American Journal of Plant Sciences
Vol.3 No.7A(2012), Article ID:21064,4 pages DOI:10.4236/ajps.2012.327123
Berberine Prolongs Life Span and Stimulates Locomotor Activity of Drosophila melanogaster
![]()
1V. N. Karazin Kharkiv National University, Kharkiv, Ukraine; 2Tufts University, Tufts Medical Center, Boston, USA.
Email: goxenkrug@tuftsmedicalcenter.org
Received June 11th, 2012; revised July 7th, 2012; accepted July 15th, 2012
Keywords: Berberine; Life Span; Drosophila melanogaster; Kynurenine; Vertical Climbing; Viability
ABSTRACT
Drosophila melanogaster mutants with deficient kynurenine (KYN) formation from tryptophan (TRP) have longer life span than wild type flies. Administration of alpha-methyl-TRP and 5-methyl-TRY, the inhibitors of TRP-KYN metabolism, prolonged life span in wild-type flies. Both inhibitors are not available for human use. Berberine, an isoquinoline alkaloid isolated from berberis aristata, is known as the herb widely used in traditional Chinese and Indian medicine. Berberin is a strong inhibitor of the enzyme catalyzing TRP conversion into KYN. Considering this particular feature we investigated the effect of berberine on lifeand health-span in wild-type Drosophila melanogaster. The results of our study showed that Berberine extended mean, median and maximum life span of female flies. Berberine did not affect the number of pupae of filial generation and decreased their lethality. Berberine increased locomotor activity (vertical climbing). The results of the study suggest that berberine prolongs lifeand improves health-span of Drosophila melanogaster. Berberine might be a candidate drug for prevention and treatment of aging and aging-associated medical and psychiatric disorders.
1. Introduction
Activation of kynurenine (KYN) pathway of tryptophan (TRP) metabolism is associated with aging and agingassociated medical and psychiatric disorders (AAMPD) [1,2]. Drosophila melanogaster is a valuable model for preclinical testing of drugs with therapeutic potential for AAMPD because of small genome size, short generation time, and shorter mean life span compared to either mice or humans. Drosophila also exhibits complex behaviors, many of which undergo age-related decline [3]. Only those drugs that show promise in Drosophila might be moved on to mammals, resulting in faster and more economical animal testing. Drosophila mutants with genetically deficient formation of KYN from TRP (vermilion and white) have longer life span than wild-type flies [4,5]. Inhibitors of KYN formation from TRP, alpha-methylTRP and 5-methyl-TRP, prolonged life span of wild-type Drosophila melanogaster [6]. Both inhibitors are not available for human use. Berberine, isoquinoline alkaloid isolated from Berberis aristata, a major herb widely used in Indian and Chinese systems of medicine, is a strong inhibitor of the rate-limiting enzyme of TRP-KYN metabolism [7]. We suggested that berberine (as an inhibitor of KYN formation from TRP) might prolong the life span. Prolongation of life span is not necessarily associated with the improvement of health span [8]. Therefore, in addition to the candidate drug effect on life span, it is important to assess its effect on health span as well. One of the biomarkers of health span is locomotor activity. Flies exhibit several forms of locomotor behavior, and the robustness of each behavior declines with age [9]. The vertical climbing (or negative geotaxis) assay measures the ability of the organism to climb the walls of a vial when startled), and is an assessment of the animal’s ability to complete a strenuous activity (climbing against gravity, which provides insight into the fly’s level of fitness) [10]. We were interested to study the effect of berberine on life span, vertical climbing and viability in wild-type Drosophila melanogaster.
2. Methods
Wild-type stock Oregon of Drosophila melanogaster from the collection of V.N. Karazin Kharkiv National University was used in the experiments. The study was carried out between April and June.
Flies were maintained at 23˚C in a 12:12 light: dark period on a standard Drosophila medium (SM) consisting of sugar, yeast, agar and semolina. Berberine (Sigma Aldrich Chemical Co, USA) was added to nutrition medium in two doses: 1 mM (0.4 mg/ml of nutrition medium) and 5 mM (2 mg/ml of medium). Berberine doses were selected based on studies using berberine to challenge Drosophila taste neurones involved in the perception of bitter substances [11].
Viability was estimated by the quality and quantity of pupae. Three pairs of parent individuals (three males and three females) were transferred into a vial containing 4 ml of SM or medium with addition of berberine. Ten vials were set up per treatment (control, berberine low and berberine high doses). Number of filial generation pupae and their lethality were calculated per vial.
Life span one day old adult flies were collected and then regularly transferred to fresh medium every 3 - 4 days. The number of dead flies was recorded at the time of transfer [6].
Locomotor activity to assess the effect of berberine on vertical climbing 20 virgin female flies were placed at the bottom of a clean 4-inch glass vial over which a second identical vial was inverted. After 30 s the two vials were separated. The climbing index was expressed as percentage of the number of flies that climbed to the top vial relative to the total number of flies tested [10]. Four climbing trials (akin to sampling with replacement) were conducted per vial. There were 35 climbing trials for control group (total number of tested flies = 700); and 25 trials for berberine group (total number of tested flies = 500).
Statistics the data were analyzed using Wilcoxon ranksum test and two ways ANOVA test.
3. Results
Viability the number of pupae of filial generation of Drosophila melanogaster exposed to the low dose (1 mM) of berberine was not different (104%) from those in the control group (P = 0.75).
The number of pupae of filial generation of Drosophila melanogaster exposed to the high dose (5 mM) of berberine was dramatically lower (22%) than those in control and low dose berberine group (Figure 1).
Lethality of pupae in group of low concentration (1 mM) of berberine (8%) was slightly lower than in control group (13%) (Figure 2).
High concentration (5 mM) of berberine increased the number of dead pupae by 31% in comparison with control and low dose of berberine groups (Figure 2).
Locomotor activity and life span Considering the toxic effects of high (5 mM) dose of berberine on the number and lethality of pupae, we used only low dose (1 mM) to study the effect of berberine on locomotor activity and life span in female flies.
Locomotor activity berberine stimulated vertical climbing of flies by 39% (Figure 3).
Life span Berberine (1 mM) increased mean (by 27%),
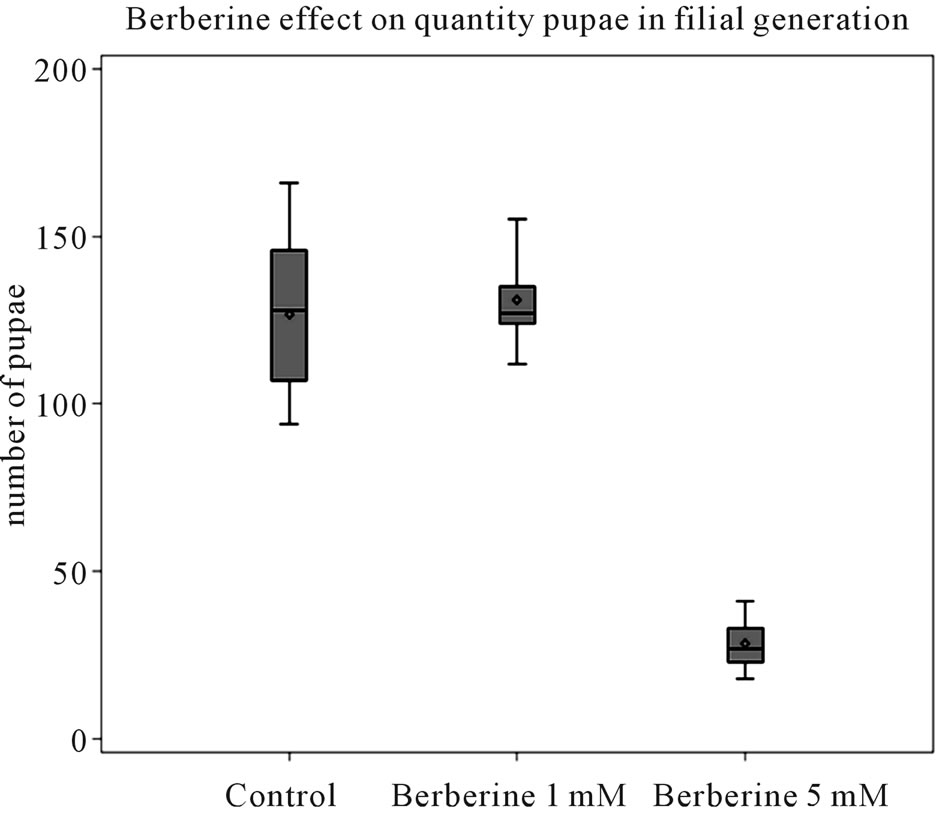
Figure 1. Berberine effect on quantity of pupae in filial generation. Quantity of pupae per vial (three pairs of parents). Ten vials per experimental group. Mean ±SEM; P < 0.01: berberine 5 mM vs control and berberine 1 mM (Wilcoxon rank-sum test).
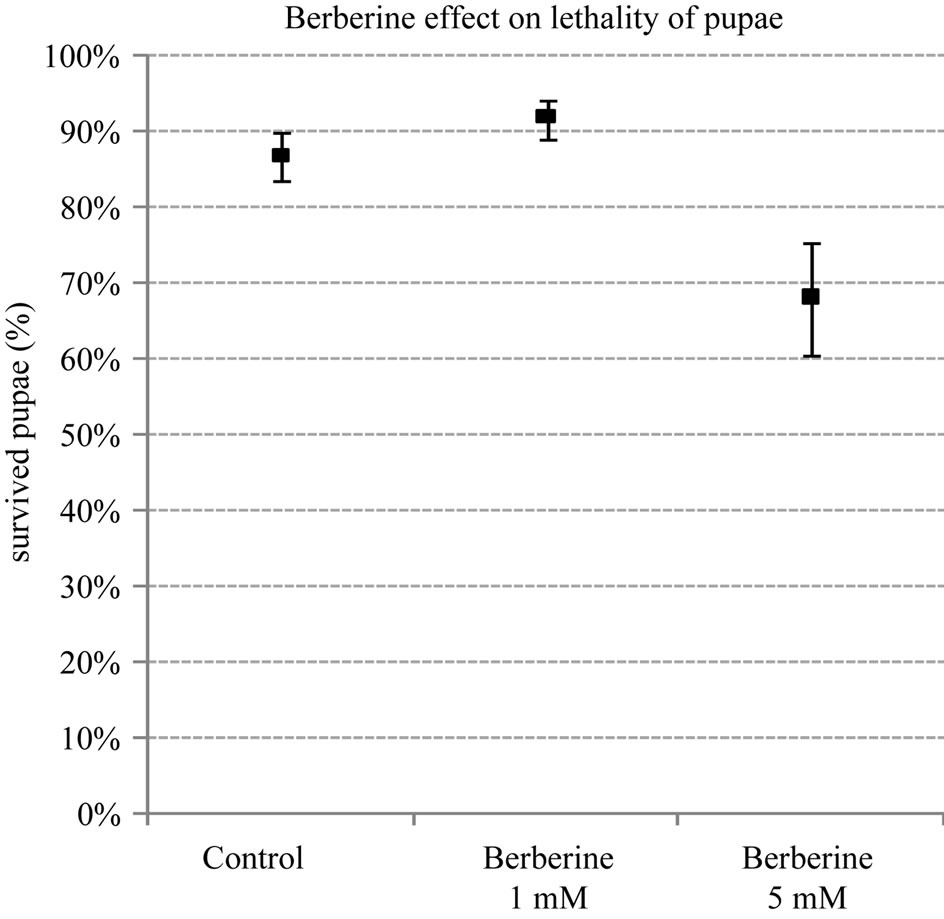
Figure 2. Berberine effect on the lethality of pupae. P < 0.02: berberine 1 mM vs control P < 0.0001: berberine 5 mM vs control and berberine 1 mM (Wilcoxon rank-sum test).
median (by 57%) and maximum (by 78%) life span of flies (Figure 4).
4. Discussion
The results of our study indicate that berberine prolongs lifeand improves health-span of wild-type Drosophila melanogaster. To the best of our knowledge this is the first study of berberine effect on lifeand health-span in Drosophila. Although berberine possesses a wide range
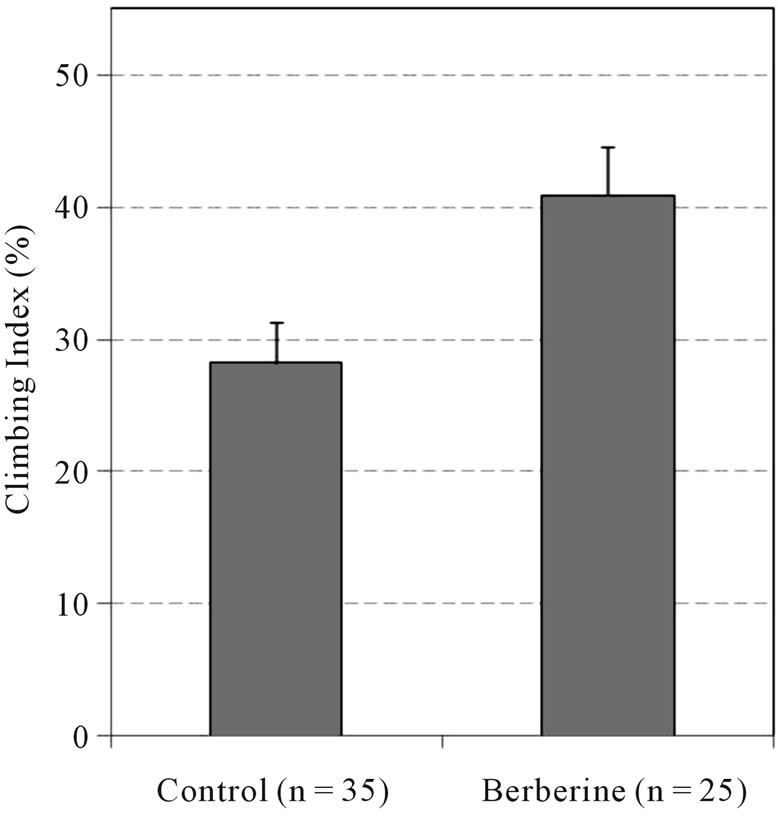
Figure 3. Berberine effect on vertical climbing. Climbing index % of flies moved to the top vial; mean ±SEM; P < 0.008: berberine vs control group, two ways ANOVA, Bonferroni adjusted.
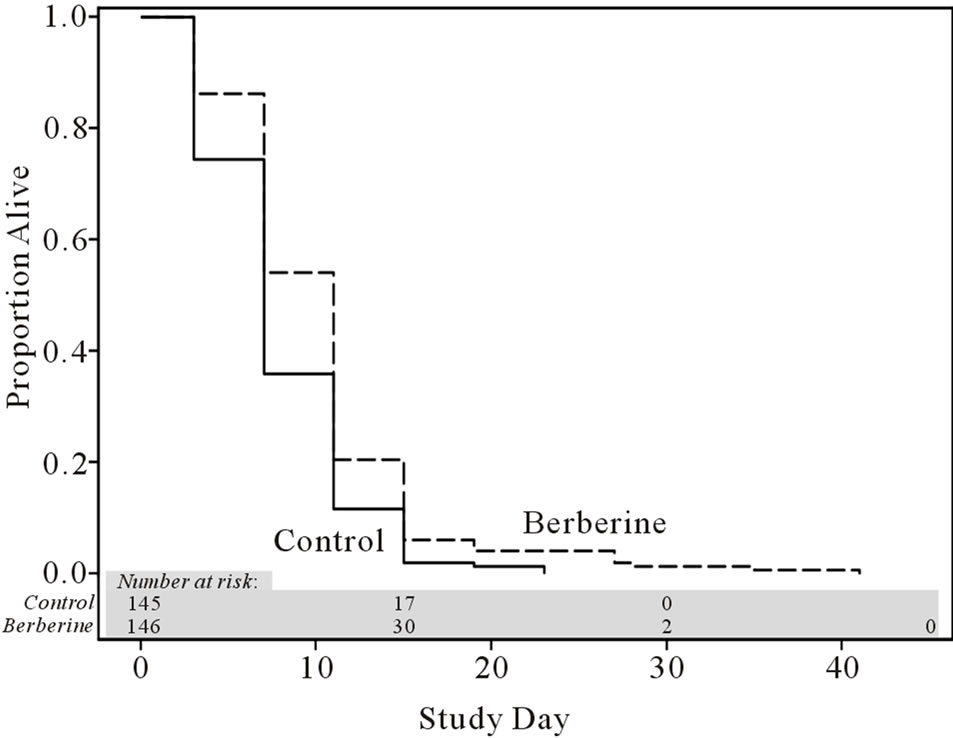
Figure 4. Berberine effect on life span in Drosophilala melanogaster. P < 0.001: berberine vs control group, Wilcoxon test adjusted for multiple comparison.
of therapeutic effects related to AAMPD (antidepressant, [12] anti-inflammatory, [13] anti-diabetic, [14] anti-adipogenic [15] and anti-tumorigenic [16]) its effect on life span has never been studied. The only mention of berberine effect on life span was the berberine-induced elevation the life span of leukemia harboring BALB/c mice [17].
Inhibition of TRP-KYN metabolism might be one of the mechanisms of observed prolongation of life span by berberine. Genetic deficiency and pharmacologic inhibition of KYN formation from TRP in Drosophila prolonged life span [4-6] and exerted neuroprotective effect in flies [18]. There are two rate-limiting enzymes of KYN formation from TRP: indoleamine 2,3-dioxygenase (IDO) and TRP-2,3-dioxygenase (TDO) [19]. Ability of berberine to inhibit human preparation of IDO is stronger than that of a most powerful IDO inhibitor, 1-methylTRP. [7] (1-methyl-TRP is not approved for human use, and had high toxicity in Drosophila (our unpublished data)). Recent studies indicate that genetic inhibition of TDO was protective against the eclosion defect in Drosophila model of Huntington’s disease (flies with impaired TRP-KYN metabolism) [20]. The increased dioxygenation of mitochondrial TRP to N-formylKYN was consistently present among conserved biomarkers across ageing models in five species [21].
However, some other mechanisms (e.g., AMP-activated protein kinase involved in life-span extension in response to caloric restriction [22]) might contribute to berberine’s effect on lifeand health-span.
Further studies (e.g., the effect of berberine on lifeand health-span in Drosophila mutants naturally knockout for rate-limiting enzyme of TRP conversion in KYN) might elucidate the role of KP metabolism in the effects of berberine on lifeand health-span.
Present results of the positive effects of berberine on studied biomarkers of health span in Drosophila suggest that berberine is a promising candidate drug for antiaging intervention and treatment of AAMPD.
5. Acknowledgements
Authors greatly appreciate invaluable statistical expertise of Robin Ruthazer. GF Oxenkrug is a recipient of NIH MH083225.
REFERENCES
- G. Oxenkrug, “Interferon-Gamma-Inducible Kynurenines/ Pteridines Inflammation Cascade: Implications for aging and Aging-Associated Medical and Psychiatric Disorders,” Journal of Neural Transmission, Vol. 118, No. 1, 2011, pp. 75-85. doi:10.1007/s00702-010-0475-7
- G. Oxenkrug, “Interferon-gamma—Inducible Inflammation: Contribution to Aging and Aging-Associated Psychiatric Disorders,” Aging and Disease, Vol. 2, No. 6, 2011, pp. 474-486.
- K. G. Iliadi and G. L. Boulianne, “Age-Related Behavioral Changes in Drosophila,” Annals of the New York Academy of Sciences, Vol. 1197, 2010, pp. 9-18. doi:10.1111/j.1749-6632.2009.05372.x
- M. G. Kamyshev, “Longevity and Its Relation to the Locomotor Activity in Tryptophan-Xanthommatin Metabolic Pathway Mutant of Drosophila,” Doklady Akademii Nauk SSSR, Vol. 253, No. 5, 1980, pp. 1476-1480.
- G. Oxenkrug, “The Extended Life Span of Drosophila melanogaster Eye-Color (White and Vermilion) Mutants with Impaired Formation of kynurenine,” Journal of Neural Transmission, Vol. 117, No. 1, 2010, pp. 23-26. doi:10.1007/s00702-009-0341-7
- G. Oxenkrug, V. Navrotskaya, L. Vorobyova and P. Summergrad, “Extension of Life Span of Drosophila melanogaster by the inhibitors of tryptophan—Kynurenine Metabolism,” Fly (Austin), Vol. 5, No. 4, 2011, pp. 307- 309. doi:10.4161/fly.5.4.18414
- C. J. Yu, M. F. Zheng, C. X. Kuang, W. D. Huang and Q. Yang, “Oren-gedoku-to and Its Constituents with Therapeutic Potential in Alzheimer’s Disease Inhibit Indoleamine 2,3-dioxygenase activity in vitro,” Journal of Alzheimer’s Disease, Vol. 22, No. 1, 2010, pp. 257-266.
- A. Avanesian, B. Khodayari, J. S. Felgner and M. Jafari, “Lamotrigine Extends Lifespan but Compromises Health Span in Drosophila melanogaster,” Biogerontology, Vol. 11, No. 1, 2010, pp. 45-52. doi:10.1007/s10522-009-9227-1
- M. A. Jones and M. Grotewiel, “Drosophila as a model for Age-Related Impairment in locomotor and other behaviors,” Experimental Gerontology, Vol. 46, No. 6, 2011, pp. 320-325. doi:10.1016/j.exger.2010.08.012
- D. C. Crowther, K. J. Kinghorn, E. Miranda , et al., “Intraneuronal Aβ, Non-Amyloid Aggregates and neurodegeneration in a Drosophila model of Alzheimer’s disease,” Neuroscience, Vol. 132, No. 1, 2005, pp. 123-135. doi:10.1016/j.neuroscience.2004.12.025
- P. Masek and K. Scott, “Limited Taste Discrimination in Drosophila,” Proceedings of the National Academy of Sciences of the United States of America, Vol. 107, No. 33, 2010, pp. 14833-14838. doi:10.1073/pnas.1009318107
- S.K. Kulkarni and A. Dhir, “On the mechanism of antidepressant-Like Action of Berberine Chloride,” European Journal of Pharmacology, Vol. 589, No. 1-3, 2008, pp. 163-172. doi:10.1016/j.ejphar.2008.05.043
- P. Bhutada, Y. Mundhada and K. Bansod, “Protection of cholinergic and Antioxidant System Contributes to the effect of Berberine Ameliorating Memory Dysfunction in Rat Model of Streptozotocin-Induced Diabetes,” Behavioural Brain Research, Vol. 220, No. 1, 2011, pp. 30-41. doi:10.1016/j.bbr.2011.01.022
- G. S. Li, X. H. Liu, H. Zhu, et al., “ Berberine-Improved Visceral White Adipose Tissue Insulin Resistance Associated with Altered Sterol Regulatory Element-Binding Proteins, Liver x receptors, and Peroxisome ProliferatorActivated Receptors Transcriptional Programs in diabetic hamsters,” Biological and Pharmaceutical Bulletin, Vol. 34, No. 5, 2011, pp. 644-654. doi:10.1248/bpb.34.644
- T. P. Pham, J. Kwon and J. Shin, “Berberine Exerts AntiAdipogenic Activity through Up-Regulation of C/EBP inhibitors, CHOP and DEC2,” Biochemical and Biophysical Research Communications, Vol. 413, No. 2, 2011, pp. 376-382. doi:10.1016/j.bbrc.2011.08.110
- K. V. Anis, N. Y. Rajeshkumar and R. Kuttan, “Inhibition of Chemical Carcinogenesis by berberine in rats and mice,” Journal of Pharmacy and Pharmacology, Vol. 53, No. 5, 2001, pp. 763-768. doi:10.1211/0022357011775901
- K. B. Harikumar, G. Kuttan and R. Kuttan, “Inhibition of progression of Erythroleukemia Induced by Friend virus in BALB/c mice by Natural Products—Berberine, Curcumin and Picroliv,” Journal of Experimental Therapeutics & Oncology, Vol. 7, No. 4, 2008, pp. 275-284.
- S. Campesan, E. W. Green, C. Breda C, K. V. Sathyasaikumar, P. J. Muchowski, R. Schwarcz and C. P. Kyriacou, “The Kynurenine Pathway Modulates Neurodegeneration in a Drosophila Model of Huntington’s Disease,” Current Biology, Vol. 21, No. 11, pp. 961-966. doi:10.1016/j.cub.2011.04.028
- G. F. Oxenkrug, “Genetic and Hormonal Regulation of the Kynurenine Pathway of Tryptophan Metabolism: New Target for Clinical Intervention in Vascular Dementia, Depression and Aging,” Annals of the New York Academy of Sciences, Vol. 1122, 2007, pp. 35-49. doi:10.1196/annals.1403.003
- E. W. Green, S. Campesan, C. Breda, K. V. Sathyasaikumar, P. J. Muchowski, R. Schwarcz, C. P. Kyriacou and F. Giorgini, “Drosophila Eye Color Mutants as Therapeutic Tools for Huntington disease,” Fly (Austin), Vol. 6, No. 2, 2012, pp. 117-120. doi:10.4161/fly.19999
- K. Groebe, F. Krause, B. Kunstmann, et al., “Differential Proteomic Profiling of mitochondria from Podospora anserina, rat and Human Reveals Distinct Patterns of ageRelated Oxidative Changes,” Experimental Gerontology, Vol. 42, No. 9, 2007, pp. 887-898. doi:10.1016/j.exger.2007.07.001
- J. T. Hwang, D. Y. Kwon and S. H. Yoon, “AMP-activated Protein Kinase: A Potential Target for the diseases prevention by Natural Occurring Polyphenols,” Biotechnol, Vol. 26, No. 1-2, 2009, pp. 17-22.

