71-73. doi:10.1002/ptr.754
- L. C. Guenka, R. C. Gomes, V. L. Melo, C. R. Kitanishi, P. S. Pereira, S. C. Franca, L. B. Couto and R. O. Beleboni, “Anti-Inflammatory and Anti-Nociceptive Effects of Zeyheria montana (Bignoniaceae) Ethanol Extract,” Memórias do Instituto Oswaldo Cruz, Vol. 103, No. 8, 2008, pp. 768-772. doi:10.1590/S0074-02762008000800004
- P. M. Nossa, L. C. Guenka, L. B. Couto and D. E. daCruz-Perez, “Effects of the Serjania erecta and Zeyheria montana Ethanol Extracts in Experimental Pulpitis in Rats: A Histological Study,” Medicina Oral Patologia Oral y Cirugia Bucal, Vol. 18, No. 2, 2012, pp. 337-342.
- L. N. Seito, A. L. Ruiz, D. Vendramini-Costa, S. V. Tinti, J. E. de Carvalho, J. K. Bastos and L. C. Di Stasi, “Antiproliferative Activity of Three Methoxylated Flavonoids Isolated from Zeyheria montana Mart. (Bignoniaceae) Leaves,” Phytotherapy Research, Vol. 25, No. 10, 2011, pp. 1447-1450. doi:10.1002/ptr.3438
- A. B. Oliveira, D. S. Raskan, M. C. M. Miraglia and A. A. L. Mesquita, “Estrutura Química e atividade biológica de Naftoquinonas de Bignociacea Brasileiras,” Química nova, Vol. 3, No. 4, 1990, pp. 302-307.
- R. L. R. P. Jácome, A. B. Oliveira, D. S. Raslan, A. Muller and H. Wagner, “Analysis of Napthoquinones in Zeyheria montana Crude Extracts,” Química Nova, Vol. 22, No. 2, 1999, pp. 175-177. doi:10.1590/S0100-40421999000200004
- G. M. Cragg and D. J. Newman, “Plants as a Source of Anti-Cancer Agents,” Journal of Ethnopharmacology, Vol. 100, No. 1-2, 2005, pp. 72-79. doi:10.1016/j.jep.2005.05.011
- M. S. Bentle, E. A. Bey, Y. Dong, K. E. Reinicke and D. A. Boothman, “New Tricks for Old Drugs: The Anticarcinogenic Potential of DNA Repair Inhibitors,” Journal of Molecular Histology, Vol. 37, No. 5-7, 2006, pp. 203- 218. doi:10.1007/s10735-006-9043-8
- G. M. Felippe, “Desenvolvimento de plantas do cerrado: uma experiência pessoal,” In: Eugen Warming e o cerrado brasileiro, um século depois, Editora da UNESP, São Paulo, 2000.
- C. M. C. Mello and M. T. S. Eira, “Conservação de sementes de jacarandá mimoso (Jacaranda acutifolia Humb & Bonpl.) Bignoceacae,” Revista Brasileira de Sementes, Vol. 17, No. 2, 1995, pp. 193-196.
- N. S. Salomão, “Tropical Seed Species’ Responses to Liquid Nitrogen Exposure,” Brazilian Journal of Plant Physiology, Vol. 14, No. 2, 2002, pp. 133-138. doi:10.1590/S1677-04202002000200008
- G. M. Felippe and J. C. S. Silva, “Estudos de germinação em espécies do cerrado,” Revista Brasileira de Botânica, Vol. 7, No. 2, 1984, pp. 157-163.
- G. Lloyd and B. McCown, “Commercially-Feasible Micropropagation of Mountain Laurel, Kalmia latifolia, by Use of Shoot-Tip Culture,” Combined Proceedings, International Plant Propagators’ Society, Vol. 30, 1981, pp. 421-427.
- W. Dillen and S. Buysens, “A Simple Technique to Overcome Vitrification in Gypsophila paniculata L.,” Plant Cell, Tissue and Organ Culture, Vol. 19, No. 3, 1989, pp. 181-188. doi:10.1007/BF00043345
- G. A. Strobel, W. M. Hess, E. Ford, R. S. Sidhu and X. Yang, “Taxol from Fungal Endophytes and the Issue of Biodiversity,” Journal of Industrial Microbiology and Biotchnology, Vol. 17, No. 5-6, 1996, pp. 417-428.
- N. Pugh, H. Tamta, P. Balachandran, X. Wu, J. L. Howell, F. E. Dayan and D. S. Pasco, “The Majority of in Vitro Macrophage Activation Exhibited by Extracts of Some Immune Enhancing Botanicals Is Due to Bacterial Lipoproteins and Lipopolysaccharides,” International Immunopharmacology, Vol. 8, No. 7, 2008, pp. 1023-1032. doi:10.1016/j.intimp.2008.03.007
- A. M. S. Pereira, A. N. Salomão, A. H. Januário, B. W. Bertoni, S. F. Amui, S. C. França, A. L. Cerdeira and R. M. Moraes, “Seed Germination and Triterpenoid Content of Anemopaegma arvense (Vell.) Stellfeld Varieties,” Genetic Resources and Crop Evolution, Vol. 54, No. 4, 2007, pp. 849-854. doi:10.1007/s10722-006-9161-x
- H. Lata, R. M. Moraes, B. W. Bertoni and A. M. S. Pereira, “In Vitro Germoplasm Conservation of Podophyllum peltatum L. under Slow Growth Conditions,” In Vitro Cellular & Developmental Biology—Plant, Vol. 46, No. 1, 2010, pp. 22-27.
- B. W. Bertoni, F. S. Astolfi, E. R. Martins, C. F. Damião Filho, S. C. França, A. M. S. Pereira, M. P. C. Telles and J. A. F. Diniz Filho, “Genetic Variability in Natural Population of Zeyheria montana Mart. From the Brazilian Cerrado,” Scientia Agrícola, Vol. 64, No. 4, 2007, pp. 409- 415. doi:10.1590/S0103-90162007000400012
NOTES
*Corresponding author.

