American Journal of Plant Sciences
Vol.3 No.8(2012), Article ID:22177,4 pages DOI:10.4236/ajps.2012.38129
Effects of Cadmium on Growth, Photosynthetic Pigments, Photosynthetic Performance, Biochemical Parameters and Structure of Chloroplasts in the Agarophyte Gracilaria domingensis (Rhodophyta, Gracilariales)
![]()
1Post-Graduate Program in Cell Biology and Development, Department of Cell Biology, Embryology and Genetics, Federal University of Santa Catarina, Florianópolis, Brazil; 2Laboratório de Bioenergética e Estresse Oxidativo, Department of Biochemistry, Federal University of Santa Catarina, Florianópolis, Brazil; 3Plant Morphogenesis and Biochemistry Laboratory, Federal University of Santa Catarina, Florianópolis, Brazil; 4Department of Botany, Federal University of Santa Catarina, Florianópolis, Brazil; 5Central Laboratory of Electron Microscopy, Federal University of Santa Catarina, Florianópolis, Brazil.
Email: *zenilda.bouzon@ufsc.br
Received May 18th, 2012; revised June 14th, 2012; accepted June 27th, 2012
Keywords: Gracilaria domingensis; Heavy Metals; Cadmium; Thylakoids; Photosynthetic Pigments; Antioxidant Systems
ABSTRACT
This paper aimed to evaluate the effects of different concentrations of cadmium on growth rates, photosynthetic pigments, photosynthetic performance, biochemical parameters and structure of chloroplasts in G. domingensis. To accomplish this, apical segments of G. domingensis were cultivated with different concentrations of cadmium, ranging from 100 to 300 μM, over a period of 16 days, and were processed for transmission electron microscopy analysis. The plants exposed to cadmium showed chloroplast alteration, especially degeneration of thylakoids and a decrease in the concentration of photosynthetic pigments, such as chlorophyll a and phycobiliproteins. However, the volume of plastoglobuli increased. As a defense mechanism, the plants treated with cadmium showed an increase in glutathione reductase activity. These results agree with the decreased photosynthetic performance and relative electron transport rate observed after exposure of algae to cadmium. Taken together, these findings strongly indicate that cadmium negatively affects the ultrastructure and metabolism of the agarophyte G. domingensis, thus posing a threat to the economic vitality of this red macroalga.
1. Introduction
Over the last few years, increasing human population and industrial development have led to an increase of contaminants in aquatic systems [1]. Accordingly, studies reporting the effects of heavy metals on aquatic organisms are currently attracting more attention, particularly those focused on industrial and urban pollution. The contamination of coastal waters with trace metals through sewage and other anthropogenic sources has become a severe problem [2]. Heavy metals, such as lead, copper, cadmium, zinc, and nickel, are among the most common pollutants found in both industrial and urban effluents [3]. In low concentrations, some heavy metals (Cu, Zn, Ni, and Mn) are essential trace elements for photosynthetic organisms; however, in high concentrations, these metals cause severe toxic effects [4]. Heavy metals affect all biological organisms, especially those in the aquatic ecosystem, in many important ways. Several studies have shown effects such as the decrease of macroalgae growth rates [2], increased activities of glutathione reductase [5], changes in photosynthetic pigments [1,6] and photosynthetic efficiency [2,6], as well as an increase in total proteins and lipid contents [1]. Finally, some reports have shown changes in the ultrastructure of the red algae Audouinella savina (F. S. Collins) Woelkerling [7] and Ceramium ciliatum (J. Ellis) Ducluzeau [8], Hypnea musciformis (Wulfen) Lamouroux [6]; the green algae Dunaliella minuta Lerche [9], Enteromorpha flexuosa (Wulfen) J. Agardh [10], Euglena gracilis Klebs [1], and the brown algae Padina gymnospora (Kützing) Sonder [11].
Cadmium (Cd) is one of the heavy metals most frequently implicated in environmental contamination. This metal is utilized in the manufacture of various products, such as batteries, chipsets, pigments, televisions, and semiconductors [4,12]. Cd can attach to sulfated groups, as well as metalloproteins and metalloenzymes, thereby neutralizing their functions [13]. However, Cd has no nutritional value for algae [9].
The genus of Gracilaria Greville is distributed worldwide from the Equator to higher latitudes [14]. As a source of agar extraction throughout the world, it has achieved significant economic importance [15]. Moreover, species of this genus have been extensively studied because of the high utilization of their phycocolloids. In fact, species of Gracilaria are some of the most useful algae in the world, combining the production of the valuable polysaccharide agar with fast growth rate, ease of vegetative reproduction and other attributes favoring their cultivation [16].
In particular, the agarophyte macroalga Gracilaria domingensis is distributed along the Brazilian coastline from Ceara State to Santa Catarina State [17]. It occurs within the intertidal zone up to the lower shore. Commonly found in areas of high wave action, it has shown considerable tolerance to environmental changes, such as salinity, temperature and water circulation [14]. In view of the effects of heavy metals on other species of algae, the present study aimed to evaluate the biological effects of cadmium on the growth, photosynthetic pigments, photosynthetic performance, chloroplast structure and biochemical activities of the red macroalga G. domingensis, a species especially important to the Brazilian economy.
2. Materials and Methods
2.1. Algal Material
G. domingensis samples were collected from Ponta das Canas Beach (27˚23′34′′S and 48˚26′11′′W), Florianopolis-SC, Brazil, in May 2010. The algal samples were collected from the rocks and were transported at ambient temperature in dark containers to LAMAR-UFSC (Macroalgae Laboratory, Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil).
Unialgal culture was established as described by Oliveira et al. [18]. To avoid contamination by the presence of epiphytes, the collected algae were meticulously cleaned with a brush and filtered seawater. The apical portions were maintained by immersing in seawater enriched with von Stosch medium (VSES/2) [19]. These segments were cultivated under the same conditions during 14 days (experimental acclimation period) before their utilization in the cadmium experiments.
2.2. Culture Conditions
The apical thalli portions were selected (±0.5 g) from the G. domingensis samples and cultivated for 16 days in Erlenmeyers flasks containing 500 mL natural sterilized seawater enriched with von Stosch medium at half strength (VSES/2) [19] with ±34 practical salinity units. Culture room conditions were 24˚C, continuous aeration, illumination from above with fluorescent lights (Philips C-5 Super 84 16W/840, Brazil) or photosynthetically active radiation (PAR) at 80 µmol photons m–2·s–1 (Licor light meter 250, USA) and 12 h photocycle (starting at 8 h).
The untreated control plants were cultivated as described above. For the treated plants, CdCl2 was added at graded concentrations of 100, 200 and 300 μM to the culture medium, as previously suggested by Talarico et al. [20] and Xia et al. [21] for Gracilaria lemaneiformis (Bory de Saint-Vincent) Greville. Four replicates were made for each experimental group.
2.3. Growth Rates (GRs)
Growth rates for treatment groups and control were calculated using the following equation: GR [%·day–1] = [(Wt/Wi) – 1] * 100/t, where Wi = initial wet mass, Wt = wet mass after 7 days, and t = internal time in days [22].
2.4. Photosynthetic Performance
Experiments were followed by measurements of chlorophyll fluorescence using a pulse amplitude-modulated (PAM) fluorometer (Diving-PAM underwater fluorometer; Walz, Effeltrich, Germany). The measurements were obtained through the application of a series of eight exposures to gradually increasing actinic irradiance levels using the “Rapid Light Curve” (RLC) option of the Diving-PAM. The RLC technique is a useful application for the rapid investigation of the photosynthetic apparatus and provides information on the overall photosynthetic performance of seaweeds [23]. PAM optimal configurations were previously evaluated for G. domingensis under in situ conditions and, once defined, they were kept constant (Gain = 4; Measuring Intensity = 6; Saturating Pulse Length = 0.8 s). The seaweeds were dark-adapted for 30 minutes before the measurements, and after dark adaptation, PAM readings were taken immediately under ambient light.
From each sample, a relative electron transport rate (rETR) was determined for each exposure, resulting in a rETR curve for every replicate. Since electrons leading to CO2 reduction in dark reactions of photosynthesis are derived from the splitting of water in photosystem II, ETR may be estimated from the effective quantum yield. Thus, ETR = ΔF/Fm′ × PAR × 0.5 × 0.16, where PAR is the actinic irradiance in μmol photons m–2·s–1, making the assumptions that photosystem II absorbs half (0.5) of the quanta of available light [24] and that 0.16 is an ETR-factor based on the average of light which is actually absorbed by red seaweeds (Diving-PAM Underwater Fluorometer Handbook of Operation, Heins Walz GmbH 1998). To compare RLCs using parametric statistics, two descriptive parameters were used: photosynthetic efficiency (α) and maximum photosynthetic rate (Pmax). These parameters were calculated by the equation of [25] with the Microcal Origin 5.0 program, using rETR values obtained for each replicate. Pmax and α were calculated by curve fitting, using all the RLC values, while α was obtained by linear fitting using the first three points of the rETR vs. irradiance curve [26].
2.5. Pigments Analysis
The content of photosynthetic pigments (chlorophyll a and phycobiliproteins) of G. domingensis was analyzed for the treatment group and control. Immediately after collection, the samples (fresh weight) were frozen by immersion in liquid nitrogen and kept at –40˚C until ready for use. All pigments were extracted in quadruplecate as previously reported [27].
2.6. Chlorophyll a (Chl a)
Chlorophyll a was extracted from approximately 1 g of tissue in 3 ml of dimethylsulfoxide (DMSO, Merck, Darmstadt, FRG) at 40˚C, during 30 min, using a glass tissue homogenizer [28,29]. Pigments were quantified spectrophotometrically according to Wellburn [30].
2.7. Phycobiliproteins
About 1 g of algae material was ground to powder with liquid nitrogen and was extracted at 4˚C in darkness in 0.1 M phosphate buffer, pH 6.4. The homogenates were centrifuged at 2000 g for 20 min. Phycobiliprotein levels [allophycocyanin (APC), phycocyanin (PC), and phycoerythrin (PE)] were determined by UV-vis spectrophotometry, and calculations were performed using the equations of Kursar et al. [31].
2.8. Biochemical Analyses
Glutathione reductase, NADH dehydrogenase activities, and protein content were assessed in the samples.
The samples of the Control and of the cadmium treatments of G. domingensis groups were homogenized in 20 mM phosphate buffer, pH 7.4, and centrifuged at 1000 × g for 10 min at 4˚C. The supernatant was separated and used for assessing glutathione reductase activity and protein content.
2.9. Glutathione Reductase Assay (GR)
Glutathione reductase (GR) activity was determined by the method described by Carlberg and Mannervik [32]. The rate of GSSG reduction was indirectly determined through monitoring the NADPH disappearance at 340 nm. Results are expressed as µg/mg protein.
2.10. Sample Preparations for Measuring the NADH Dehydrogenase Activity
The samples of the Control and of the cadmium treatments of G. domingensis groups were homogenized in 10 volumes of 50 mM phosphate buffer, pH 7.4, containing 0.3 M sucrose, 5 mM MOPS, 1 mM EGTA and 0.1% bovine serum albumin. The homogenates were centrifuged at 1000 × g for 10 min at 4˚C; the pellet was then discarded, and the supernatants were used for measuring NADH dehydrogenase activity [33].
2.11. Determination of NADH Dehydrogenase Activity
NADH dehydrogenase activity was assessed in supernatants by the rate of NADH-dependent ferricyanide reduction at 420 nm (1 mM−1·cm−1), as previously described in Cassina and Radi [34]. The method described to determine this activity was slightly modified, as detailed in a previous report by Latini et al. [35]. Enzyme activity was calculated as nmol/minute/mg protein.
2.12. Protein Determination
The amount of protein in the samples was determined according to Lowry et al. [36].
2.13. Transmission Electron Microscope (TEM)
For observation under the transmission electron microscope (TEM), samples approximately 5 mm in length were fixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.2) plus 0.2 M sucrose overnight [37]. The material was post-fixed with 1% osmium tetroxide for four hours, dehydrated in a graded acetone series, and embedded in Spurr’s resin. Thin sections were stained with aqueous uranyl acetate, followed by lead citrate, according to Reynolds [38]. Four replicates were made for each experimental group; two samples per replication were then examined under TEM JEM 1011 (JEOL Ltd., Tokyo, Japan, at 80 kV). Similarities based on the comparison of individual treatment with replicates suggested that the ultrastructural analyses were reliable.
2.14. Data Analysis
Data were analyzed by unifactorial Analysis of Variance (ANOVA) and Tukey’s a posteriori test. Unifactorial statistical analyses were performed using the Statistica software package (Release 6.0), considering p ≤ 0.05. Analyses were performed in order to evaluate the effects on the growth rates, concentration of photosynthetic pigments, photosynthetic parameters (α and Pmax), biochemical analyses for control (PAR-only), and cadmiumtreated plants.
3. Results
3.1. Growth Rates and Morphology
After 16 days in culture, G. domingensis showed statistical differences (ANOVA, p < 0.05) in growth rates (GRs) between control plants (no cadmium) and thalli cultured with different concentrations of cadmium (Figure 1). Cadmium stress caused a significant reduction in GRs. The sample control showed increased dichotomy in apical segments at the end of the experiment (Figures 2(a) and (b)). However, for the apical segments cultivated with 100 (Figure 2(c)), 200 (Figure 2(d)) and 300 µM of cadmium (Figure 2(e)), a reduced dichotomy in the apical segments of G. domingensis was detected. During 16 days, the exposure to 200 and 300 M of cadmium caused a bleaching of the apical segments (Figures 2(d) and (e)). This process, which ultimately led to weight loss, significantly affected GRs. The control had the highest GRs at 7.6% day–1, compared to 100 µM at 2.1% day–1, 200 µM at 1.6% day–1 and 300 µM at 1.1% day–1 from the average 16-day cultivation.
3.2. Pigments
Cadmium treatment affected the content of photosynthetic pigments in G. domingensis (Table 1). Cadmium
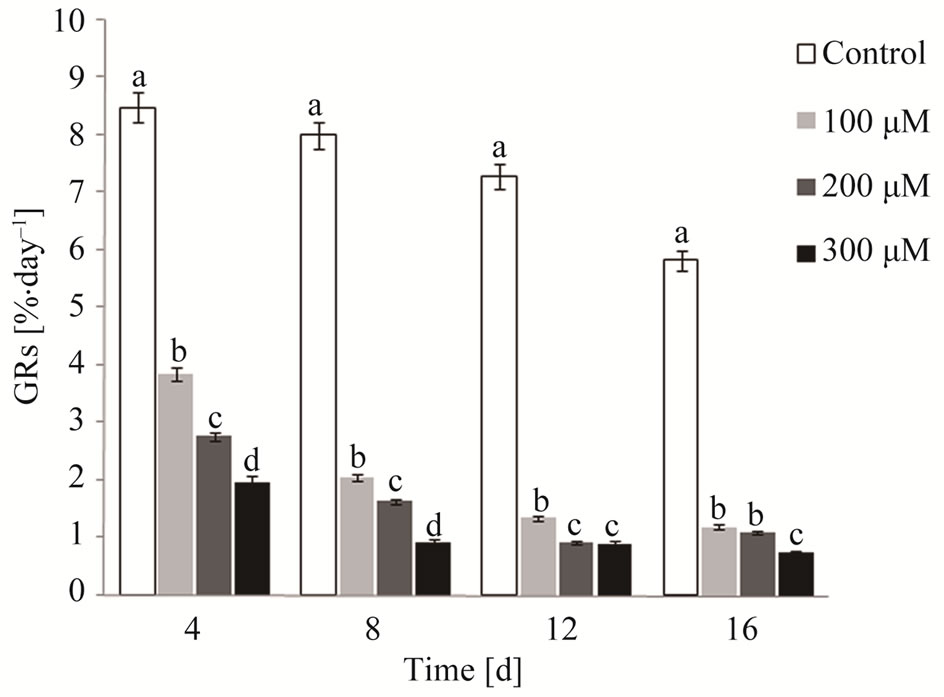
Figure 1. Growth rates (GRs) of G. domingensis under cadmium treatment and control. Vertical bars represent ±SD for means (n = 4). Letters indicate significant differences according to Tukey’s range test (p < 0.05).

Figure 2. Apical segments of G. domingensis according to the treatments. (a): Control initial; (b): Control after 16 days of culture; (c): Apical segments after 16 days of treatment with 100 μM of the cadmium; (d): Tallus after 16 days of treatment with 200 μM of the cadmium; (e): Apical segments after 16 days of treatment with 300 μM of the cadmium. Scale bars = 1 cm.
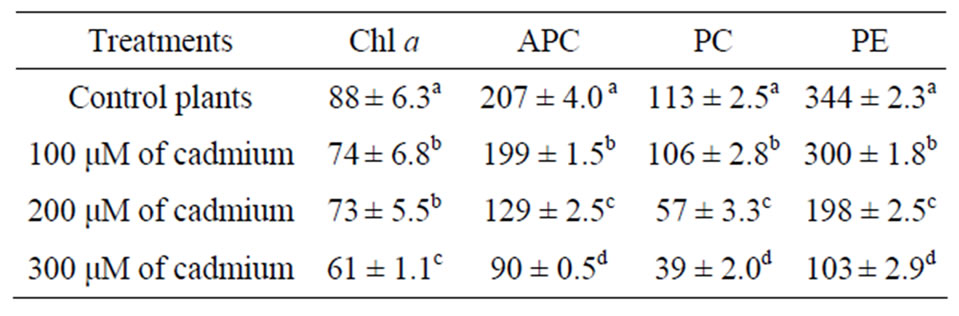
Table 1. Changes in photosynthetic pigments [µg/g – 1(FM)] of G. domingensis under cadmium treatment. The values refer to mean ± SD, n = 4. Different letters indicate signifycant differences according to Tukey’s range test (p < 0.05).
treatment decreased chlorophyll a level compared to control algae, as well as the amounts of phycobiliprotein contents (APC, PC, and PE). The values of concentration of all photosynthetic pigments were significantly different for the control and cadmium-treated algae.
3.3. Photosynthetic Performance
The rETR values decreased after culture with cadmium (Figure 3) when compared to control samples of the G. domingensis (Figure 3). However, no meaningful difference (p < 0.05) was detected among the cadmiumtreated plants compared to control. Maximum photosynthetic rate (Pmax) and photosynthetic efficiency (α) values decreased after culture with cadmium (Table 2).
3.4. Biochemical Responses
G. domingensis plants treated with cadmium showed significantly increased GR activity (Figure 4(a)) when
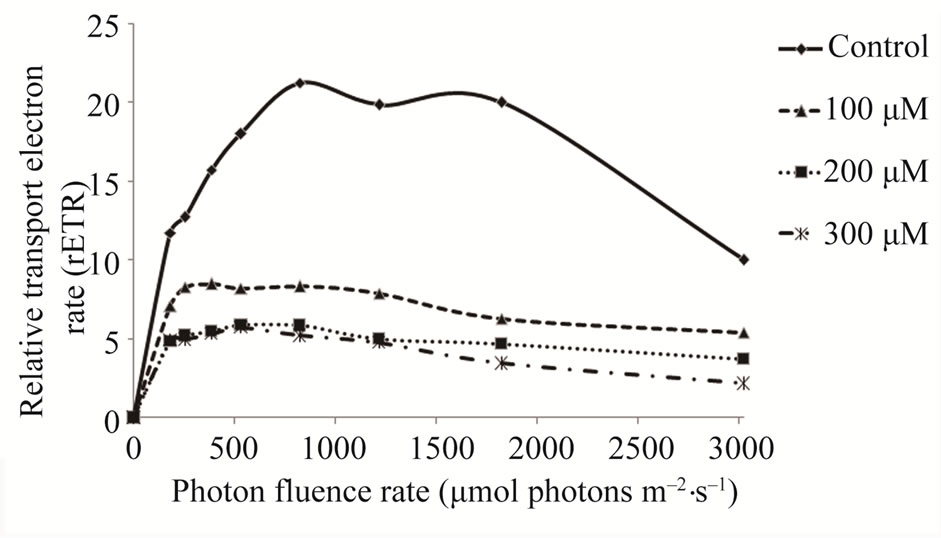
Figure 3. Relative electron transport rate of G. domingensis exposed to cadmium treatments for a period of 16 days. Data are means of quadruplicates. Means ± SD, n = 4.
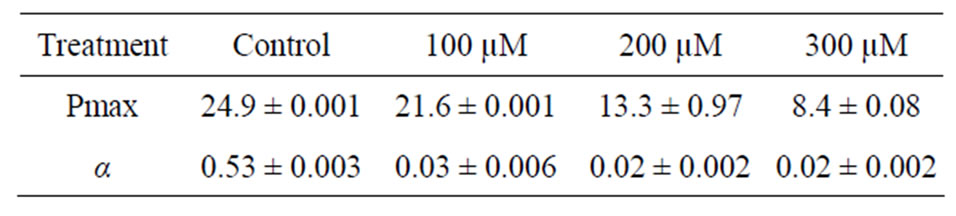
Table 2. Photosynthetic efficiency of G. domingensis cultivated with different concentrations of cadmium over a period of 16 days. Means ± SD, n = 4. Different letters indicate significant differences according to Tukey’s range test (p < 0.05).
compared with control plants (p < 0.05). On the other hand, NADH dehydrogenase activity and protein content were not altered by Cd treatment (Figures 4(b) and (c)).
3.5. Observations under TEM
When observed by transmission electron microscopy, the chloroplasts assumed the typical internal organization of the red algae with unstacked, evenly spaced thylakoids (Figure 5(A)). Electron-dense lipid droplets described as plastoglobuli were observed between the thylakoids (Figure 5(A)). After 16 days of culture with 100 μM (Figure 5(B)), 200 μM (Figure 5(C)) and 300 μM (Figure 5(D)) of cadmium, G. domingensis chloroplasts showed visible changes in ultrastructural organization with irregular morphology (Figures 5(B)-(D)). The thylakoids were disrupted (Figures 5(B)-(D)), and the number of plastoglobuli increased in the chloroplasts (Figures 5(B)-(D)).
4. Discussion
The present study showed that the architecture and metabolism of the agarophyte G. domingensis are affected by cadmium exposure. The treatment induced changes in chloroplast morphology and growth rates, in addition to decreased photosynthetic pigments and photosynthetic performance, but increased glutathione reductase activity.
G. domingensis treated with cadmium showed a decrease
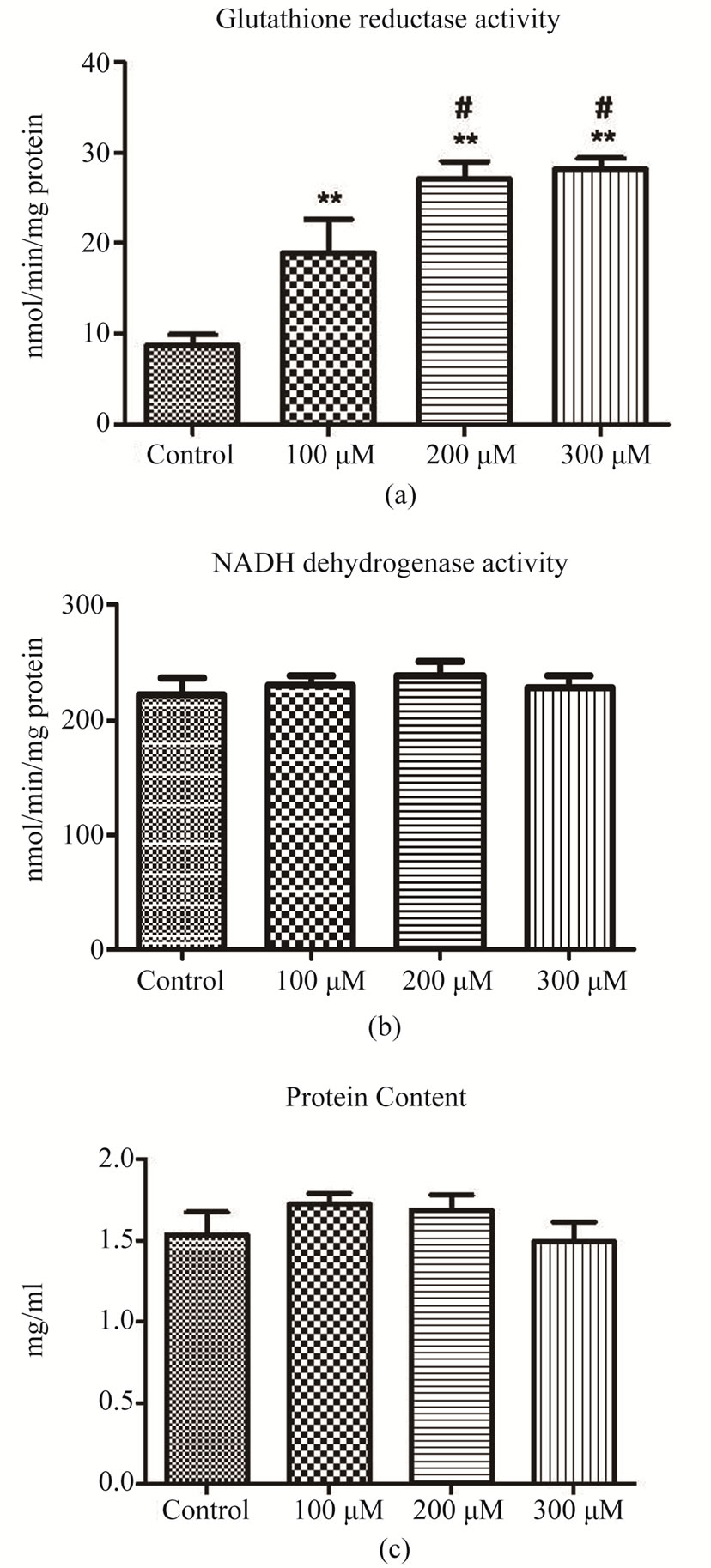
Figure 4. Biochemical responses of control and Cd-treated G. domingensis plants. (a): Glutathione reductase activity; (b): NADH dehydrogenase activity; (c): Protein content. The symbols indicate significant differences according to Tukey’s range test (p < 0.05).
in growth rates, indicating that cadmium stress is a key factor limiting growth. Similar results were observed by Bouzon et al. [6] with the carragenophyte H. musciformis after exposure to cadmium during 7 days. The apical segments of G. domingensis cultivated with 200 and 300 μM of cadmium showed bleaching and depigmentation after 16 days in culture. In G. domingensis, Schmidt et al. [17] also observed that bleaching and depigmentation occur in apical segments after submission to ultraviolet radiation-B during 21days.
According to Xia et al. [21], cadmium is a nonessen-
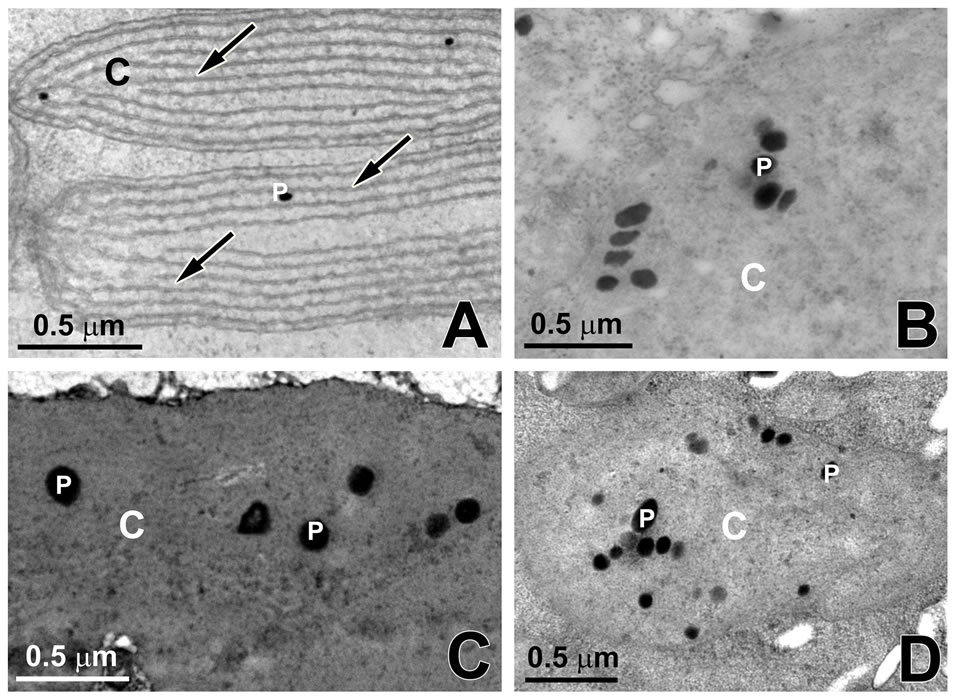
Figure 5. Transmission electron microscopy of G. domingensis chloroplasts (C) under cadmium treatment. A: Control. Observe the thylakoids (arrows). Note the presence of plastoglobuli (P); B: Treatment with 100 mM of cadmium; C: Treatment with 200 mM of cadmium; D: Treatment with of 300 mM cadmium.
tial element for macroalgae growth, development, and physiological processes. The algae may actively exclude or sequester the cadmium to minimize toxicity. The decrease in growth rates observed in G. domingensis studied in this report may be related to the use of energy for activation of adaptation mechanisms and repair of damage induced by cadmium stress. According to Collén et al. [39], heavy metal in exposed algae induces the production of reactive oxygen species (ROS). The ROS, in turn, induce changes in several molecules, including lipids, proteins, and nucleic acids. As a strategy to prevent the damaging effects of ROS, photosynthetic organisms induce antioxidant defenses, such as flavonoids, tocopherols, carotenoids, and enzymes.
The increased GR activity observed in G. domingensis after cadmium exposure could be related to the increased antioxidant defenses that result from Cd-induced oxidative stress. These results agree with those of Kumar et al. [5], who demonstrated that the green alga Ulva lactuca also increased in GR activity after exposure to cadmium.
In the present study, we observed a dramatic reduction in values of photosynthetic efficiency and photosynthetic pigments of G. domingensis after exposure to cadmium. In the green alga Dunaliella minuta exposed to cadmium, Visviki and Rachlin [9] demonstrated a reduction in chloroplast volume and photosynthetic potential. Chlorophyll a levels decreased drastically in G. domingensis after exposure to cadmium. This reduction could be associated with Mg and Fe deficiency in the biosynthetic process of chlorophyll a [21,40]. On the other hand, the reduction could be related to the inhibition of enzyme activity, i.e., photochlorophyllide reductase [21,41]. A similar result was observed by Bouzon et al. [6] with the carragenophyte H. musciformis after exposure to cadmium during 7 days.
The amounts of phycobiliproteins decreased in G. domingensis treated with cadmium. The phycobiliproteins are located in the phycobilisomes outside the chloroplast thylakoids. Our results demonstrated that phycobiliprotein levels, including APC, PC, and PE, decreased in G. domingensis after cadmium treatment. According to Xia et al. [21], a high concentration of cadmium altered phycobilisome structure, and these changes resulted in a decline of absorbed light energy, thus inhibiting photosynthesis. We found a decrease in the phycobiliprotein levels similar to the findings of Xia et al. [21] who studied the red macroalga Gracilaria lemaneiformis cultivated with cadmium during 4 days and those of Bouzon et al. [6] with H. musciformis after exposure to cadmium during 7 days. This indicates that cadmium strongly inhibited the accumulation of phycobiliproteins.
In red algae, the thylakoids that are not associated with each other are free in chloroplasts. The chloroplasts of control G. domingensis showed a structure very similar to that of normal red algae, having one peripheral thylakoid surrounded by parallel thylakoids. The number of parallel thylakoids is variable, and this number mainly depends on the spatial location of the cell in the algae [17]. In contrast, the chloroplasts of G. domingensis exposed to cadmium showed significant structural changes, including modification in the quantity, size, and organization of thylakoids. Similar results were observed with the red macroalga Ceramium ciliatum exposed to cadmium, where the chloroplast appeared with disrupted thylakoids and an increase in plastoglobuli volume [8], and with H. musciformis after exposure to cadmium during 7 days [6]. However, it should be noted that Talarico [7] demonstrated only a few changes in the chloroplast organization of Audouinella saviana after exposure to cadmium. Finally, when analyzed by TEM, Cd-exposed G. domingensis revealed an increase in the number of the plastoglobuli in the chloroplast. This increase in the number of lipids can interpreted as a change in metabolism, which, in turn, results in a reduction of cell proliferation and a decrease in GRs. According to Holzinger et al. [42], when algae are subjected to stress, nitrogen limitation and the synthesis of lipids are observed. These phenomena occur because the pathways to form protein-containing cell structures are suppressed.
Our results indicated that the concentrations of cadmium utilized in the experiments were directly related to decreased photosynthetic mechanism, as a consequence of inhibited growth rates and increased enzymatic defense, showing that cadmium is highly toxic to G. domingensis.
5. Acknowledgements
The authors would like to acknowledge the staff of the Central Laboratory of Electron Microscopy (LCME), Federal University of Santa Catarina, Florianopolis, Santa Catarina, Brazil, for the use of their scanning and transmission electron microscopes. This study was supported, in part, by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CAPES, Brazil), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil), and Fundação de Apoio à Pesquisa Cientifica e Tecnológica do Estado de Santa Catarina (FAPESC).
REFERENCES
- I. Rocchetta, P. I. Leonardi, G. M. Amado Filho, M. del C. R. de Molina and V. Conforti, “Ultrastructure and X-ray Microanalysis of Euglena Gracilis (Euglenophyta) under Chromium Stress,” Phycologia, Vol. 46, No. 3, 2007, pp. 300-306.
- H. B. Pratap, F. A. Mamboya, M. S. P. Mtolera and M. Björk, “The Effect of Copper on the Daily Growth Rate and Photosynthetic Efficiency of the Brown Macroalga Padina Boergensenii,” Proceedings of the Conference on Advances on Marine Sciences in Tanzania, Bilateral Marine Science Programme, 1999, pp. 185-192.
- P. X. Sheng, Y. Ting, J. P. Chen and L. Hong, “Sorption of Lead, Copper, Cadmium, Zinc and Nickel by Marine Algal Biomass: Characterization of Biosorptive Capacity and Investigation of Mechanisms,” Journal of Colloid and Interface Science, Vol. 275, No. 1, 2004, pp. 131-141.
- S. X. Hu, C. H. Tang and M. Wu, “Cadmium Accumulation by Several Seaweeds,” Science of the Total Environment, Vol. 187, No. 2, 1996, pp. 65-71. doi:10.1016/0048-9697(96)05143-1
- M. Kumar, P. Kumari, V. Gupta, P. A. Anisha, C. R. K. Reddy and B. Jha, “Differential Responses to Cadmium Induced Oxidative Stress in Marine Macroalga Ulva Lactuca (Ulvales, Chlorophyta),” Biometals, Vol. 23, No. 2, 2010, pp. 315-325.
- Z. L. Bouzon, E. C. Schmidt, A. C. de Almeida, N. S. Yokoya, M. C. de Oliveira and F. Y. Chow, “Cytochemical Characterization and Ultrastructural Organization in Calluses of the Agarophyte Gracilariopsis Tenuifrons (Gracilariales, Rhodophyta),” Micron, Vol. 42, No. 1, 2011, pp. 80-86. doi:10.1016/j.micron.2010.07.012
- L. Talarico, “Fine Structure and X-Ray Microanalysis of a Red Macrophyte Cultured under Cadmium Stress,” Environmental Pollution, Vol. 120, No. 3, 2002, pp. 813-821. doi:10.1016/S0269-7491(02)00156-2
- B. E. Diannelidis and S. G. Delivopoulos, “The Effects of Zinc, Copper and Cadmium on the Fine Structure of Ceramium Ciliatum (Rhodophyceae, Ceramiales),” Marine Environmental Research, Vol. 44, No. 2, 1997, pp. 127- 134. doi:10.1016/S0141-1136(96)00106-7
- I. Visviki and J. W. Rachlin, “Ultrastructural Changes in Dunaliella Minuta Following Acute and Chronic Exposure to Copper and Cadmium,” Archives of Environmental Contamination and Toxicology, Vol. 23, No. 4, 1992, pp. 420-425.
- L. R. Andrade, M. Farina, and G. M. Amado Filho, “Effects of Copper on Enteromorpha Flexuosa (Chlorophyta) in Vitro,” Ecotoxicology and Environmental Safety, Vol. 58, No. 1, 2004, pp. 117-125. doi:10.1016/S0147-6513(03)00106-4
- L. R. de Andrade, M. Farina and G. M. A. Filho, “Role of Padina Gymnospora (Dictyotales, Phaeophyceae) Cell Walls in Cadmium Accumulation,” Phycologia, Vol. 41, No. 1, 2002, pp. 39-48.
- M. A. Hashim and K. H. Chu, “Biosorption of Cadmium by Brown, Green and Red Seaweeds,” Chemical Engineering Journal, Vol. 97, No. 2-3, 2004, pp. 249-255. doi:10.1016/S1385-8947(03)00216-X
- E. Pinto, T. C. S. Sigaud-Kutner, M. A. S. Leitão, O. K. Okamoto, D. Morse and P. Colepicolo, “Heavy MetalInduced Oxidative Stress in Algae,” Journal of Phycology, Vol. 39, No. 6, 2003, pp. 1008-1018.
- E. C. Oliveira and E. M. Plastino, “Gracilariaceae,” In: I. Akatsuka, Ed., Biology of Economic Algae, SPB Academic Publishing, The Hague, 1994, pp. 185-226.
- R. Armisen and A. Postal, “World-Wide Use and Importance of Gracilaria,” Journal of Applied Phycology, Vol. 7, No. 3, 1995, pp. 231-243.
- J. M. Kain and C. Destombe, “A Review of the Life History, Reproduction and Phenology of Gracilaria,” Journal of Applied Phycology, Vol. 7, No. 3, 1995, pp. 269- 281.
- E. C. Schmidt, R. Santos, P. A. Horta, M. Maraschin and Z. L. Bouzon, “Effects of UVB Radiation on the Agarophyte Gracilaria Domingensis (Rhodophyta, Gracilariales): Changes in Cell Organization, Growth and Photosynthetic Performance,” Micron, Vol. 41, No. 8, 2010, pp. 919-930. doi:10.1016/j.micron.2010.07.010
- E. C. Oliveira, E. J. Paula, E. M. Plastino and R. Petti, “Metodologías Para el Cultivo no Axenico de Macroalgas Marinas in Vitro,” In: K. Alveal, M. Ferrario, E. Oliveira and E. SAR, Eds., Manual de Métodos Ficológicos, Universidad de Concepción, Concepción-Chile, 1995, pp. 429-447.
- P. Edwards, “Illustrated Guide to the Seaweeds and Sea Grasses in the Vicinity of Porto Arkansas,” In: Dr. Tracy and A. Villareal, Eds., Contributions in Marine Science, Marine Science Institute, Texas, 1970, pp. 1-228.
- L. Talarico, S. Bozo and G. Maranzana, “Preliminary Observations on Audouinella Saviana (Meneghini) Woelkerling (Nemaliales, Rhodophyta) Cultured at Increasing Cd Concentrations,” Phycologia, Vol. 36, 1997, p. 111.
- J. R. Xia, Y. J. Li, J. Lu and B. Chen, “Effects of Copper and Cadmium on Growth, Photosyntesis, and Pigment Content in Gracilaria Lemaneiformis,” Bulletin of Environmental Contamination and Toxicology, Vol. 73, No. 6, 2004, pp. 979-986.
- C. A. Penniman, A. C. Mathieson and C. E. Penniman, “Reproductive Phenology and Growth of Gracilaria tikvahiae McLachlan (Gigartinales, Rhodophyta) in the Great Bay Estuary, New Hampshire,” Botanica Marina, Vol. 29, No. 2, 1986, pp. 147-154.
- A. J. White and C. Critchley, “Rapid Light Curves: A New Fluorescence Method to Assess the State of the Photosynthetic Apparatus,” Photosynthesis Research, Vol. 59, No. 1, 1999, pp. 63-72.
- R. J. Jones, T. Kildea and O. Hoegh-Gudberg, “PAM Chlorophyll Fluorometry: A New in Situ Technique for Stress Assessment in Scleractinian Corals, Used to Examine the Effect of Cyanide from Cyanide Fishing,” Marine Pollution Bulletin, Vol. 38, No. 10, 1999, pp. 864- 874. doi:10.1016/S0025-326X(98)90160-6
- T. Platt, C. L. Gallegos and W. G. Harrison, “Photoinhibition of Photosynthesis in Natural Assemblages of Marine Phytoplankton,” Journal of Marine Research, Vol. 38, 1980, pp. 687-701.
- N. S. Yokoya, O. Necchi, A. P. Martins, S. F. Gonzalez and E. M. Plastino, “Growth Responses and Photosynthetic Characteristics of Wild and Phycoerythrin-Deficient Strains of Hypnea Musciformis (Rhodophyta),” Journal of Applied Phycology, Vol. 19, No. 3, 2007, pp. 197-205. doi:10.1007/s10811-006-9124-9
- E. C. Schmidt, M. Maraschin and Z. L. Bouzon, “Effects of UVB Radiation on the Carragenophyte Kappaphycus Alvarezii (Rhodophyta, Gigartinales): Changes in Ultrastructure, Growth, and Photosynthetic Pigments,” Hydrobiologia, Vol. 649, No. 1, 2010, pp. 171-182. doi:10.1007/s10750-010-0243-6
- J. D. Hiscox and G. F. Israelstam, “A Method for the Extraction of Chlorophyll from Leaf Tissue without Maceration,” Canadian Journal of Botany, Vol. 57, No. 12, 1979, pp. 1332-1334.
- E. C. Schmidt, B. G. Nunes, M. Maraschin and Z. L. Bouzon, “Effect of Ultraviolet-B Radiation on Growth, Photosynthetic Pigments, and Cell Biology of Kappaphycus Alvarezii (Rhodophyta, Gigartinales) Macroalgae Brown Strain,” Photosynthetica, Vol. 48, No. 2, 2010, pp. 161- 172.
- A. R. Wellburn, “The Spectral Determination of Chlorophyll A and Chlorophyll B, as well as Total Carotenoids, Using Various Solvents with Spectrophotometers of Different Resolution,” Journal Plant Physiology, Vol. 144, No. 3, 1994, pp. 307-313.
- T. A. Kursar, J. van Der Meer and R. S. Alberte, “LightHarvesting System of the Red Alga Gracilaria tikvahiae,” Plant Physiology, Vol. 73, 1983, pp. 353-360.
- I. Carlberg and B. Mannervik, “Glutathione Reductase,” Methods in Enzymology, Vol. 113, 1985, pp. 484-490.
- E. C. Schmidt, B. Pereira, C. L. M. Pontes, R. dos Santos, F. Scherner, P. A. Horta, R. de P. Martins, A. Latini, M. Maraschin and Z. L. Bouzon, “Alterations in Architecture and Metabolism Induced by Ultraviolet Radiation-B in the Carragenophyte Chondracanthus teedei (Rhodophyta, Gigartinales),” Protoplasma, Vol. 249, No. 2, 2012, pp. 353-367. doi:10.1007/s00709-011-0286-1
- A. Cassina and R. Radi, “Differential Inhibitory Action of Nitric Oxide and Peroxynitrite on Mitochondrial Electron Transport,” Archives of Biochemistry and Biophysics, Vol. 328, No. 2, 1996, pp. 309-316.
- A. Latini, M. Rodriguez, R. B. Rosa, K. Scussiato, G. Leipnitz, D. R. de Assis, G. da C. Ferreira, C. Funchal, M. C. Jacques-Silva, L. Buzzin, R. Giugliani, A. Cassina, R. Radi and M. Wajner, “3-Hydroxyglutaric Acid Moderately Impairs Energy Metabolism in Brain of Young Rats,” Neuroscience, Vol. 135, No. 1, 2005, pp. 111-120.
- O. H. Lowry, N. J. Rosebough, A. L. Farr and R. J. Randall, “Protein Measurement with the Folin Phenol Reagent,” The Journal of Biological Chemistry, Vol. 193, No. 1, 1951, pp. 265-275.
- E. C. Schmidt, L. A. Scariot, T. Rover and Z. L. Bouzon, “Changes in Ultrastructure and Histochemistry of Two Red Macroalgae Strains of Kappaphycus alvarezii (Rhodophyta, Gigartinales), as a Consequence of Ultraviolet B Radiation Exposure,” Micron, Vol. 40, No. 8, 2009, pp. 860-869.
- E. S. Reynolds, “The Use of Lead Citrate at High pH as an Electron Opaque Stain in Electron Microscopy,” The Journal of Cell Biology, Vol. 17, No. 1, 1963, pp. 208- 212.
- J. Collén, E. Pinto, M. Pedersén and P. Colepicolo, “Induction of Oxidative Stress in the Red Macroalga Gracilaria tenuistipitata by Pollutant Metals,” Archives of Environmental Contamination and Toxicology, Vol. 45, No. 3, 2003, pp. 337-342.
- M. Greger and E. Ogren, “Direct and Indirect Effects of Cd2+ on Photosynthesis in Sugar Beet (Beta vulgaris),” Plant Physiology, Vol. 83, No. 1, 1991, pp. 129-135.
- A. K. Stobart, W. T. Griffiths, I. Ameen-Bukhari and R. P. Sherwood, “The Effects of Cd2+ on the Biosynthesis of Chlorophyll in Leaves of Barley,” Physiology Plantarum, Vol. 63, No. 3, 1985, pp. 293-298.
- A. Holzinger, M. Y. Roleda and C. Lütz, “The Vegetative Arctic Freshwater Green Alga Zygnema Is Insensitive to Experimental UV Exposure,” Micron, Vol. 40, No. 8, 2009, pp. 831-838.
NOTES
*Corresponding author.

