American Journal of Plant Sciences
Vol.3 No.7(2012), Article ID:20717,4 pages DOI:10.4236/ajps.2012.37105
Not All Shrivels Are Created Equal—Morpho-Anatomical and Compositional Characteristics Differ among Different Shrivel Types That Develop during Ripening of Grape (Vitis vinifera L.) Berries
![]()
1Department of Horticulture and Landscape Architecture, Washington State University Tri-Cities, Richland, USA; 2Irrigated Agriculture Research and Extension Center, Washington State University, Prosser, USA.
Email: *bbondada@wsu.edu
Received April 30th, 2012; revised May 28th, 2012; accepted June 7th, 2012
Keywords: Grape; Bunch Stem; Dehydration; Necrosis; Ripening; Shrivel; Sunburn; Vitis vinifera
ABSTRACT
An understanding of physiological disorders associated with ripening of fruits triggered by abiotic stress relies on anatomical and physico-chemical analyses, as it provides insights into their origin and probable causes. The objective of this study was to analyze different ripening disorders of grape (Vitis vinifera L.) berries by dissecting their morphoanatomy, shriveling nature, and composition. Four well-defined disorders—sunburn, prolonged dehydration (PD), lateseason bunch stem necrosis (LBSN), and berry shrivel (BS) were analyzed in field-grown grapevines of the cultivar Cabernet Sauvignon. Early bunch stem necrosis (EBSN) that occurred before ripening was also included in the study. Unlike healthy spherical berries, the pericarp of disordered berries except for sunburn shriveled causing concomitant reductions in fresh weight and volume. The exocarp of PD berries developed well-ordered indentations as distinct from the wrinkles in LBSN berries, whereas BS berries were flaccid with numerous skin folds. The epicuticular wax occurred as upright platelets in all shrivel forms excluding the sun-exposed hemisphere of sunburned berries. A chlorophyllous inflorescence framework persisted in all shrivel forms but in LBSN, wherein the necrotic regions developed tylosis. Unlike the translucent mesocarp of healthy, sunburned, and PD berries, the mesocarp was collapsed in BS and LBSN berries, nevertheless all had well-developed seeds. The composition of healthy berries was optimal, whereas the disordered berries were compositionally distinct from each other, which as a whole differed from the healthy berries. The BS berries had the lowest sugar content, and although sugar concentration was higher in LBSN, sunburned and PD berries, sugar amount per berry was highest in the healthy berries, the same was true for hexoses. Healthy and BS berries exhibited highest amounts of tartaric acid followed by sunburn and PD berries, whereas the LBSN berries had the lowest values. Conversely, healthy and PD berries had the highest amounts of malic acid followed by LBSN, sunburn and BS berries, which collectively displayed similar amounts. The PD berries exhibited the highest calcium content followed by LBSN, healthy, and finally BS and sunburned berries. A linear relationship existed between potassium (K) and pH of the berries. The PD berries had the highest amounts of K followed by healthy, sunburn, LBSN, and BS berries. Overall, the results reported here provided combined morpho-anatomical and compositional analyses of different shrivel types that occurred during a single growing season. Such analysis is needed to make a progress on understanding these ripening disorders culminating in the development of remedial measures.
1. Introduction
Of all phenological events, the ripening phenomenon especially of fleshy fruits has been a topic of intense investigation because of the specificity of this developmental process to plant biology and the practical importance of ripening to the human diet and health [1]. Ripening of fleshy fruits is a dynamic transitional period that encompasses a coordinated series of changes in color, texture, volatile expression, and the accumulation of sugars [2]. Collectively, these changes indicate suitability for consumption and dispersal by vertebrate frugivores [3]. Provided that fruits follow the highly coordinated events of ripening, a soft edible ripe fruit with desirable organoleptic qualities develops as the ultimate end product [4]. Conversely, perturbations in the cascade of ripening events result in various ripening disorders [5]. For instance, apples develop various disorders such as internal browning, core browning, soft scald, and superficial scald, which limits the commercial storage periods [6]. The extensively researched tomato fruit develops numerous disorders, one particularly concerned with ripening includes blotchy ripening wherein the fruit fails to develop color uniformly [7]. Stone fruit disorders such as split and shattered pits and double fruit of peaches [8], and albino and malformed fruits of strawberries [9] reduces fresh market quality. These ripening disorders are considered to be physiological in nature resulting from altered metabolism and disruption of normal ripening and senescence processes eventually compromising fruit quality attributes and yield; what precisely causes them are not known [5]. Nevertheless, examinations of their internal and surface morphologies coupled with compositional analysis have led to great advances in the understanding of how these disorders develop and deteriorate fruit quality [5].
As opposed to other fruit crops, grape (Vitis vinifera) is unique in that not only is it among the most ancient and widely cultivated fruit crops, but also is the only fleshy fruit crop to have its genome sequenced [10]. Hence, the keen interest in the understanding of grape berry ripening is justified by the economic relevance of the quality of grapes and their processed products, such as wine, juice and dried fruit [4]. In view of the availability of its complete genomics toolkits and the specificity of its ripening process to plant biology and its comercial significance, the grape berry has emerged as a model organism for investigating fleshy fruit development and acid fruit physiology [4,11]. The ultimate goal of grapevine as a model plant is to optimize commercial maturity of grapes by understanding the physiological, hormonal, and molecular basis of ripening [10,12]. Thus, ripening of the grape berry is a key growth phase as it immediately precedes harvesting determining the nature of the material used in the production of wine, juice, and table grapes. Commercial maturity (soluble solids in the range of 15 - 30 Brix) of grapes is typically evaluated at harvest and like any fleshy fruit involves observation of changes in skin color, and the measurement of acid concentrations and soluble solid content [13]. Since ripening of fruit is associated with an increased risk of physiological disorders [2], grapevines akin to any other fruit crop develop their own ripening oddities discernible in various forms of shrivel mostly prior to harvest. These disorders have been classified morphologically into four well-defined forms: sunburn, prolonged dehydration (PD), berry shrivel (BS), and late-season bunch stem necrosis (LBSN); one more type, the early-season bunch stem necrosis (EBSN) occurs before ripening [14-17]. The prolonged dehydration refers to the type of berry weight loss that typically occurs in Shiraz berries during late in the ripening a few weeks before harvest [18,19]. Each shrivel type has a different derivation evolving with distinct morphological and compositional characteristics; the only commonality among them is the loss of volume occurring during the ripening phase of the berry growth curve.
Viticulturists use the French word véraison (a term denoting the change in color of the skin) to describe the initiation of berry ripening, which represents one of the modules of berry’s double sigmoidal growth pattern resulting from two successive periods of vacuolar swelling during which the size and nature of accumulated solutes drastically change [13]. In the first part of the biphasic growth (growth stage I), berry volume increases via a series of mitotic division and cell enlargement. The first phase is followed by a lag period (lag phase, growth stage II) and it is during this slowing down phase that the embryo and endosperm within the seed differentiate and the seed matures. The second sigmoid curve (growth stage III) characterizes the ripening process marked by accumulation of anthocyanins in the exocarp of pigmented red and black varieties, increased deformability accompanied by fruit softening, a decline in titratable acidity, massive accumulation of hexose sugars, metabolism of malate, and production of vast array of flavor and aroma compounds [4,16,20]. This is a key growth phase since it determines the quality of the final product such as wine or table grape. The boundary between stages II and III is called “véraison” and by just about this time not only the fruit ripening process begins but also different disorders initiate [15,17,21-23]. The most striking feature of these disorders is the shriveling of the pericarp. Although the various forms of shrivel visually appear identical, shrivels themselves are distinctive and for this reason each is quite sharply different from the other with respect to morpho-anatomy and composition [24]. It is hypothesized that each shrivel form develops with distinct characteristics resulting distinguishable features in composition and morpho-anatomy of the berry. Compared to extensive studies on physiological disorders of other fruit crops species such as apple, tomato, cherry [5], limited literature exists for the various shrivel forms in grape berry. The objective of this study was to analyze morpho-anatomical and compositional basis of distinguished features among different shrivel forms and provide insights into probable causes of each form.
2. Materials and Methods
2.1. Plant Material
A commercial vineyard (lat. 46˚15'47.48''N, long. 119˚29' 16.09''W) with mature own-rooted V. vinifera cv. Cabernet Sauvignon located in the Red Mountain AVA (American Viticultural Area) at Benton City, WA was chosen for dissecting various ripening disorders during the 2007-2009 growing seasons. This vineyard was chosen as these vines consistently exhibited incidences of several ripening disorders. The vineyards had a vine by row spacing of 1.83 m by 2.74 m. The soil types in this AVA include Warden (Mesic Xeric Haplocambids), Hezel (Mesic Xeric Torriorthents), and Scooteney (Mesic Xeric Haplocambids) series derived from Quaternary glacial sediments and wind-blown loess overlying Miocene basalt. Vines were trained to a bilateral cordon (an extension of the trunk), which entailed training the vines in both directions from the trunk along the cordon wire and were drip-irrigated during the growing season. Training in viticulture parlance refers to the design and development of a grapevine framework. The shoots emerging from spurs (a bearing unit with 2 - 4 buds) on cordon were positioned vertically using catch wires. Vines were spur-pruned during winter, i.e., canes (a mature woody and lignified stem from previous season’s shoot) were cut back to two count nodes/buds (the readily visible buds on a dormant cane, not including the small base buds); the noncount shoots (shoots arising from base buds of the spur) were removed at the beginning of bloom that approximately equated to 20 shoots per meter. Throughout the growing season, the vines were continually monitored for the inception of all disorders especially during the ripening period. Following the appearance of disorders, the symptomatic vines were identified by tagging the vines and their clusters, i.e. fertilized inflorescence, which later in the season become the fruiting structures. Thereafter, the progression of each disorder was monitored for analyzing morphological and compositional characteristics at harvest.
2.2. Berry Volume and Fresh Weight
Fruits were de-pediceled using a sharp razor blade and their fresh weights were measured using a pre-tared 1 mg sensitivity electronic balance. Following fresh weight measurements, the volume of whole berry was measured by the water displacement method based on Archimedes’ principle. Forty berries were immersed in a 100 ml measuring cylinder filled with 60 ml of water. The increase in volume of water displaced by berries was recorded, which according to Archimedes principle is equal to the weight of the volume of water the berries displaced. Since the recorded quantity was in volume units, it was numerically equal to the volume of berries. Per berry volume was calculated from the volume of water displaced by 40 berries.
2.3. Berry Composition
All compositional attributes of berries pertaining to soluble solids, glucose + fructose, tartaric and malic acids, calcium, and potassium were analyzed by a commercial laboratory (ETS Laboratories, Walla Walla, WA, USA).
2.4. Light Microscopy
To examine the gross morphological and anatomical features of berries, berries from healthy and afflicted clusters were excised from the rachis. Berries were then either cross-sectioned at the proximal (pedicel) and distal (stylar) end, or were sectioned longitudinally through the center to examine the pericarp with stereomicroscope (Stemi 2000-C, Carl Zeiss, Thornwood, NY, USA) attached with digital camera (DXM 1200C; Nikon Instruments, Melville, NY, USA), which was used for capturing digital images. Whole berries from each shrivel type, and the dewaxed healthy berries prepared by gently rubbing the berries with kimwipes were also examined with the same stereomicropscope.
To examine the anatomy of rachis and pedicels, the standard free-hand sectioning technique [25] was used. All sections were cut using a new double-sided razor blade and stained with safranin O (Fisher Scientific, Pittsburgh, PA), 0.5% (w/v) dissolved in 50% EtOH. After each cutting, the cut sections that collected on the blade were transferred to a Petri dish containing safranin O. Following staining the sections were either viewed with bright field microscopy (Carl Zeiss Inc., Thornwood, NY, USA) or with wide field epifluorescence microscopy (Carl Zeiss Inc., Thornwood, NY, USA) attached with a digital camera (DXM 1200C).
2.5. Scanning Electron Microscopy
Small pieces of the berry exocarp from the equatorial region were cut using a sharp razor blade. The tissue samples were fastened to aluminum specimen mounts and placed in a desiccator for air-drying the samples. Prior to viewing, the dried samples were coated with platinum. To observe epicuticular wax morphology, a Focused-Ion-Beam/Scanning Electron Microscopy (FIB/ SEM) dual beam microscope of the microscopy facility located at EMSL-PNNL, Richland, WA was used. The mounted samples were transferred to dual beam system for focused ion beam (FIB)/SEM operation (Helios NanoLab DualBeam focused ionbeam/scanning electron microscope, FEI, Corp., Hillsboro, OR, USA). SEM imaging was performed by means of the field emission gun electron column available in the same system using a SEM beam voltage of 5 kV with a 1 nm spot size.
2.6. Data Analysis
All quantitative data were statistically analyzed by oneway ANOVA using SPSS (SPSS statistical package 11; SPSS, Chicago, IL, USA). Regression analysis was performed on selected variables using SPSS software. Significance among mean values was determined by least significant difference (LSD) values where P = 0.05.
3. Results
3.1. Morpho-Anatomical Characteristics
3.1.1. Healthy Berry
The berries were borne on a cluster framework with a proximal-to-distal decrease comprising of peduncle (hypoclade)—the stalk of the cluster, rachis—the main framework and axis of the cluster, and pedicels, the stalk of the berries (Figure 1(A)). The proximal end of the chlorophyllous pedicel was thin whereas the distal end was swollen into a receptacle (Figure 1(B)). Numerous lenticels as very small heaps of loosely arranged suberized cells (Figure 1(A), inset) were distributed on the surface giving the pedicel a warty appearance (Figure 1(B), inset). Where the rim of the receptacle contacted the berry surface, an annular waxy margin was formed (Figure 1(B), inset). Conversely, the distal end of the berry had an unbound small conspicuous brownish mass of dead tissues (remnants of style and stigma) (Figures 1(B), (C)). Individual berries were spherical, firm yet soft to touch and elastic, and purple colored (Figures 1(A), (B), Table 1). The outer surface of the berry appeared as powdery bloom to the naked eye (Figure 1(B)). The surface of the berry after the wax had been rubbed off with fingers revealed a taut cuticle giving the berry a shine or luster, and appeared dark colored reflecting the accumulation of anthocyanin pigments in the vacuoles of exocarp (skin) cells (Figure 1(C)). The powdery bloom was due to the presence of fine, loose, shiny, and offwhite aggregates of epicuticular wax crystals (Figure 1(D)). A nanoscale resolution revealed the crystalline shape of the epicuticular wax in the form of upright platelets consisting of a reticulum of microfilaments (Figure 1(E)).
A longitudinal section of the berry revealed a bilateral symmetry of tissues, which confined three tissue strata: the outer stratum, exocarp; the relatively thick stratum below it—a translucent, hydrated and soft mesocarp (pulp or flesh) with colorless juice; and the membranous Inner stratum—endocarp (Figure 1(F)). The exocarp resting on the softer foundation of mesocarp was purple colored and its exterior was rough due to the presence of epicuticular wax platelets that were randomly distributed throughout the surface (Figure 1(D)). The middle stratum or the mesocarp represented the largest portion of the berry volume (Figure 1(F)). The internal stratum or endocarp acted as a single inner wall between the mesocarp
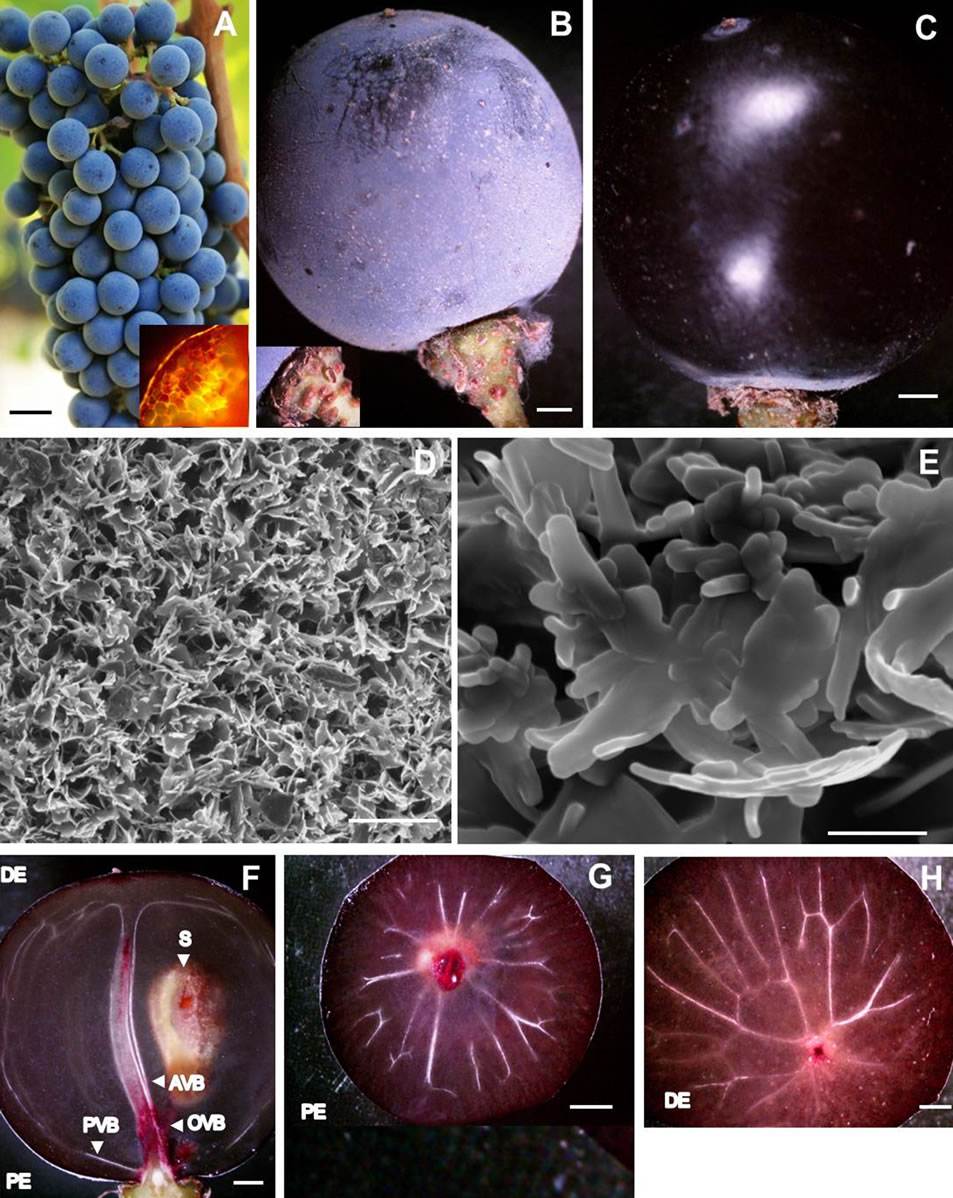
Figure 1. Photographs of (A) Healthy Cabernet Sauvignon grape cluster showing spherical berries attached to rachis with healthy pedicels, and cross section of lenticel (inset); (B) A single healthy spherical berry covered with bloom resting on the distal swollen receptacle of chlorophyllous pedicel replete with lenticels (inset); (C) De-waxed surface of healthy berry revealing a taught cuticle and a shine or luster resembling a polished dark colored grape; (D) Scanning electron micrograph of bloom showing fine, loose, shiny, and offwhite aggregates of epicuticular wax crystals; (E) Nanoscale resolution scanning electron micrograph of epicuticular wax platelet showing microtubules; (F) A stereomicrograph showing longitudinal section of a healthy berry with bilateral symmetry of pericarp embedded with axial, peripheral and ovular vascular tissues, and a seed; (G) A stereomicrograph of healthy berry showing the highly ramified peripheral or dorsal vascular bundle at the proximal end; (H) A stereomicrograph of healthy berry showing peripheral bundle united with the axial vascular bundle at its distal end. Scale bars: 10 mm (A-C, F-H), 5 µm (D), 500 nm (E). AVB—Axial vascular bundle, DE—Distal end, OVB— Ovular vascular bundle, PE—Proximal end, PVB—peripheral vascular bundle, S—Seed.
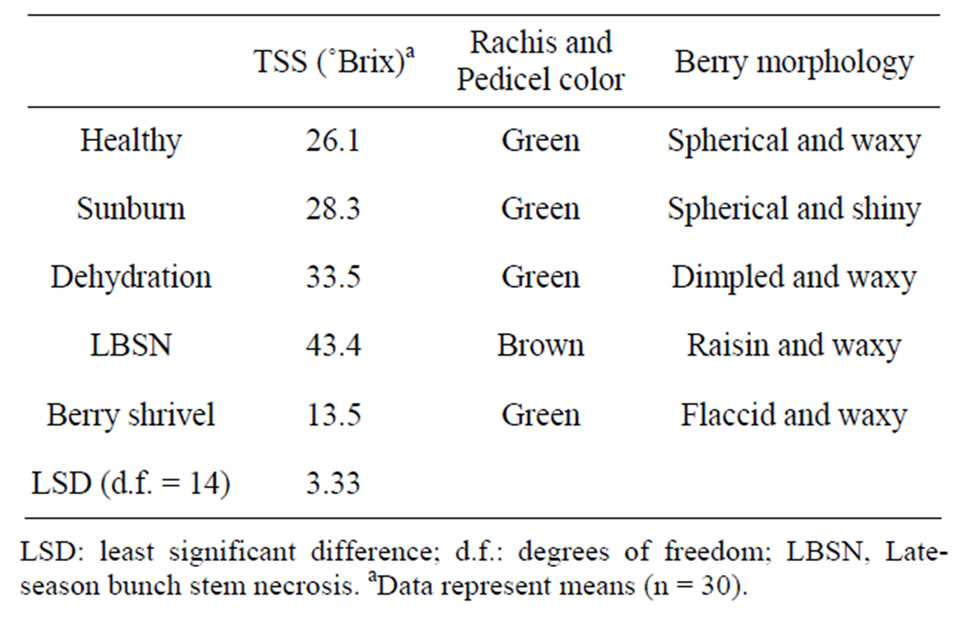
Table 1. Total soluble solids (TSS) and morphological features of berries afflicted with different ripening disorders.
and seeds. The seeds were confined to the locules in the center of the mesocarp. One of the seeds is shown in Figure 1(F). Together these three tissues comprised the pericarp, which was fed via vascular bundles that entered the berry from the pedicel as a group consisting of three components (Figure 1(F)). One of the components, the axial vascular bundle extended into the distal pole of the berry (Figure 1(F)), the second component, the highly ramified peripheral or dorsal vascular bundle branching out from the axial vascular bundle at the proximal end (Figures 1(F), (G)) and then again uniting with the axial vascular bundle at its distal end (Figures 1(F), (H)) was located just beneath the exocarp. The dorsal vascular bundle separated the mesocarp into two zones, inner and outer zones. The third component entailed the ovular vascular bundles that served the seeds (ovules) (Figure 1(F)).
The cross section of pedicel at the distal end was angular due to the presence of lenticels (Figure 2(A)). Its anatomy was much like that of a young stem comprising an epidermis, typical cortex, pith, and vascular system consisting of discrete bundles of xylem and phloem elements. However, in this cross section (post-veraison berry), the individual vascular bundles were thickened by secondary growth into pie-shaped sectors separated by rays (Figures 2(A), (B)). The course of vascular bundles remained unchanged until the region of receptacle was reached where they branched (Figures 2(C)-(E)) and served the berry as described in the previous section.
3.1.2. Sunburn
Sunburn was mostly observed in clusters that were exposed to direct solar radiation on the west aspect of the canopy in this vineyard. In a given cluster, all sun-exposed berries succumbed to sunburn (Figure 3(A)), wherein one hemisphere remained constantly exposed to the sun (except on cloudy days), while the other hemisphere developed in shade (Figures 3(A), (B)). Most of
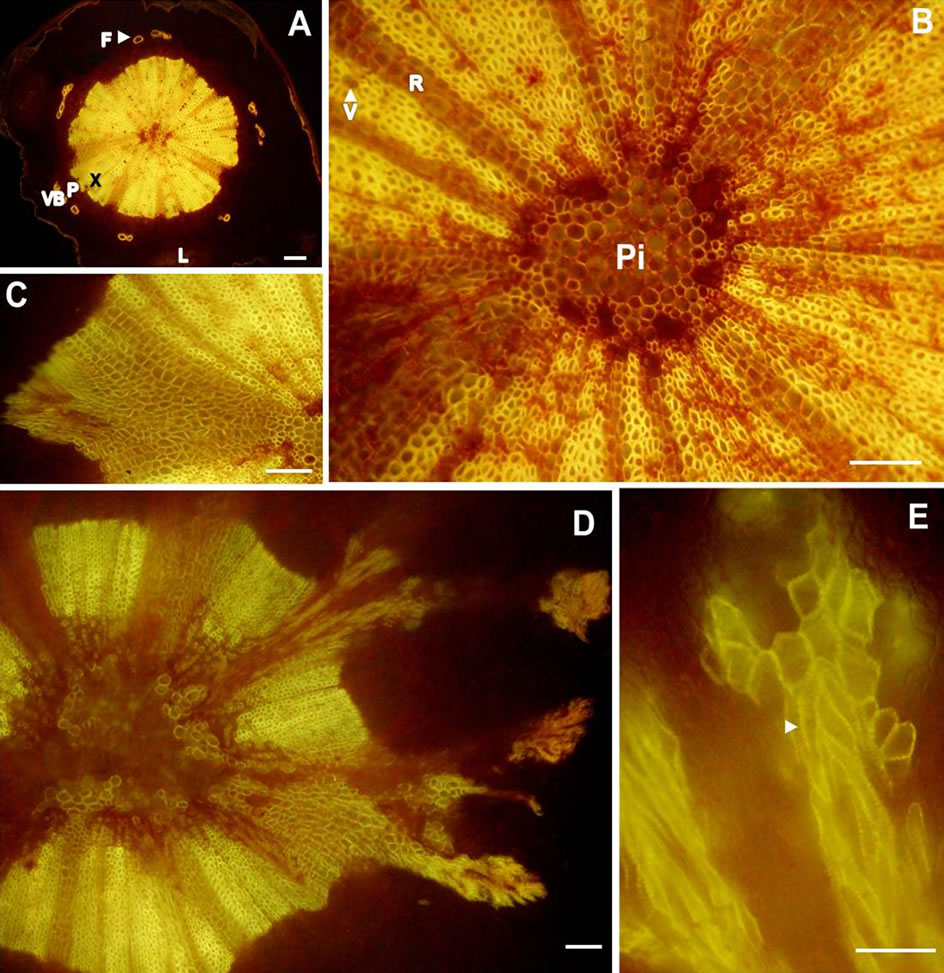
Figure 2. Transverse light micrographs of (A) A pedicel from healthy berry showing lenticel, cortex, phloem fibers and discrete bundles of xylem and phloem elements; (B) High magnification of the individual vascular bundles from (A) that were thickened by secondary growth into pieshaped sectors separated by rays, (C), (D), and (E) showing the course of branching from the receptacle. Arrow head indicates secondary cell wall of xylem vessels. Scale bars: 100 µm (A)-(D), 50 µm (E). F-Phloem fibers, L—Lenticel, P—Phloem, Pi-Pith, R—Ray parenchyma, V—Vessel, VB— Vascular bundle, X—Xylem.
the injury occurred on the exposed hemisphere manifested as loss of crystalline structure of epicuticular wax resulting in a shiny surface (Figure 3(A), Table 1). The wax platelets were degraded and transitioned into amorphous masses creating a rough surface morphology (Figure 3(B)) with poor development of color (Figure 3(A), inset). By contrast, the exocarp of the corresponding hemisphere on the opposite shaded side developed normally; there was no injury to this surface. On clusters that were exposed to constant sunlight, only the berries facing the sun developed sunburn symptoms; there was no injury to the cluster framework (peduncle, rachis and pedicels) (Figure 3(A)).
Longitudinal sections of the berries revealed healthy seeds (Figure 3(C)). There was no apparent injury to any of the vascular systems. The mesocarp appeared to be firm and intact (Figure 3(C)).
3.1.3. Prolonged Dehydration (PD)
The PD clusters were observed on both east-west aspects of the canopy, but mostly on the west aspect of the canopy in the vineyard. In a given cluster afflicted with PD, the taut exocarp in dehydrated berries systematically

Figure 3. Photograph of (A) A cluster of sunburned berries with chlorophyllous pedicel and rachis showing loss of crystalline structure of epicuticular wax from berries exposing the cuticle and epidermal surface rendering the exposed surface a smooth and shiny appearance; (B) A scanning electron micrograph of the exposed rough surface revealing amorphous mass of degraded wax platelets; (C) A stereomicrograph showing longitudinal section of sunburned berry with bilateral symmetry of pericarp embedded with axial, peripheral and ovular vascular tissues, and a seed (D) Photograph of a cluster of berries with prolonged dehydration; (E) A single dehydrated berry from (D) showing the distinct polygonal indentations throughout the outer mesocarp zone resembling that of a golf ball; and (F) A stereomicrograph showing longitudinal section of a dehydrated berry with bilateral symmetry of pericarp embedded with axial, peripheral and ovular vascular tissues, and seeds. Scale bars: 10 mm (A), (C)-(F), 3 µm (B). AVB—Axial vascular bundle, DE—Distal end, PE—Proximal end, PVB— peripheral vascular bundle, S—Seed.
developed small polygonal indentations throughout the outer zone of the mesocarp (Figures 3(D), (E)). To the naked eye, the dehydrated berry with its dimpled exocarp was reminiscent of a golf ball (Figures 3(D), (E), Table 1). Dehydration did not alter the wax morphology. Longitudinal section revealed a translucent mesocarp with well-developed seeds and intact vasculature, although the dorsal vascular bundle at the proximal end appeared to be curved round the periphery of the outer mesocarp (Figure 3(F)). There was no injury to the cluster framework (Figure 3(D)).
3.1.4. Berry Shrivel (BS)
BS did not follow any discernible pattern with respect to shoot position on the cordon, cluster location on the shoot, and the vineyard site. BS clusters were observed throughout the vineyard. Not all clusters on a shoot displayed BS, if one cluster was affected, the other clusters of the same shoot either remained healthy and normal or afflicted with BS. Furthermore, the shoot next to a shoot with BS cluster either developed normal clusters or afflicted with BS. In addition, the vine next to the vine bearing BS clusters was either had normal clusters or developed BS clusters. The BS berries were flaccid and soft (Figures 4(A), (B) with their exocarp wrinkled and pulled away from the receptacle forming deep grooves into the mesocarp reminiscent of a deflated soccer ball (Figure 4(B), Table 1). These modifications; however, did not alter the wax morphology. Longitudinal sections of the BS berry revealed a collapsed mesocarp, yet the seeds were well developed (Figure 4(C)). The collapsed mesocarp was responsible for the flaccidity of the berries. The peduncle, rachis and pedicels appeared to be healthy (Figure 4(A)).
3.1.5. Late-Season Bunch Stem Necrosis (LBSN)
LBSN occurred wherever the clusters were shaded by the shoot system, this predominantly occurred on the east aspect of the canopy. In a given cluster, although the shoulder (wing) of the cluster exhibited necrosis, it was the distal end of the rachis that frequently developed necrosis. In viticulture parlance, the bunch stem is a collective term for the peduncle, rachis, and pedicel. In clusters afflicted with LBSN, darkened lesions developed toward either the distal end of the rachis or on the shoulder (upper lateral branches of the cluster), the proximal end of rachis or on both ends. The Figure 4(D) shows such lesions in the distal rachis region of the bunch stem. These lesions expanded and girdled the rachis causing necrosis and loss of green color. The girdling of the rachis blocked the supply of water, nutrients, and sugar to the berries distal to the necrotic region. Consequently, berries on the affected portions of the cluster dried up, shriveled, and eventually developed raisin-like wrinkles (Figure 4(E), Table 1). The rachis and pedicels in the region distal to the necrosis also dehydrated and turned brown (Figure 4(D)). On the contrary, all structures proximal to the necrotic region remained healthy (Figure 4(D)). The necrosis of rachis contributed to a significant loss in firmness, eventually shriveled the berries. Thus, in a textural context, LBSN promoted raisin like characteristics by modifying berry shape and dimension, wherein no harm was done to the seeds but the mesocarp was completely collapsed as evident in the longitudinal sections. The striking feature of LBSN was that the xylem vessels in the necrotic region developed tylosis (Figure 5(A)), no tylosis was observed in the healthy part of the
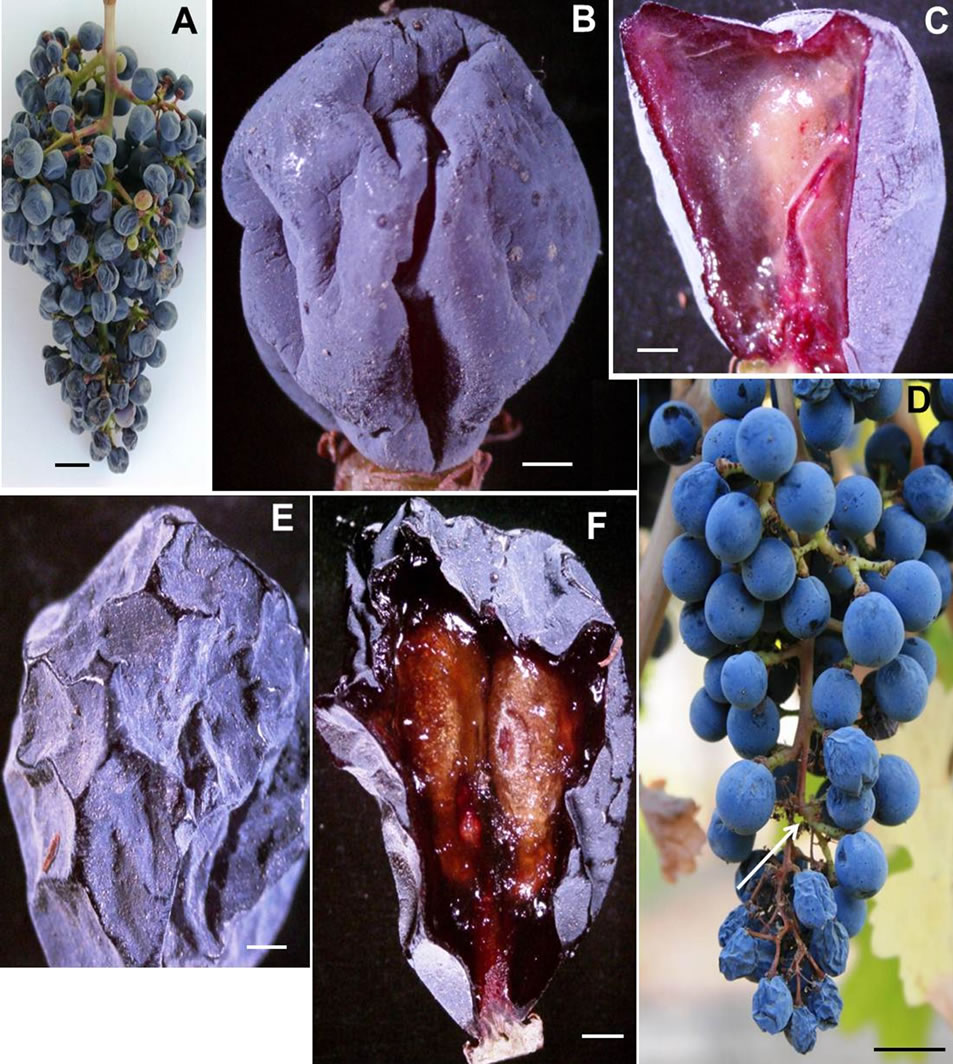
Figure 4. Photograph of (A) A cluster of berries afflicted with berry shrivel attached to chlorophyllous rachis via chlorophyllous pedicel (B) A stereomicrograph of a single berry afflicted with berry shrivel showing the distinct wrinkling of the exocarp that pulled away from the receptacle forming deep grooves into the mesocarp reminiscent of a deflated soccer ball, (C) A stereomicrograph of a longitudenal section of a berry afflicted with berry shrivel showing collapsed mesocarp and healthy seed, (D) Photograph of a cluster showing healthy proximal part with spherical berries attached to the chlorophyllous rachis via chlorophylllous pedicel whereas the distal part afflicted with LBSN bearing raisined berries with necrotic rachis and pedicels, (E) Stereomicrograph of a single LBSN berry displaying shriveled pericarp resembling a raisin, and (F) Stereomicrograph of longitudinal section of a single LBSN berry displaying dried up mesocarp and healthy seeds. Scale bars: 10 mm (A), (D), 3 mm (B), 1 mm (C)-(F).
cluster (Figure 5(B)).
3.1.6. Early-Season Bunch Stem Necrosis (EBSN)
The occurrence pattern of EBSN was similar to LBSN. However, as opposed to LBSN, EBSN occurred before ripening during flowering. While the end result was the same as poor fruit set, the expression of symptoms was different. Different parts of the bunch stem developed necrosis, which eventually shriveled, dried and turned brown, but remained attached to the bunch stem (Figures 5(C), (D)). Consequently, the whole bunch stem had fewer healthy branches resulting in poor fruit set (Figures 5(F)-(H)). The berries that developed on the remaining healthy part of the branch developed all qualities similar to a healthy cluster (Figure 5(E)).
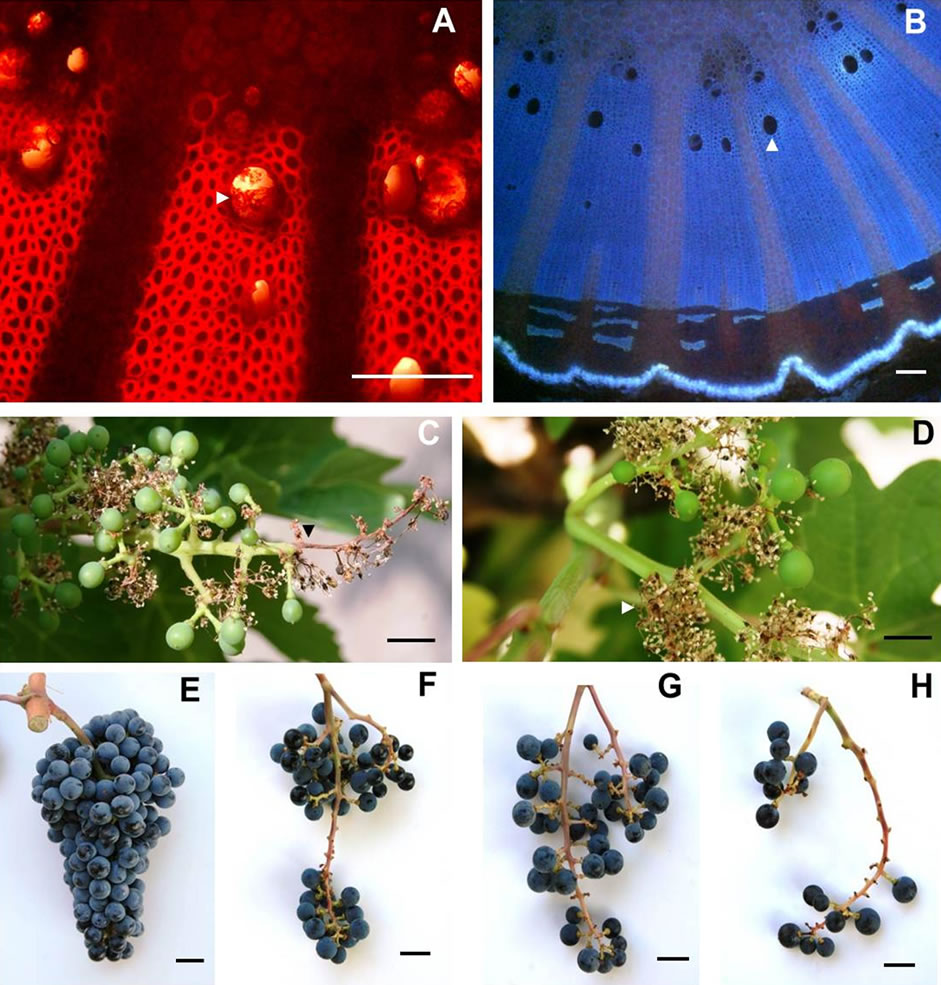
Figure 5. Transverse light micrographs of (A) LBSN shoulder (upper lateral branch) showing tylosed xylem vessels and (B) healthy shoulder showing open xylem vessels, and young clusters afflicted with EBSN showing necrosis confined to distal portion of the rachis (C) and (D) secondary branches of the rachis, photographs of (E) healthy cluster and (F)-(H) poor berry set caused by varying degrees of EBSN. Scale bars: 100 µm (A), (B), 10 mm (C)-(H). The white arrow heads in (A) indicates a tylosed vessel and open vessel in B.
3.2. Physical and Compositional Characteristics of Berries
Berry size as measured by fresh weight and volume was greatest in healthy berries followed by sunburn, PD, BSN, and BS berries (Figure 6). Except for the browning and necrosis of the distal part of LBSN cluster, the inflorescence framework in all other clusters was covered with chlorophyllous pigments (Table 1, Figures 1, 3, 4, and 5). With regard to berry morphology, healthy berries were spherical (Table 1). The sunburned and PD berries were also spherical but the PD berries had dimpled surface (Table 1). The berries from LBSN affliction developed raisin like characteristics whereas BS berries were flaccid (Table 1). Except for the exposed surface of sunburned berries, berries of all shrivel forms were waxy (Table 1).
The Brix values, a measure of total soluble solids (primarily sugars) were generally greater in those shriveled berries, which shriveled (excluding BS berries) followed by healthy berries. These in increasing order of Brix included LBSN, PD, which were higher than healthy and sunburned berries; the BS berries had the lowest concentrations (Table 1). However, converting Brix values to sugar concentrations on a per berry basis, the healthy
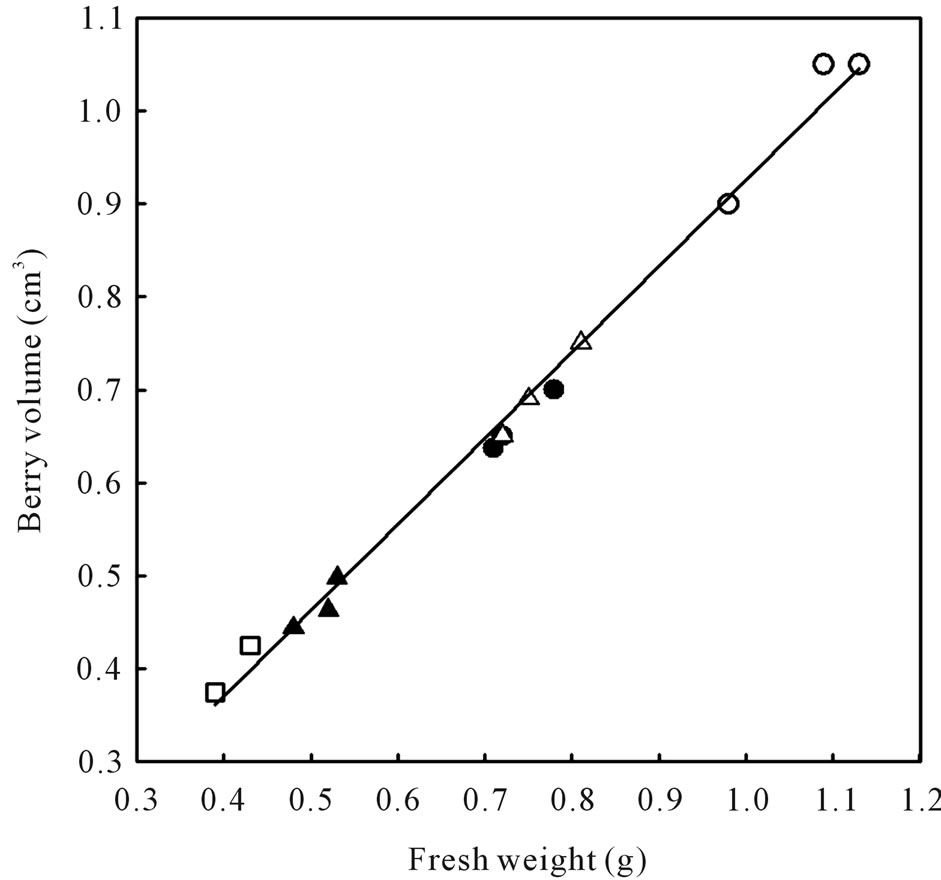
Figure 6. Relationship between berry volume and berry fresh weight of healthy berries and berries afflicted with various ripening disorders. Each data point represent single berry volume calculated from the volume of water displaced by 40 berries according to Archimedes principle. The regression analysis was made with the SPSS software. ○—Healthy berries, ●—Sunburn, ∆—PD, ▲—LBSN, and □—BS. y = 0.925x + 0.0071, r2 = 0.99, P < 0.001.
berries accumulated higher sugars than SB, LBSN, and BS berries (Table 2). The same trend was true for glucose + fructose amounts except that it was not different between LBSN and healthy berries (Table 2). Among the shriveled berries, the trend in sugars on per berry basis was different from the one observed for Brix. The PD berries had the highest sugar concentrations followed by LBSN and sunburned berries; the BS berries displayed the lowest amount (Table 2). With regard to organic acids, healthy and BS berries had similar amounts of tartaric acid, which were the highest of all berry types. Collectively their amounts were greater than the amounts in SB, PD, and LBSN berries (Table 2). Among the SB, PDand LBSN berries, the tartaric acid was almost identical between SB and PD berries; however, collectively, their concentrations were higher than those in the concentrations in LBSN berries (Table 2). Conversely, the amount of malic acid was higher in healthy and PD berries; however, their concentrations were not only the highest of all berry types but also were higher than SB, LBSN and BS berries among which the malic acid concentrations did not differ (Table 2). Nutritionally, the PD and LBSN berries contained higher amounts of calcium than the healthy, SB, and BS berries (Table 2). A close linear relationship existed between potassium (K) and pH of the berries (Figure 7). The PD berries contained the highest amounts of K followed by healthy, sunburn, LBSN, and BS berries (Figure 7).
4. Discussion
4.1. Organoleptic Qualities of Grape— Morpho-Anatomy of Healthy Berries
The bilateral symmetry of grapes [26,27] as observed in the healthy berries represents the culmination of a successful completion of all events of flower formation followed by pollination and fertilization and the double sigmoid course of berry development [28]. Furthermore, the healthy berry shaped slightly like a flattened sphere indicated cell expansion roughly equal in all directions ensuing from the typical double-sigmoid course of berry development [26] unlike, for example in coleoptiles, which expand predominantly longitudinally [29]. The protective exocarp encasing the mesocarp is a common feature in all fruits; however, the physical properties of mesocarp vary [28]. For instance, the translucency of mesocarp in the healthy berries indicated that it is either largely free from intercellular air spaces or the free spaces are flooded with apoplastic sap. According to a recent study [30], it is the latter since the apoplast of grape berries accumulate solutes as part of their normal development to avoid fruit cracking/splitting. This is also
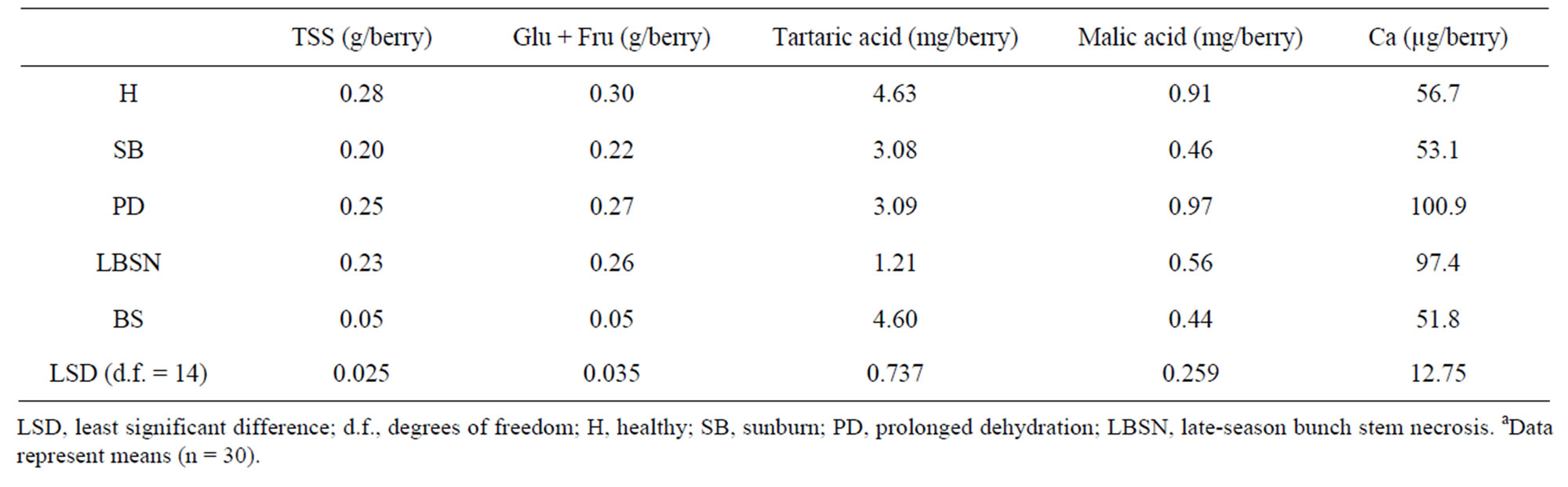
Table 2. Fruit composition of berries afflicted with different ripening disordersa.
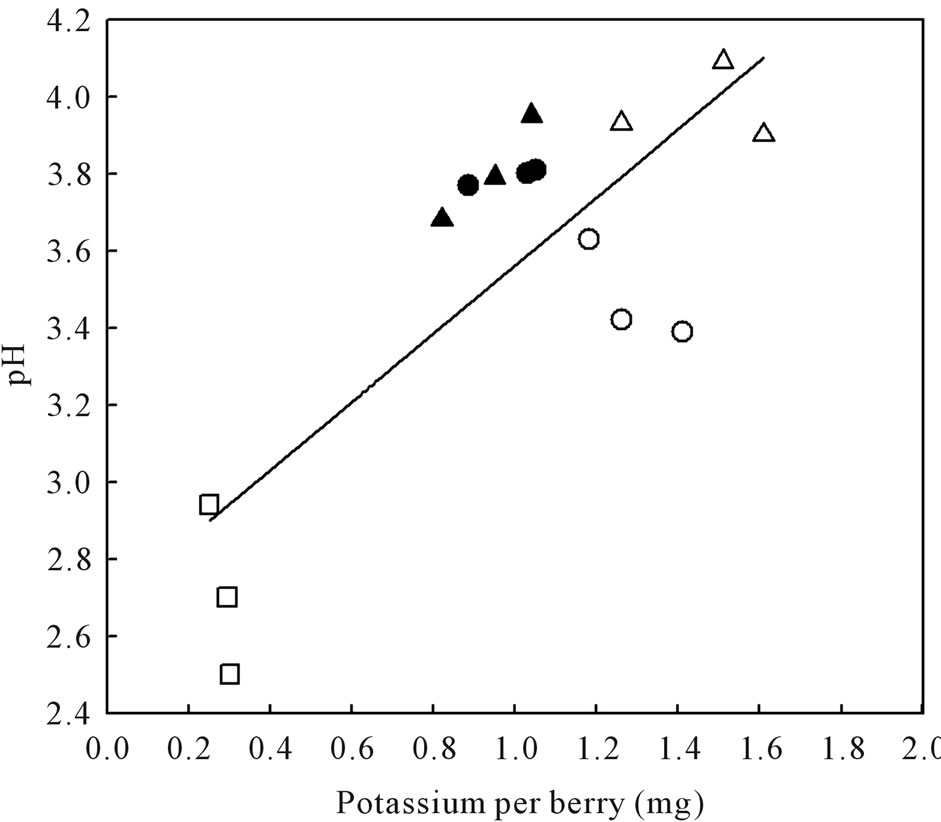
Figure 7. Relationship between pH and potassium content of healthy berries and berries afflicted with various ripening disorders. The regression analysis was made with the SPSS software. ○—Healthy berries, ●—Sunburn, ∆—PD, ▲—LBSN, and □—BS. y = 0.89x + 2.67, r2 = 0.63, P < 0.001.
a reflection of phloem unloading switching from symplastic to apolastic during veraison [31]. By contrast, although apple fruit utilizes an apoplasmic phloem unloading pathway [32] similar to grape [31], the flesh of apple has large proportion of intercellular air spaces, which reflect and diffuse light strongly and render the flesh appear white [33]. Furthermore, if the translucency increases due to an increase in volume of intercellular spaces, which generally occurs during storage, then it is considered a physiological disorder, e.g. apples afflicted with water core [34], or in pineapple wherein translucency of flesh is not at all a desired characteristic [35]. Conversely, the table grapes (e.g. Redglobe), which are same species as wine grapes but much firmer, have opaque colored flesh attributable to its high turgidity and firmness [36]. Despite these differences, the fleshy mesocarp in all fruits characterizes the most considerable fraction of their weight. For instance, as evident in this study, the mesocarp represented about 75% - 85% of the weight of which water accumulation accounts for about 70% - 80% of the weight [37]. Collectively, the growth of mesocarp and its water balance determined by supply of sap through peripheral vascular bundles, back flow to other organs, and by cuticular transpiration has dominant effect on the overall growth of the berry [13,31,38,39]. The same is true for apricots, peach, and apple and hence could be regarded as the general feature of the growth of many fleshy fruits [40] with a minor variation in grape berry in that the mesocarp is separated by the dorsal vascular bundles into inner and outer zones, the inner and outer mesocarp [41-43].
4.2. Cluster Framework and Composition of Healthy Berries
The proximal-to-distal decrease within the clusters of fruit as observed in the healthy clusters is a commonly observed phenomenon in plants that use inflorescence as reproductive structures and has frequently been attributed to competition among developing fruits for resources [44]. Nevertheless, all healthy berries shared similar physical characteristics and composition, wherein water supplied by ventral (axial) vascular bundles [22] predominantly contributed to their volumetric growth. Since along with water, soluble solids such as sugars, organic acids and mineral nutrients (Ca and K) are brought in, they too are integral components of growth [45]. This was evident in the healthy berry’s composition, which according to a recent study [46] corresponded to optimal range. Typically, in berries with such composition, the hexose sugars (glucose and fructose) accumulate solely in the course of expansive growth during ripening, whereas the accumulation of organic acids primarily tartaric and malic acids parallels with early growth triggered by both mitotic activity and cell expansion [4,13,20]. This type of accumulation pattern is also typical in other fruits accumulating sugars and acids such as apple [47] and peach [48]. However, unlike apple, a predominant accumulator of malic acid, and peach, which accumulate malic and citric acids as the dominant acids [40,48], grape berries accumulate the nonfermentable tartaric acid, and malic acid, the respiratory substrate, both accounting for 90% or more of the total acidity [13,49]. Between the two acids, 80% of the acid content is tartaric acid [13] with ~10% malic acid in mature grapes. These values are in complete agreement with the acidity observed in the healthy berries. The same is true for commercially important juice grape species such as V. labrusca and V. rotundifolia [49]. However, this acidic phenomenon of Vitis spp. lies in sharp contrast to other tartaric acid accumulating fruits such as loquat (Eriobotrya japonica Lindl.) wherein the malic acid predominates over tartaric acid [50].
Furthermore, the sugars and organic acids in the healthy berries accounted for the major portion of total soluble solids (TSS) similar to other fleshy fruits wherein it increases dramatically during ripening [40]. Because of this reason, sugar content and acid concentration are the properties generally utilized to determine fruit maturity in most species [28]. However, with respect to grapes, even though acids are present in TSS at maturity, it is the sugars (glucose and fructose) that represent major portion of TSS in wine grape berries, these are also the primary sugars in other grape species (V. labrusca and V. rotundifolia) and French hybrids [40]. Hence, the accumulation of hexoses is one of the main features of the ripening process in grape berries and is a major commercial consideration for the grape grower, winemaker and dried fruit producer [51]. Conversely, in apple and pears, fructose was found to be dominant sugar [52]. The peach is one of the few fruits in which the amount of sucrose exceeds the reducing sugar content [40].
Concomitant with an influx of water and sugars, a number of mineral elements move into fruits that contribute to their growth and development and organoleptic qualities. Because of this reason, K and Ca in particular were analyzed as these two act as the predominant cellular cations in most fruits including grape [13,53]. Between the two, K accumulation was higher indicating that K is a major component of the mineral pool in grape berries. It constitutes about 50% - 70% of the total cations and at harvest K in grape berries has been estimated to be approximately 60% of total vine K [54] as opposed to 85% in tomatoes [55]. The marked increase in K is a reflection of its multiple roles in fruit quality by influencing the growth and development of the berry. For instance, K contributes to charge balance and is involved in the translocation of solutes into the berry through its roles in phloem loading and unloading, which explains its strong correlation with changes in TSS [56]. From a viticultural and enological perspectives, K concentrations in grape berries is of great importance because its accumulation has a direct influence on the pH of the wine and tartrate stability [54]. Compared to K, Ca concentrations were low. This is due to its low mobility in the phloem [57]. Nevertheless, like K, Ca is involved in a range of fundamental physiological functions such as preserving cell membrane integrity, delaying senescence and abscission [58], and most importantly, it is the major cation integral to the pectin rich middle lamella conferring rigidity to cell wall [59]. With reference to ripening, maintenance of relatively high calcium concentrations in fruit tissue results in a slower rate of ripening, as seen in lower respiration rates, reduced ethylene production, and slower softening of fruit flesh [60].
4.3. Shriveling Pattern and Morpho-Anatomy of Disordered Berries
Wrinkles form when an applied compressive force acts on a rigid skin that rests on a softer foundation [61]. For example, the ripening disorders characterized in this study, all developed with a thin exocarp that surrounded a soft hydrated thick mesocarp. While they were ripening, the mesocarp was not maintained at full hydration causing reductions in volume and fresh weight. Consequently, there was too much of exocarp, which eventually shriveled reflecting compositional and textural changes referring to microand macro-structural characteristics of berries. Analysis of the shriveled exocarp led to the identification of four major ripening disorders with unique characteristics. These included: sunburn, PD, LBSN, and BS berries; each disorder exhibited a distinctive shriveling pattern indicating differential hydration properties of the mesocarp.
4.4. Sunburn
Even though the sunburned fruits were spherical, they dehydrated discernible as reduced volume revealing that despite protective covering in the form of upright epicuticular wax platelets, sunburn still occurs. The same is true in other fruit crops [62]. As evident from the wax morphology in this study, this was due to phase transition from crystalline to amorphous structure by intense sunlight and high temperature. Such transition also occurs in other sunburned fruits, for instance in sunburned apple [63]. These changes in the physical structure of epicuticular wax will enhance water loss from berries as crystalline form of wax serves as a formidable barrier to water permeability than amorphous wax [64] accounting for the weight (volume) loss in sunburned berries. Moreover, sunburned berries had reduced color development similar to tomato fruit [65] indicating that sunburn inhibited color by degrading anthocyanin, which occurs when mRNA transcription of anthocyanins biosynthetic genes is inhibited [66]. By contrast, sunburn caused brown lesions in white grape varieties [23] attributable to cell death in the epidermal layers of the exocarp [67]. On the other hand, in green pigmented fruits such as apple [68] and avocado [69], sunburn reduced photosynthetic activity. Although sunburn caused extensive damage to exocarp, the mesocarp visually appeared to be fairly intact. Despite no visual injury of mesocarp, gradients in tissue temperature, cell structure and shape affecting textural features between shaded and exposed surfaces of the berries are expected. For instance, in sun-exposed cantaloupe fruit, a temperature gradient existed among different tissues in the pericarp with the epidermis loading most and the central tissues least of the heat [70]. Higher flesh firmness has been found on the exposed surface of “Pacific Rose” apple [71] and avocado fruit [69]. Whether these differences are due to differing cell wall composition, cell number, or cell turgor properties are not known [72].
Regardless of the manner (persistent or sudden exposure) in which sunburn occurs in fruits, sunburn primarily results from their heat budget, i.e. the heat received or lost is linked to the environmental factors: radiation, wind, air humidity and air temperature; and its thermal and optical properties [73]. Of these meteorological variables, the two most important variables affecting temperature in grape berries include wind velocity and solar radiation with temperature increasing linearly with incident radiation [74]. Growth conditions that favor these factors and increase the propensity for sunburn in grapes include open canopies induced by deficit irrigation, leaf removal, and reduced use of nitrogen fertilizer [75]. Though increased sunlight exposure of grape clusters is generally believed to improve fruit composition [76]; however, if the concomitant increase in berry temperature exceeds a threshold temperature, then sunburn occurs. This threshold varies among different fruit crops, for instance, it is 45˚C - 49˚C for apple cultivars [77], which show different forms of sunburn. These include sunburn necrosis, sunburn browning and photoxidative sunburn, each occurring at a different temperature regime [78]. By contrast, grape berry sunburn does not have such classification except for sunburn occurring at different growth stages and exposed surface turning light brown in color [23]. Most importantly, grape berries do not tolerate such high temperatures as in apples and exhibit sunburn at a temperature about 40˚C [79] i.e. an increase of about 13˚C in berry temperature above the ambient temperature [75], slightly lower than watermelon, a pepo fruit whose upper pericarp heats to 15˚C [70].
4.5. Prolonged Dehydration
It is not uncommon for fruits including grapes to transpire and shrivel diurnally and seasonally with the stage of development [7,80]. Fruits undergo this rhythmic phenomenon as it lowers fruit water potential, thus creating the conditions for enhancing vascular import into the fruit [81]. For instance, it has been estimated that 60% - 95% of water enters through the phloem, while only 5% - 40% of water enters through the xylem, in fruits of tomatoes [82], grapes [83], apples [84], cereals [85], and legumes [86]. However, none of these fruit species (except for grapes) exhibit a prolonged transpiretional loss of water during advanced stages of ripening. Indeed these fruits shrivel, but these are short term diurnal shrinkage with no effect on fruit quality. Conversely, sustained transpirational water loss in Cabernet Sauvignon of this study is similar to Shiraz berries, which is known to lose about 7% of berry volume per day [87]. Although PD has been known to afflict Cabernet Sauvignon [88], comparatively, Shiraz is notorious for developing PD year after year in a range of climates [57] and under different irrigation regimes causing concentration of sugars and thus a reduction in the osmotic potential [89]. In comparison, apples under storage lose as little as a 5% of mass and develop an unattractive shriveled and wilted appearance eventually affecting texture [90]. Another interesting aspect of PD is that except Vitis spp., as yet, this form of shrivel has not been reported in other fruit crops, even though intact fruits of other species such as tomato exhibit enhanced water loss. However, the exocarp does not form the distinct pattern of indentations of dehydrated grape berries but rather wrinkles erratically [91]. Thus, the dimpled exocarp associated with berry transpiration can be considered to be a unique phenomenon exclusively of grape berries.
It is noteworthy that unlike the sunburned berries, PD occurred in the presence of a dense crystalline epicuticular wax; precisely how this might have occurred is unclear. The same is true for Shiraz berries [19]. These authors were of the opinion that either the composition of the cuticle and the epicuticular wax may be more important than the thickness or other factors are involved in determining transpiration rates. One of these other factors could be orientation of the wax platelets, which lie upright on the berry surface as opposed to horizontal on leaf surface [27] and thus it is possible that the berry surface is not completely sealed causing sustained water loss. Other authors quantified backflow from the berry (Shiraz and Chardonnay) to the parent vine via the xylem [87] indicating significant intraspecific variation in xylem back flow and perhaps other aspects of berry development. Nonetheless, the actual mechanisms of berry dehydration are still unresolved.
4.6. LBSN and EBSN
Of this unique assemblage of shrivel forms, LBSN is the most researched disorder known since the 1920’s [92]. The prominent feature of LBSN was the development of necrotic lesions, which have been reported to develop around the stomata destroying the guard cells, subsidiary cells, collenchyma and parenchyma tissues, and eventually the phloem tissues in the affected region of the cluster [93,94]. Furthermore, in the affected cells and cell walls, polyphenols are oxidized producing the typical visible necrotic areas wherein girdling occurs [95]. Following girdling of the rachis, dehydration of berries occurs due to loss of inflow of water via the phloem tissue and continued transpiration or xylem back flow. However, the rachis and pedicels below the girdled area would lose water much more rapidly on a weight-perunit-volume basis than do the berries due to high surface to volume ratio, and also because pedicels have high density of lenticels through which water vapor readily escapes. This explains the complete desiccation of rachis and its brown coloration, which either abscises [96] or remains on the cluster in a dry condition as observed in this study. The berries on the desiccated rachis would be forced to undergo drying due to a lack of xylem and phloem influx coupled with a continued loss of water from the berry cuticles. This scenario is identical to that occurs when fruits undergo drying wherein loss of water occurs from the vacuolar compartment of mesocarp tissues with minor changes in the water content of either the cytoplasm or the cell wall compartments causing cellular shrinkage [97]. This explains the collapse of parenchymatous mesocarp and the significant reduction in volume of the LBSN berries, which basically modified the global structure of the berry affecting their macroscopic characteristics. No other species develops LBSN or symptoms that closely resemble this ripening disorder. The only other fruit known to develop similar characteristics that may resemble some of the symptoms of LBSN include table grapes, which are the same species as wine grapes, wherein the rachis dries up and turns brown resulting berry shatter, wilting and shriveling of berries; however, these occur during postharvest handling of the berries [98] unlike LBSN. Even though a high concentration of sugars occurred in LBSN berries, generally they are not suitable for making wine, nevertheless such clusters can be harvested and used for making wines of different style depending upon its timing of inception.
Although LBSN damages phloem tissues [17,95], this study revealed that the xylem vessels developed tyloses possibly in a way similar to pruned grapevine stems that develop tyloses induced by biosynthesis of ethylene [99]. The extent of development and amount of obstruction caused by tyloses determines the formation of necrotic area [100]. Whether or not necrosis of inflorescence or necrosis-induced tylose formation occurs in inflorescence/cluster of fruits of other species is not known. Nevertheless a similar phenomenon, but not quite the same occurs naturally in pineapple after fruit formation wherein tylose-like structures form in the withered and necrotic stylar canals [101]. Likewise, walnut trees afflicted with apoplexy disorder develop extensive tylose formation throughout the crown with wilted and chlorotic scorched leaves of symptomatic trees [102].
On a whole plant level, LBSN has been attributed to wide range of vineyard conditions such as over-cropping, high humidity, Mg and/or Ca deficiency, ammonium toxicity, sever hedging, and cluster shading [14]. At the plant physiological level, the causal factors have been contradictory. According to one study [95], nitrogen and ammonium (NH4) have been the most consistent nutriational factors to be linked to LBSN. However, there are reports that show that both excessive and deficient concentrations of nitrogen as possible causes or cures [103]. Other nutrients that have been involved as either cause or correction are Ca, K, and Mg [14], which also account for physiological disorders in other fruit crops such as apple [77] and tomato [7]. These contradictory results suggest that additional abiotic forces work in tandem with nutrients in influencing LBSN.
Unlike LBSN, the remaining few berries on EBSN clusters developed normally. Both disorders initiate as necrosis of bunch stem; however, ontogenetic differences occur between the two. EBSN is an early-season disorder, whereas LBSN is a post-veraison disorder. Although temporal variation exists between the two, both forms are induced by necrotic lesions in the cluster indicating that both could be forms of the same disorder displayed at different developmental times [104]. Causes of EBSN are unknown but similar symptoms can be induced by cation sprays of Ca2+ and 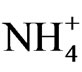 before flowering, but no response was found to K+ and Mg2+ [105].
before flowering, but no response was found to K+ and Mg2+ [105].
4.7. BS
BS is also known as SAD (Sugar Accumulation Disorder) [106], SOUR (Suppression of Uniform Ripening) berry [24], and Traubenwelke in Germany [22] and had the smallest volume of all shrivel forms. Increase in fruit volume largely results from the development of pericarp tissues achieved through two important cellular processes: the production of new cells and cell expansion [107]. Since BS initiates after veraison [106], the volume reduction is mostly due to a lack of cell expansion brought about by sugar-driven turgor pressure [13] rather than cuticular transpirational loss of water alone. Even though the exocarp maintained its structural integrity in BS berries, the berries as a whole lacked firmness indicating a collapsed mesocarp wherein the parenchyma cells lost their membrane integrity leading to loss of cell vitality [43,88] and eventually turgor pressure [15]. A similar scenario occurs in pears afflicted with internal browning, a physiological disorder wherein the leaking of cell liquid into the intercellular space following membrane damage reduced the gas diffusivity [108]. In grape berries such as Chardonnay and Shiraz, leakiness of cell membranes in the mesocarp and breaking down of cell compartmentation have been reported but this generally occurs at or near the time when the berries attain maximum weight and continue to decline until normal harvest dates, not at the inception of ripening [38]. What may trigger BS is not known at this time. Thus far, this is the only species known to exhibit this kind of problem. Even though BS berries have viable seeds [17], they cannot be used for making wine or any other product due to low sugars, and even the birds are not attracted to them. Hence berries afflicted with BS cannot serve as a profitable reproductive strategy for seed dispersal in vine’s natural setting. Furthermore, even though the inflorescence framework appeared visually healthy, cell death do occurs in the rachis axil [17].
4.8. Physical Changes in Disordered Berries
During post-veraison, cell expansion ensuing predominantly from water accumulation is solely responsible for the volumetric growth, which eventually determines final yield and fruit quality [13]. So, considering the low respiration rate and the nonclimateric nature of grapes [45], it is logical to delineate the significant reductions in fresh weight and volume accompanied by shriveling of pericarp in clusters of sunburn, PD, and LBSN in the context of water loss. While they all shriveled (except sunburn), the distinctive topography of the pericarp was a strong reflection of a divergent water flux pattern. For instance, the PD was a manifestation of an imbalance between inflows of fluids from its dorsal vascular system fed through the pedicel xylem and phloem and outflows through the exocarp ensuing from parenchyma cells in the outer mesocarp. In other words, water moving through the dehydrating grape berry followed the peripheral vascular bundles in the outer mesocarp diffused through the exocarp and evaporated on the surface. Since turgor pressure is responsible for cell shape and fruit firmness [109,110], the dehydration-induced dimpling of the exocarp unequivocally ensued from loss of turgor and perhaps plasmolysis in the outer mesocarp cells. In such situations, a reduction in tissue and cell volumes occur, but their cell contents including sugars concentrate, which increases cellular osmotic stress [18]. Then again, the smooth appearance of the exocarp in the sunburned berries indicated that despite water loss, some influx into the berry may have occurred. This contrasts with LBSN berries wherein the influx into the berry was completely cut off after the rachis had been girdled following necrosis forcing the berry mesocarp to undergo drying with the end result being the formation of raisins. A plausible scenario like this might not have occurred in the BS, sunburned, and PD berries as their cluster framework retained chlorophyllous tissues whose internal structures were analogous to healthy cluster framework. Quite the contrary occurred in the flaccid berries of BS. Since phloem influx serves as the predominant source of water and solutes for the ripening berries to gain weight and volume after veraison [111], and the fact that BS berries accumulated meager amount of solutes (sugars), their shriveling phenomenon reflected more of a loss of phloem functionality after verasion [17,88] rather than sole involvement of water efflux either by cuticular transpiration or backflow into the vine as suggested for PD berries. Accordingly, the flaccidity of the pericarp originnated from a lack of continual turgor pressure required for expansive growth [112] and sugar accumulation [83], which failed to occur due to a decrease in mesocarp cell viability [106].
4.9. Compositional Changes in Disordered Berries
From a quality perspective, fruit water loss is not a desirable characteristic as water loss not only renders the fruits unattractive and affects texture, but also causes large and significant changes in the metabolism and composition of fruits and vegetables [113]. Particularly in grapes, weight loss impacts sugar metabolism and flavor development [114], mineral nutrient concentrations [57], and the practical estimation of final yields by viticulturists [18]. For instance, even with no visual injury, berry volume (weight), TSS, Glucose + Fructose, and acids declined in sunburned berries analogous to heat-exposed Cardinal and Pinot noir [115] and Semillon [116] grapes, and sunburned apple [77]. Taken together, these studies reinforce the fact that fruit composition during ripening is primarily altered by temperature. In grape berries, the temperature effect on composition is usually described in respiratory context [117]. So, when berries respire, malic acid diffuses from the interior to the periphery where it is metabolized [118]. If the berry temperature rises during ripening, then concomitant increases in malic acid respiration also occur [117], which accounts for the low acidity of the sunburned berries. Such phenomenon implicitly suggests up-regulation of respiration as a temperature-sensitive malate metabolic pathway, due to the involvement of malate in this pathway during ripening [119]. Similar phenomenon ensuing from temperature-induced malic acid degradation occurs in peach [120] and apple [52]. In addition to dramatic decreases in malic acid, the sugar content also decreased in sunburned berries. This sensitivity of sugar accumulation to temperature is not clearly understood [121]; however, the heat-induced impediment of sugar accumulation has been attributed to several factors including restriction of phloem unloading into the bunch, reduced transport of sugars to the bunch or reduced supply at the source [116]. Grapes that are likely to develop composition similar to the ones observed in sunburned berries generally belong to warmer viticultural regions. Such grapes if used for winemaking, produce unbalanced, flat wines that are more susceptible to oxidative and microbial spoilage [122]. As opposed to sunburned berries, the low malic acid in BS berries was associated with their flaccidity. In such situations, tonoplast leakage is likely to increase as membranes have been proposed to be the sites of damage [123] and when it does, the leakage becomes prime cause of malate vacuolar decompartmentation resulting changes in malic acid [124].
As a result of malic acid breakdown, pH of the cell sap increases during ripening [125] and becomes abnormally high in sunlit berries [126]. This explains the high pH of sunburned berries, which was same as reported for sunexposed Merlot berries [127]. The next highest pH occurred in the LBSN berries attributable unequivocally to its low malic acid concentrations. What precisely degraded malic acid in LBSN berries is unknown. However, in view of increased respiration during tissue necrosis [128], it is possible that the necrosis of rachis may have accelerated malate respiration in the berries. Alternatively, since malic acid serves as an intermediate in many metabolic biochemical pathways [96], there is a strong possibility that malic acid was metabolized through a non-respiratory means indicating an altered ripening pattern rather than a failure to ripen. Other than sunlight [126], pH of the juice is strongly affected by K concentrations which reduces free tartaric acid resulting in an increase in pH [54]. This accounted for the highest pH in the PD berries. On the other hand, such high K concentrations in the PD berries would decrease the rate of malic acid degradation through malate respiration resulting in an increase in their malic acid as observed in this study. This is generally achieved by impeding malate transfer from the vacuole storage pools to the cytoplasm, the site of malate degradation [129]. Conversely, the lowest pH in BS berries was associated with their low K content and high amounts of significantly stronger tartaric acid whose synthesis has been reported to be sensitive to day temperature and light intensity [117]. Thus, except for BS berries, the low tartaric acids in other shriveled, especially the LBSN berries indicated that they either developed a reduced ability to synthesize tartaric acids with maturity or incurred reduced translocation from leaves. Other possibility is the altered metabolic processes in the berries [96] involving the conversion of tartaric to malic acid as it has been reported to occurs in grape leaves [130] indicating that tartaric acid is not biochemically inert [131].
The high Ca content in LBSN and dehydrated berries indicated that despite the disordered ripening, Ca content continued to accumulate whereas it slowed in healthy and other shriveled berries. The same was true for K accumulation only in dehydrated berries, not in LBSN berries. The slow accumulation of K and continued accumulation of Ca in LBSN berries during ripening is similar to the accumulation patterns reported in waterberries (LBSN) of Thompson Seedless [96,103] and Riesling [132]. Apparently, the continued xylem transport of Ca constitutes a significant feature of LBSN berries. Then again, it is not clear as to how this phenomenon might relate to the concurrent occurrence of rachis necrosis of this disorder. One possibility is that the rachis symptoms are the result, not the cause, of altered metabolic processes in the fruit [103]. The relatively exorbitant concentrations of both Ca and K in PD berries provided compelling evidence of a continuous vascular influx of these ions from the parent grapevine into enlarging berries until probably the maximum berry fresh weight occurred. The berries of grape cultivar, Shiraz afflicted with PD exhibited a similar pattern [57]. Since the vascular flow, especially the phloem accelerates at veraison [133], further accumulations of these ions continued by means of a sustained accelerated vascular flow despite late-season dehydration. Whether this was driven by increased transpiration (dehydration), sugar concentration, energized transport system, or some other factor is not known. Nevertheless, for the same disorder in Shiraz grape, berries continued to accumulate K and Ca by gaining an access via some non-vascular connection [57]. Based on these observations, it is reasonable to infer that a cessation of vascular flow to the berry may not contribute to prolonged dehydration of either variety. On the other hand, the vascular system in BS berries did not function to its fullest capacity resulting low accumulation of sugars (glucose and fructose) and K, and it all culminated into flaccidity of berries. This is because, massive accumulation of glucose and fructose, and K along with water drives post-veraison cell and fruit expansion by generating turgor pressure [13,54,134]. Since in wine grapes, sugar (glucose and fructose) concentration determines alcohol level, and shapes the flavor profile in the final wine [13, 135], the clusters afflicted with BS are culled at harvest by dropping them on the ground. Generally, the surge of accumulation in grape berries plateaus at concentrations of 0.18 g to 0.25 g of sugar per gram fresh weight or 0.30 g in some small berries [45]. This explains that the higher Brix concentrations in PD, LBSN, and sunburned berries primarily originated from a concentration effect following continual loss of water. Since these berries have high concentrations of sugars, they can be used for making certain style of wine.
To conclude, during fruit growth and ripening, there is a high risk of physiological disorders, which reduce organoleptic qualities of fruits necessitating examination of ripening maladies from morpho-anatomical and compositional perspectives as a step toward making progress on their understanding and eventually sustaining fruit quality. This relatively in-depth concurrent analysis of all physiological disorders of grape berries associated with ripening is unprecedented in terms of the range and combination of analytical techniques.The analyses revealed four major forms of shrivel wherein each shrivel form possessed distinctive appearance owing to their unique shriveling pattern, morpho-anatomy, and compositional profile with the most drastic changes occurring in the berries afflicted with BS. Unlike BS berries, the sunburned, PD, and LBSN berries concentrated sugars but their acid concentrations were lower than BS berries. Because of the pronounced shriveling patterns in each form, it is possible to predict their compositional characteristics without subjecting them to analysis and thus a decision can be made in the field as to which clusters to harvest for making wine or other products. Pinning down the precise causal factors of these physiological ripening disorders is a challenge; however, this work represents an exciting leap forward as these ripening disorders have hardly ever been explored en masse in a given variety during a single growing season. The information provided in this study has considerable implications for gaining insights into the elusive physiological and molecular manifestations of ripening processes in grapes leading toward the development of production practices that can minimize the incidences of these disorders. Hence, the results are of vital interest for viticulturists and grape industry since the reported analyses of differential shrivel forms not only serve as a guide to correctly identify seemingly similar disorders but also aid them in minimizing fruit quality and yield losses.
5. Acknowledgements
Financial support for this study was provided by the Washington State Grape and Wine Research Program. The authors would like to thank Sushan Ru and Katherine Wang for their assistance with sampling of the clusters and Mr. Bruce Arey of EMSL microscopy facility for technical assistance.
REFERENCES
- L. Adams-Phillips, C. Barry and J. Giovannoni, “Signal Transduction Systems Regulating Fruit Ripening,” Trends in Plant Science, Vol. 9, No. 7, 2004, pp. 331-338. doi:10.1016/j.tplants.2004.05.004
- C. J. Brady, “Fruit Ripening,” Annual Review of Plant Physiology, Vol. 38, 1987, pp. 155-178. doi:10.1146/annurev.pp.38.060187.001103
- R. Dudley, “Ethanol, Fruit Ripening, and Historical Origins of Human Alcoholism in Primate Frugivory,” Integrative and Comparative Biology, Vol. 44, No. 9, 2004, pp. 315-323. doi:10.1093/icb/44.4.315
- C. Conde, P. Silva, N. Fontes, A. C. P. Dias, R. M. Tavares, M. J. Sousa, A. Agasse, S. Delrot and H. Gerós, “Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality,” Food, Vol. 1, No. 1, 2007, pp. 1-22.
- C. B. Watkins, “The Effect of 1-MCP on the Development of Physiological Storage Disorders in Horticultural Crops,” Stewart Postharvest Review, Vol. 2, No. 11, 2007, pp. 1-6. doi:10.2212/spr.2007.2.11
- B. Ehsani-Moghaddam and J. DeEll, “Correlation and Path-Coefficient Analyses of Ripening Attributes and Storage Disorders in ‘Ambrosia’ and ‘Empire’ Apples,” Post Harvest Biology and Technology, Vol. 51, 2009, pp. 168-173. doi:10.1016/j.postharvbio.2008.07.006
- S. Guichard, N. Bertin, C. Leonard and C. Gary, “Tomato Fruit Quality in Relation to Water and Carbon Fluxes,” Agronomie, Vol. 21, No. 2, 2001, pp. 385-392. doi:10.1051/agro:2001131
- M. J. Ceponis, R. A. Cappellini, J. M. Wells and G. W. Lightner, “Disorders in Plum, Peach, and Nectarine Shipments to the New York Market,” 1972-1985,” Plant Disease, Vol. 71, No. 10, 1987, pp. 947-952.
- R. Singh, R. R. Sharma and S. K. Tyagi, “Pre-Harvest Foliar Application of Calcium and Boron Influences Physiological Disorders, Fruit Yield and Quality of Strawberry (Fragaria × ananassa Duch.),” Scientia Horticulturae, Vol. 112, No. 2, 2007, pp. 215-220. doi:10.1016/j.scienta.2006.12.019
- O. Jaillon, J. M. Aury, B. Noel, et al., “The Grapevine Genome Sequence Suggests Ancestral Hexaploidization in Major Angiosperm Phyla,” Nature, Vol. 449, No. 7162, 2007, pp. 463-468. doi:10.1038/nature06148
- S. Pilati, M. Perazzolli, A. Malossini, A. Cestaro, L. Demattè, P. Fontana, A. D. Ri, R. Viola, R. Velasco and C. Moser, “Genome-Wide Transcriptional Analysis of Grapevine Berry Ripening Reveals a Set of Genes Similarly Modulated during Three Seasons and the Occurrence of an Oxidative Burst at Vèraison,” BMC Genomics, Vol. 8, No. 428, 2007.
- M. Giribaldi and M. M. Giuffrida, “Heard It through the Grapevine: Proteomic Perspective on Grape and Wine,” Journal of Proteomics, Vol. 73, No. 9, 2010, pp. 1647- 1655. doi:10.1016/j.jprot.2010.05.002
- B. G. Coombe, “Research on Development and Ripening of the Grape Berry,” American Journal of Enology and Viticulture, Vol. 43, No. 1, 1992, pp. 101-110.
- E. Hughes, A. Reynolds and B. Bondada, “Bunch Stem Necrosis,” Wine East, March-April, Vol. 35, pp. 18-25.
- M. Krasnow, N. Weis, R. J. Smith, M. J. Benz, M. A. Matthews and K. A. Shackel, “Inception, Progression, and Compositional Consequences of a Berry Shrivel Disorder,” American Journal of Enology and Viticulture, Vol. 60, No. 1, 2009, pp. 24-34.
- M. Keller, “The Science of Grapevines—Anatomy and Physiology,” Elsevier, Academic Press, Burlington, 2010.
- G. Hall, B. R. Bondada and M. Keller, “Loss of Rachis Cell Viability Is Associated with Ripening Disorders in Grapes,” Journal of Experimental Botany, Vol. 62, No. 3, 2011, pp. 1145-1153. doi:10.1093/jxb/erq355
- M. G. McCarthy, “Weight Loss from Ripening Berries of Shiraz Grapevines (Vitis vinifera L. cv. Shiraz),” Australian Journal of Grape and Wine Research, Vol. 5, No. 1, 1999, pp. 10-16. doi:10.1111/j.1755-0238.1999.tb00145.x
- S. Y. Rogiers, J. M. Hatfield, V. G. Jaudzems, R. G. White and M. Keller, “Grape Berry cv. Shiraz Epicuticular Wax and Transpiration during Ripening and Preharvest Weight Loss,” American Journal of Enology and Viticulture, Vol. 55, No. 2, 2004, pp. 121-127.
- M. A. Matthews and K. A. Shackel, “Growth and Water Transport in Fleshy Fruit,” In: N. M. Holbrook and M. A. Zwieniecki, Eds., Vascular Transport in Plants, Elsevier, Academic Press, Boston, 2005, pp. 189-197. doi:10.1016/B978-012088457-5/50011-3
- H. A. A. Hifny and G. Alleweldt, “Untersuchungen zur Stiellähme der Reben. I. Die Symptomatologie der Krankhei,” Vitis, Vol. 10, 1972, pp. 298-313.
- M. Knoll and D. Achleitner, “Sugar Accumulation in Zweigelt Grapes as Affected by Traubenwelke,” Vitis, Vol. 49, No. 3, 2010, pp. 101-106.
- M. N. Krasnow, M. A. Matthews, R. J. Smith, J. Benz, E. Weber and K. A. Shackel, “Distinctive Symptoms Differentiate Four Common Types of Berry Shrivel Disorder in Grape,” California Agriculture, Vol. 64, No. 3, 2010, pp. 155-159. doi:10.3733/ca.v064n03p155
- B. Bondada, “To Shrivel or Not to Shrivel—Toward an Understanding of Ripening Related Physiological Disorders of Grape Berry,” Proceedings of the International Symposium GiESCO, Asti-Alba, 2011, pp. 473-475.
- S. E. Ruzin, “Plant Microtechnique and Microscopy,” Oxford University Press, Oxford, 1999.
- W. J. Hardie, T. P. O’Brien and V. G. Jaudzems, “Morphology, Anatomy and Development of the Pericarp after Anthesis in Grape, Vitis vinifera L.,” Australian Journal of Grape and Wine Research, Vol. 2, No. 2, 1996, pp. 97-142. doi:10.1111/j.1755-0238.1996.tb00101.x
- B. R. Bondada, “Anomalies in Structure, Growth Characteristics, and Nutritional Composition as Induced by 2, 4-D Drift Phytotoxicity in Grapevine (Vitis vinifera L.) Leaves and Clusters,” Journal of the American Society for Horticultural Science, Vol. 136, No. 3, 2011, pp. 165- 176.
- B. G. Coombe, “The Development of Fleshy Fruits,” Annual Review of Plant Physiology, Vol. 27, 1976, pp. 507-528. doi:10.1146/annurev.pp.27.060176.001231
- R. I. Grange, “Water Relations and Growth of Tomato Pericarp Tissue,” Plant Cell and Environment, Vol. 18, No. 11, 1995, pp. 1311-1318. doi:10.1111/j.1365-3040.1995.tb00190.x
- H. Wada, K. A. Shackel and M. A. Matthews, “Fruit Ripening in Vitis vinifera: Apoplastic Solute Accumulation Accounts for Pre-Veraison Turgor Loss in Berries,” Planta, Vol. 227, No. 6, 2008, pp. 1351-1361. doi:10.1007/s00425-008-0707-3
- L. Y. Zhang, Y. B. Peng, S. Pelleschi-Travie, Y. Fan, Y. F. Lu, Y. M, Lu, X. P. Gao, Y. Y. Shen, S. Delrot and D. P. Zhang, “Evidence for Apoplasmic Phloem Unloading in Developing Apple Fruit,” Plant Physiology, Vol. 135, No. 1, 2004, pp. 574-586. doi:10.1104/pp.103.036632
- X. Y. Zhang, X. L. Wang, X. Wang, G. H. Xia, Q. H. Pan, R. C. Fan, F. Q. Wu, X. C. Yu and D. P. Zhang, “A Shift of Phloem Unloading from Symplasmic to Apoplasmic Pathway Is Involved in Developmental Onset of Ripening in Grape Berry,” Plant Physiology, Vol. 1, No. 142, 2006, pp. 220-232. doi:10.1104/pp.106.081430
- D. S. Skene, “The Fine Structure of Apple, Pear, and Plum Fruit Surfaces, Their Changes during Ripening, and Their Responses to Polishing,” Annals of Botany, Vol. 27, No. 4, 1963, pp. 581-587.
- J. H. Bowen and C. B. Watkins, “Fruit Maturity, Carbohydrate and Mineral Content Relationships with Watercore in ‘Fuji’ Apples,” Postharvest Biology and Technology, Vol. 11, No. 1, 1997, pp. 31-38. doi:10.1016/S0925-5214(97)01409-9
- C. P. Sideris and B. H. Krauss, “Physiological Studies on the Factors Influencing the Quality of Pineapple Fruits. I. Physicochemical Variations in the Tissue of Ripe Pineapple Fruits,” Pineapple Quarterly, Vol. 3, 1933, pp. 82- 114.
- B. W. du Plessis, “Cellular Factors That Affect Table Grape Berry Firmness,” MS Thesis, Stellenbosch University, Stellenbosch, 2008.
- S. M. Glidewell, B. Williamson, B. A. Goodman, J. A. Chudek and G. Hunter, “An NMR Microscopic Study of Grape (Vitis vinifera L.),” Protoplasma, Vol. 198, No. 1-2, 1997, pp. 27-35. doi:10.1007/BF01282128
- J. Tilbrook and S. D. Tyerman, “Cell Death in Grape Berries: Varietal Differences Linked to Xylem Pressure and Berry Weight Loss,” Functional Plant Biology, Vol. 35, No. 3, 2008, pp. 173-184. doi:10.1071/FP07278
- M. Keller, J. P. Smith and B. R. Bondada, “Ripening Grape Berries Remain Hydraulically Connected to the Shoot,” Journal of Experimental Botany, Vol. 57, No. 11, 2006, pp. 2577-2587. doi:10.1093/jxb/erl020
- J. F. Gallander, “Chemistry of Grapes and Other Fruits as the Raw Materials Involved in Winemaking,” In: A. D. Webb, Ed., Advances in Chemistry Series No. 137, American Chemical Society, Washington DC, 1974, pp. 11-49.
- M. Fougere-Rifot, H. S. Park and J. Bouard, “Berry Pericarp Ontogenesis from Fertilization to Maturity of Vitis vinifera L. var. Merlot,” The Journal International des Sciences de la Vigne et du Vin, Vol. 31, No. 3, 1997, pp. 109-118.
- B. R. Bondada, M. A. Matthews and K. A. Shackel, “Functional Xylem Exists in Post-Veraison Grape Berry,” Journal of Experimental Botany, Vol. 56, No. 421, 2005, pp. 2949-2957. doi:10.1093/jxb/eri291
- B. Bondada and M. Keller, “Morpho-Anatomical Symptomatology and Osmotic Behavior of Grape Berry Shrivel,” Journal of the American Society for Horticultural Science, Vol. 137, No. 1, 2012, pp. 1-11.
- P. K. Diggle, “Architectural Effects and the Interpretation of Patterns of Fruit and Seed Development,” Annual Review of Ecology and Systematics, Vol. 26, 1995, pp. 531- 552. doi:10.1146/annurev.es.26.110195.002531
- B. G. Coombe, “Grape Berry as a Sink,” Acta Horticulturae, Vol. 239, 1989, pp. 149-158.
- Z. W. Dai, P. Vivin, F. Barrieu, N. Ollat and S. Delrot, “Physiological and Modelling Approaches to Understand Water and Carbon Fluxes during Grape Berry Growth and Quality Development: A Review,” Australian Journal of Grape and Wine Research, Vol. 16, No. S1, 2010, pp. 70-85. doi:10.1111/j.1755-0238.2009.00071.x
- J. Ackermann, M. Fischer and R. Amado, “Changes in Sugars, Acids, and Amino Acids during Ripening and Storage of Apples (cv. Glockenapfel),” Journal of Agricultural and Food Chemistry, Vol. 40, No. 7, 1992, pp. 1131-1134. doi:10.1021/jf00019a008
- K. Boudehri, A. Bendahmane, G. Cardinet, C. Troadec, A. Moing and E. Dirlewanger, “Phenotypic and Fine Genetic Characterization of the D Locus Controlling Fruit Acidity in Peach,” BMC Plant Biology, Vol. 9, 2009, Article No. 59. doi:10.1186/1471-2229-9-59
- W. M. Kliewer, “Concentration of Tartrates, Malates, Glucose and Fructose in the Fruits of the Genus Vitis,” American Journal of Enology and Viticulture, Vol. 18, 1967, pp. 87-96.
- A. Amorós, P. Zapata, M. T. Pretel, M. A. Botella and M. Serrano, “Physico-Chemical and Physiological Changes during Fruit Development and Ripening of Five Loquat (Eriobotrya japonica Lindl.) Cultivars,” Food Science and Technology International, Vol. 9, No. 1, 2003, pp. 43-51. doi:10.1177/1082013203009001007
- C. Davies and S. P. Robinson, “Sugar Accumulation in Grape Berries. Cloning of Two Putative Vacuolar Invertase cDNAs and Their Expression in Grapevine Tissues,” Plant Physiology, Vol. 111, No. 1, 1996, pp. 275-283. doi:10.1104/pp.111.1.275
- G. E. Neal and A. C. Hulme, “The Organic Acid Metabolism of Bramley’s Seedling Apple Peel,” Journal of Experimental Botany, Vol. 9, No. 1, 1958, pp. 142-157. doi:10.1093/jxb/9.1.142
- S. Y. Rogiers, D. H. Greer, J. M. Hatfield, B. Orchard and M. Keller, “Mineral Sinks Within Ripening Grape Berries,” Vitis, Vol. 45, No. 3, 2006, pp. 115-123.
- B. Mpelasoka, D. P. Schachtman and M. T. Treeby, “A Review of Potassium Nutrition in Grapevines with Special Emphasis on Berry Accumulation,” Australian Journal of Grape and Wine Research, Vol. 9, No. 3, 2003, pp. 154-168. doi:10.1111/j.1755-0238.2003.tb00265.x
- D. B. Bradley, “Varietal and Location Influence on Acid Composition of Tomato Fruit,” Agricultural and Food Chemistry, Vol. 12, No. 3, 1964, pp. 213-216. doi:10.1021/jf60133a006
- A. Lang, “Turgor Regulated Translocation,” Plant, and Cell & Environment, Vol. 6, No. 9, 1983, pp. 683-689.
- S. Rogiers, M. Keller, B. Holzapfel and J. M. Virgona, “Accumulation of Potassium and Calcium by Ripening Berries on Field Vines of Vitis vinifera (L.) cv. Shiraz,” Australian Journal of Grape and Wine Research, Vol. 6, 2000, pp. 240-243. doi:10.1111/j.1755-0238.2000.tb00184.x
- F. J. M. Maathuis, “Physiological Functions of Mineral Macronutrients,” Current Opinion in Plant Biology, Vol. 12, No. 3, 2009, pp. 250-258. doi:10.1016/j.pbi.2009.04.003
- K. J. Nunan, I. M. Sims, A. Bacic, S. P. Robinson and G. B. Fincher, “Isolation and Characteristics of the Mesocarp of the Mature Grape Berries (Vitis vinifera L.),” Planta, Vol. 203, No. 1, 1997, pp. 90-100.
- I. B. Ferguson, “Calcium in Plant Senescence and Fruit Ripening,” Plant, Cell and Environment, Vol. 7, No. 6, 1984, pp. 477-489. doi:10.1111/j.1365-3040.1984.tb01438.x
- J. Huang, M. Juszkiewicz, W. H. de Jeu, E. Cerda, T. Emrick, N. Menon and T. P. Russell, “Capillary Wrinkling of Floating Thin Polymer Films,” Science, Vol. 317, No. 650, 2007, pp. 650-653. doi:10.1126/science.1144616
- H. E. Nordby and R. E. McDonald, “Variations in Chilling Injury and Epicuticular Wax Composition of White Grapefruit with Canopy Position and Fruit Development during the Season,” Journal of Agriculture and Food Chemistry, Vol. 43, No. 7, 1995, pp. 1828-1833. doi:10.1021/jf00055a015
- D. Knuth and R. Stosser, “Comparison of the Sun-Exposed and Shaded Side of Apple Fruit I. Cuticle, Epidermal Cell Size and Surface Waxes,” Gartenbauwissenschaft, Vol. 52, 1987, pp. 49-57.
- J. V. Possingham, “Surface Wax Structure in Fresh and Dried Sultana Grapes,” Annals of Botany, Vol. 36, No. 5, 1972, pp. 993-996.
- C. A. Torres, P. K. Andrews and N. M. Davies, “Physiological and Biochemical Responses of Fruit Exocarp of Tomato (Lycopersicon esculentum Mill.) Mutants to Natural Photo-Oxidative Conditions,” Journal of Experimental Botany, Vol. 57, No. 9, 2006, pp. 1933-1947. doi:10.1093/jxb/erj136
- K. Mori, N. Goto-Yamamoto, M. Kitayama and K. Hashizume, “Loss of Anthocyanins in Red Wine Grape Varieties under High Temperature,” Journal of Experimental Botany, Vol. 58, No. 8, 2007, pp. 1935-1945. doi:10.1093/jxb/erm055
- D. H. Greer, S. Y. Rogiers and C. C. Steel, “Susceptibility of Chardonnay Grapes to Sunburn,” Vitis, Vol. 45, No. 3, 2006, pp. 147-148.
- J. N. Wünsche, J. W. Palmer and D. H. Greer, “Effect of Crop Load on Fruiting and Gas Exchange Characteristics of ‘Braeburn’/M.26 Apple Trees at Full Canopy,” Journal of the American Society for Horticultural Science, Vol. 125, No. 1, 2000, pp. 93-99.
- A. B. Woolf, J. H. Bowen and I. B. Ferguson, “Preharvest Exposure to the Sun Influences Postharvest Responses of ‘Hass’ Avocado Fruit,” Postharvest Biology and Technology, Vol. 15, No. 2, 1999, pp. 143-153. doi:10.1016/S0925-5214(98)00077-5
- C. A. Schroeder, “Temperature Relations in Fruit Tissues under Extreme Conditions,” Proceedings of the American Society for Horticultural Science, Vol. 87, 1965, pp. 199- 203.
- L. U. Opara and T. Tadesse, “Fruit Growth and Mineral Element Accumulation in Pacific Rose Apple in Relation to Orchard Management Factors and Calyx-End Splitting,” Journal of Plant Nutrition, Vol. 23, No. 8, 2000, pp. 1079-1093. doi:10.1080/01904160009382083
- A. B. Woolf and I. B. Ferguson, “Postharvest Responses to High Fruit Temperatures in the Field,” Postharvest Biology and Technology, Vol. 21, No. 1, 2000, pp. 7-20. doi:10.1016/S0925-5214(00)00161-7
- M. Saudreau, H. Sinoquet, O. Santin, et al., “A 3D Model for Simulating the Spatial and Temporal Distribution of Temperature within Ellipsoidal Fruit,” Agricultural and Forest Meteorology, Vol. 147, No. 1-2, 2007, pp. 1-15. doi:10.1016/j.agrformet.2007.06.006
- R. E. Smart and T. R. Sinclair, “Solar Heating of Grape Berries and Other Spherical Fruit,” Agricultural Meteorology, Vol. 17, No. 4, 1976, pp. 241-259. doi:10.1016/0002-1571(76)90029-7
- S. E. Spayd, J. M. Tarara, D. L. Mee and J. C. Ferguson, “Separation of Sunlight and Temperature Effects on the Composition of Vitis vinifera cv. Merlot Berries,” American Journal of Enology and Viticulture, Vol. 53, No. 3, 2002, pp. 171-182.
- R. E. Smart, “Principles of Grapevine Canopy Microclimate Manipulation with Implications for Yield and Quality. A Review,” American Journal of Enology and Viticulture, Vol. 36, No. 3, 1985, pp. 230-239.
- L. E. Schrader, J. Zhang, J. Sun, J. Xu, D. C. Elfving and C. Kahn, “Postharvest Changes in Internal Fruit Quality in Apples with Sunburn Browning,” Journal of the American Society for Horticultural Science, Vol. 134, No. 1, 2009, pp. 148-155.
- D. A. Felicetti and L. E. Schrader, “Changes in Pigment Concentrations Associated with the Degree of Sunburn Browning of ‘Fuji’ Apple,” Journal of the American Society for Horticultural Science, Vol. 133, No. 1, 2008, pp. 27-34.
- A. J. Winkler, J. A. Cook, W. M. Kliewer and L. A. Lider, “General Viticulture,” University of California Press, Berkeley, 1974.
- M. D. Greenspan, K. A. Shackel and M. A. Matthews, “Developmental Changes in the Diurnal Water Budget of the Grape Berry Exposed to Water Deficits,” Plant Cell Environment, Vol. 17, No. 7, 1994, pp. 811-820. doi:10.1111/j.1365-3040.1994.tb00175.x
- L. M. McFadyen, R. J. Hutton and E. W. R. Barlow, “Effects of Crop Load in Fruit Water Relations and Fruit Growth in Peach,” Journal of Horticultural Science, Vol. 71, No. 3, 1996, pp. 469-480.
- L. C. Ho, R. I. Grange and A. J. Picken, “An Analysis of the Accumulation of Water and Dry Matter in Tomato Fruit,” Plant, Cell and Environment, Vol. 10, No. 2, 1987, pp. 157-162.
- A. Lang and M. R. Thorpe, “Xylem, Phloem and Transpiration Flows in a Grape: Application of a Technique for Measuring the Volume of Attached Fruits to High Resolution Using Archimedes Principle,” Journal of Experimental Botany, Vol. 40, No. 219, 1989, pp. 1069- 1078. doi:10.1093/jxb/40.10.1069
- A. Lang, “Xylem, Phloem and Transpiration Flows in Developing Apple Fruits,” Journal of Experimental Botany, Vol. 41, No. 6, 1990, pp. 645-651. doi:10.1093/jxb/41.6.645
- H. Cook and K. J. Oparka, “Movement of Fluorescein into Isolated Caryopses of Wheat and Barley,” Plant, Cell and Environment, Vol. 6, No. 3, 1983, pp. 239-242.
- J. S. Pate, P. J. Sharkey and C. A. Atkins, “Nutrition of a Developing Legume Fruit. Functional Economics in Terms of Carbon, Nitrogen, and Water,” Plant Physiology, Vol. 59, No. 3, 1977, pp. 506-510. doi:10.1104/pp.59.3.506
- J. Tilbrook and S. D. Tyerman, “Hydraulic Connection of Grape Berries to the Vine: Varietal Differences in Water Conductance into and out of Berries, and Potential for Backflow,” Functional Plant Biology, Vol. 36, No. 6, 2009, pp. 541-550. doi:10.1071/FP09019
- B. R. Bondada, M. Keller and G. Hall, “Compositional and Anatomical Characterization of SOUR Berry. Proceedings of the International GiESCO Symposium,” Davis, CA, 2009.
- M. G. McCarthy, “The Effect of Transient Water Deficit on Berry Development of cv. Shiraz (Vitis vinifera L.),” Australian Journal of Grape and Wine Research, Vol. 3, No. 3, 1997, pp. 102-108. doi:10.1111/j.1755-0238.1997.tb00128.x
- S. G. S. Hatfield and M. Knee, “Effects of Water Loss on Apple in Storage,” International Journal of Food Science and Technology, Vol. 23, No. 6, 1988, pp. 575-583. doi:10.1111/j.1365-2621.1988.tb01043.x
- G. Vogg, S. Fischer, J. Leide, E. Emmanuel, R. Jetter, A. A. Levy and M. Riederer, “Tomato Fruit Cuticular Waxes and Their Effects on Transpiration Barrier Properties: Functional Characterization of a Mutant Deficient in a Very-Long-Chain Fatty Acid/3-Ketoacyl-CoA Synthase,” Journal of Experimental Botany, Vol. 55, No. 401, 2004, pp. 1401-1410. doi:10.1093/jxb/erh149
- F. Bioletti, “Blackmeasles, Waterberries and Related Troubles,” California Agricultural Experiment Station Bulletin, Vol. 358, 1923, pp. 1-15.
- R. Theiler, “Anotomische Untersuchungen an Traubenstielen im Zusmmenhang mit der Stiellähme,” Wein-Wiss, Vol. 25, 1970, pp. 381-417.
- G. Brendel, F. Stellwaag-Kittler, et al., “Die PathoPhysiologischen Kriterien der Stiellähme,” Mitt Klosterneuburg, Vol. 33, 1983, pp. 100-104.
- E. R. Capps, “The Relationship between Mineral Nutrition and Late-Season Bunch Stem Necrosis of Cabernet Sauvignon (Vitis vinifera L.) Grapes,” MS Thesis, Virginia Polytechnic Institute and State University, Blacksburg, 1999.
- J. C. Morrison and M. Lodi, “The Influence of Water Berry on the Development and Composition of Thompson Seedless Grapes,” American Journal of Enology and Viticulture, Vol. 41, No. 4, 1990, pp. 301-305.
- B. P. Hills and B. Remigereau, “NMR Studies of Changes in Subcellular Water Compartmentation in Parenchyma Apple Tissue during Drying and Freezing,” International Journal of Food Science and Technology, Vol. 32, No. 1, 1997, pp. 51-61. doi:10.1046/j.1365-2621.1997.00381.x
- D. K. Salunke and B. B. Desaie, “Postharvest Biotechnology of Fruits,” Vol. 1, CRC Press, Florida, 1984, pp. 95-109.
- Q, Sun, T. L. Rost, M. S. Reid and M. A. Matthews, “Ethylene and Not Embolism Is Required for WoundInduced Tylose Development in Stems of Grapevines,” Plant Physiology, Vol. 145, No. 4, 2007, pp. 1629-1636. doi:10.1104/pp.107.100537
- A. N. Kasimatis, “Some Factors Influencing the Development of Water Berries in Thompson Seedless Grapes Grown for Table Use,” MS Thesis, University of California, Davis, 1957.
- M. C. Okimoto, “Anatomy and Histology of the Pineapple Inflorescence and Fruit,” Botanical Gazette, Vol. 110, No. 2, 1948, pp. 217-231. doi:10.1086/335530
- A. J. McElrone, J. A. Grant and D. A. Kluepfel, “The Role of Tyloses in Crown Hydraulic Failure of Nature Walnut Trees Afflicted by Apoplexy Disorder,” Tree Physiology, Vol. 30, No. 6, 2010, pp. 761-772. doi:10.1093/treephys/tpq026
- P. Christensen and J. Boggero, “A Study of Mineral Nutrition Relationships of Waterberry Relationship in Thompson Seedless,” American Journal of Enology and Viticulture, Vol. 36, No. 1, 1985, pp. 57-64.
- D. I. Jackson, “Environmental and Hormonal Effects on Development of Early Bunch Stem Necrosis,” American Journal of Enology and Viticulture, Vol. 42, No. 4, 1991, pp. 290-294.
- D. I. Jackson and B. G. Coombe, “Early Bunchstem Necrosis—A Matter of Nomenclature,” American Journal of Enology and Viticulture, Vol. 46, No. 4, 1988, pp. 579- 580.
- M. Krasnow, M. A. Matthews and K. A. Shackel, “Evidence for Substantial Maintenance of Membrane Integrity and Cell Viability in Normally Developing Grape (Vitis vinifera L.) Berries throughout Development,” Journal of Experimental Botany, Vol. 59, No. 4, 2008, pp. 849-859. doi:10.1093/jxb/erm372
- L. C. Ho and J. D. Hewitt, “Fruit Development,” In: J. G. Atherton and J. Rudich, Eds., The Tomato Crop, Chapman and Hall, UK, 1986, pp. 201-240. doi:10.1007/978-94-009-3137-4_5
- Q. T. Ho, B. E. Verlinden, P. Verboven and B. M. Nicolaï, “Gas Diffusion Properties at Different Positions in the Pear,” Postharvest Biology and Technology, Vol. 41, No. 2, 2006, pp. 113-120. doi:10.1016/j.postharvbio.2006.04.002
- K. A. Shackel, C. Greve, J. M. Labavitch and H. Ahmadi, “Cell Turgor Changes Associated with Ripening in Tomato Pericarp Tissue,” Plant Physiology, Vol. 97, No. 1, 1991, pp. 814-816. doi:10.1104/pp.97.2.814
- T. R. Thomas, M. A. Matthews and K. A. Shackel, “Direct in Situ Measurement of Cell Turgor in Grape (Vitis vinifera L.) Berries during Development and in Response to Plant Water Deficits,” Plant, Cell and Environment, Vol. 29, No. 5, 2006, pp. 993-1001. doi:10.1111/j.1365-3040.2006.01496.x
- M. G. McCarthy and B. G. Coombe, “Is Weight Loss in Ripening Grape Berries cv. Shiraz Caused by Impeded Phloem Transport?” Australian Journal of Grape and Wine Research, Vol. 5, No. 1, 1999, pp. 17-21. doi:10.1111/j.1755-0238.1999.tb00146.x
- T. R. Thomas, K. A. Shackel and M. A. Matthews, “Mesocarp Cell Turgor in Vitis vinifera L. Berries throughout Development and Its Relation to Firmness, Growth, and the Onset of Ripening,” Planta, Vol. 228, No. 6, 2008, pp. 1067-1076. doi:10.1007/s00425-008-0808-z
- S. J. Kays and R. E. Paull, “Postharvest Biology,” Exon Press, Athens, 2004.
- B. G. Coombe and M. G. McCarthy, “Dynamics of Grape Berry Growth and Physiology of Ripening,” Australian Journal of Grape and Wine Research, Vol. 6, No. 2, 2000, pp. 131-135. doi:10.1111/j.1755-0238.2000.tb00171.x
- W. M. Kliewer and L. A. Lider, “Effects of Day Temperature and Light Intensity on Growth and Composition of Vitis vinifera Fruits,” Journal of the American Society for Horticultural Science, Vol. 95, 1970, pp. 766-769.
- D. H. Greer and C. Weston, “Heat Stress Affects Flowering, Berry Growth, Sugar Accumulation and Photosynthesis of Vitis vinifera cv. Semillon Grapevines Grown in a Controlled Environment,” Functional Plant Biology, Vol. 37, No. 3, 2010, pp. 206-241. doi:10.1071/FP09209
- W. M. Kliewer, “Effect of Day Temperature and Light Intensity on Concentration of Malic and Tartaric Acids in Vitis vinifera L. Grapes,” Journal of the American Society for Horticultural Science, Vol. 96, 1971, pp. 372-377.
- H. Steffan and A. Rapp, “Ein Beitragzum Nachweis Unterschiedlicher Malatpools in Beeren der Rebe,” Vitis, Vol. 18, 1979, pp. 100-105.
- C. Sweetman, L. G. Deluc, G. R. Cramer, C. M. Ford and K. L. Soole, “Regulation of Malate Metabolism in Grape Berry and Other Developing Fruits,” Phytochemistry, Vol. 70, No. 11-12, 2009, pp. 1329-1334. doi:10.1016/j.phytochem.2009.08.006
- P. Lobit, M. Genard, P. Soing and R. Habib, “Modelling Malic Acid Accumulation in Fruits: Relationships with Organic Acids, Potassium, and Temperature,” Journal of Experimental Botany, Vol. 57, No. 6, 2006, pp. 1471- 1483. doi:10.1093/jxb/erj128
- M. S. Buttrose, C. R. Hale and W. M. Kliewer, “Effect of Temperature on the Composition of ‘Cabernet Sauvignon’ Berries,” American Journal of Enology and Viticulture, Vol. 22, No. 2, 1971, pp. 71-75.
- H. P. Ruffner, “Metabolism of Tartaric and Malic Acids in Vitis: A Review—Part A,” Vitis, Vol. 21, 1982, pp. 247-259.
- F. M. DuPont, “Effect of Temperature on the Plasma Membrane and Tonoplast ATPases of Barley Roots. Comparison of Results Obtained with Acridine Orange and Quinacrine,” Plant Physiology, Vol. 89, No. 4, 1989, pp. 1401-1412. doi:10.1104/pp.89.4.1401
- N. Terrier, F. X. Sauvage, A. Ageorges and C. Romieu, “Changes in Acidity and in Proton Transport at the Tonoplast of Grape Berries during Development,” Planta, Vol. 213, No. 1, 2001, pp. 20-28. doi:10.1007/s004250000472
- H. P. Ruffner, D. Possner, S. Brem and D. M. Rast, “The Physiological Role of Malic Enzyme in Grape Ripening,” Planta, Vol. 160, No. 5, 1984, pp. 444-448. doi:10.1007/BF00429761
- W. M. Kliewer and L. A. Lider, “Influence of Cluster Exposure to the Sun on the Composition of Thompson Seedless Fruit,” American Journal of Enology and Viticulture, Vol. 19, No. 3, 1968, pp. 175-184.
- J. M. Tarara, J. Lee, S. E. Spayd and C. F. Scagel, “Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes,” American Journal of Enology and Viticulture, Vol. 59, No. 3, 2008, pp. 235-247.
- C. R. Parra, L. M. L. Sarmiento, G. C. Salinas and G. G. Giraldo, “The Effect of Hail and Wind on the Development and Quality of the Fruits of Dominico Harton and FHIA-21 Plantain,” Info Musa, Vol. 10, 2001, pp. 13-17.
- C. R. Hale, “Relation between Potassium and the Malate and Tartrate Contents of Grape Berries,” Vitis, Vol. 16, 1977, pp. 9-19.
- K. Takimoto, K. Saito and Z. Kasai, “Conversion of Tartarate to Malate and Monoethyl Tartarate in Grape Leaves,” Phytochemistry, Vol. 16, No. 11, 1977, pp. 1641-1645. doi:10.1016/0031-9422(71)85061-6
- K. Takimoto, K. Saito and Z. Kasai, “Diurnal Changes in Tartarate Dissimilation during the Ripening of Grapes,” Plant Physiology and Biochemistry, Vol. 15, No. 6, 1976, pp. 927-930.
- W. Wienhaus, “Reaktionen in Organen der weinrebe auf Applikation von Stoffwechselhemmstoffen und Entkopplern wahrend der Reifephase,” Vitis, Vol. 12, 1973, pp. 105-118.
- N. Ollat and J. P. Gaudillère, “Investigation of Assimilate Import Mechanisms in Berries of Vitis vinifera var. ‘Cabernet Sauvignon’,” Acta Horticulturae, Vol. 427, 1996, pp. 141-149.
- C. Davis, R. Shin, W. Liu, M. R. Thomas and D. P. Schachtman, “Transporters Expressed during Grape Berry (Vitis vinifera L.) Development Are Associated with an Increase in Berry Size and Berry Potassium Accumulation,” Journal of Experimental Botany, Vol. 57, No. 12, 2006, pp. 3209-3216. doi:10.1093/jxb/erl091
- M. A. Matthews and V. Nuzzo, “Berry Size and Yield Paradigms on Grapes and Wines Quality,” Acta Horticulturae, Vol. 754, 2007, p. 423.
NOTES
*Corresponding author.

