American Journal of Plant Sciences
Vol.3 No.1(2012), Article ID:16627,11 pages DOI:10.4236/ajps.2012.31006
In Vitro Propagation of Ocimum gratissimum L. (Lamiaceae) and Its Evaluation of Genetic Fidelity Using RAPD Marker
![]()
1Department of Botany, Cytogenetics and Plant Biotechnology Research Unit, University of Kalyani, Nadia, India; 2Department of Botany, Microbiology Research Laboratory, University of Kalyani, Nadia, India.
Email: pdgbot@yahoo.co.in
Received July 5th, 2011; revised August 2nd, 2011; accepted August 15th, 2011
Keywords: Micropropagation; Multiple Shoot; Nodal Explant; Histology; Genetic Fidelity
ABSTRACT
An efficient plant propagation system through nodal explants was established in Ocimum gratissimum L, a medicinally important herbaceous perennial herb belonging to the family Lamiaceae. Axillary shoot bud proliferation was initiated from nodal explants cultured on Murashige and Skoog (MS) medium supplemented with various concentrations of N6- benzyladenine (BA) (0.5 - 3.0 mg/l), Kinetin (KN) (0.5 - 3.0 mg/l) and 2-isoPentenyladenine (2-iP) (0.5 - 3.0 mg/l). Maximum numbers of shoots (5.17 ± 0.04) with average length (2.50 ± 0.07) were induced on medium containing 1.0 mg/l BA. Shoot multiplication was maintained by repeated subculturing the original nodal explants on shoot multiplication medium after each harvest of newly formed shoots. Histological study shows that the organogenesis occurs directly, without callus formation on epidermal and sub epidermal layer of the explants. Rooting of shoots was achieved on half strength MS medium supplemented with 1.5 mg/1 Indole-3-butyric acid (IBA) and 2% sucrose. Well-developed complete plantlets were transferred to plastic pots containing a mixture of (1:1) soil and vermiculite showed 82.5% survival rate. Genetic fidelity was assessed by chromosome analysis and DNA fingerprinting using random amplified polymorphic DNA (RAPD) of in vitro and in vivo plants. Nine arbitrary decamers displayed same banding profile showed no genomic alterations, indicating homogeneity among the tissue culture regenerates and genetic uniformity with that of donor plants. The present study provides high fidelity micro-propagated system for efficient and rapid micro-propagation protocol of this important medicinal plant and great use in conserving without risk of genetic instability.
1. Introduction
Medicinal and aromatic plants are an important source of medicines and play a significant role in world health care system. Today medicinal plants are important to the global economy, as well as source of income for rural people in developing countries. About 70% - 80% of the people worldwide rely on herbal medicines derived from plants for their primary healthcare needs [1]. This awakening has led to a sudden rise in demand for herbal medicines. Generally, herbal preparations are produced from field-grown plants and are susceptible to infestation by bacteria, fungi, and insects that can alter the medicinal content of the preparations [2]. But it is difficult to ensure the quality control as the medicinal preparations are multi-herb preparations and also difficult to identify and quantify the active constituents [3]. There is significant evidence to show that the supply of plants for traditional medicines is failing to satisfy the demand [4]. Therefore, the success of any health care system depends on the availability of suitable drugs on a sustainable basis. Although synthetic drugs and antibiotics are essential for current medical practice, plants provide a major contribution to the pharmaceutical industry [5]. However, due to excessive human exploitation, non-regulated collection, unresolved inherent problems of seed viability and seed germination, this priority many species have become threatened or endangered [6,7].
Ocimum gratissimum L. is an economically important multi purpose medicinal herbaceous perennial herb, popularly known as “Shrubby basil” in English. It belongs to the family Lamiacea and widely distributed in tropical and warm temperate regions. Traditionally, this plant is being widely used for the treatment of various ailments including rheumatism, paralysis, epilepsy, high fever, diarrhea, sunstroke, influenza, gonorrhea and mental illness [8]. The plant exhibits antimicrobial [9], Antifungal [10], antibacterial [11], antimalarial [12] and antiprotozoal [13] activities. The active compounds present as volatile oil from the leaves consist mainly of eugenol, thymol, citrol, geraniol and linalool [8]. It is has also been used in toothpaste and mouth washes as well as some topical ointments [11,13-14]. It is used an excellent gargle for sore throats and tonsillitis. The plant extract is used against gastrointestinal helminthes of animals and man [15].
The major difficulty in the use of Lamiaceae species for pharmaceutical purpose in the individual variability, due to genetic and biochemical heterogenecity [16]. The conventional method of propagation of this species through seeds, the seed viability is very poor, season dependence and low germination rate (<10%) potential restricts its multiplication [8,17]. Moreover, seed derived progenies are does not allow the production of homogeneous populations, due to cross pollination [18].
In vitro culture techniques represents an excellent option for the study and conservation of rare, threatened or endangered medicinal plants [19,20], as well as tool for efficient rapid clonal propagation of important plants allowing production of genetically stable and true-to-true type progeny [21]. Therefore, the interest in using these techniques for rapid and large-scale propagation of medicinal and aromatic plants has been significantly increased [22-24]. Until now, there is only a single report on the micropropagation of Ocimum gratissimum L. [25], but the attempts were poorly defined and the data provided were inadequate. In the present study was therefore, undertaken to establish an efficient protocol for rapid large-scale regeneration of plantlets in vitro from nodal explants of Ocimum gratissimum L. with maintaining stable gene pool fidelity of the regenerants as assessed by RAPD.
2. Materials and Methods
2.1. Plant Material
Young disease free nodal explants (2 - 2.5 cm) were collected from 1 year old plant growing in the medicinal and aromatic plant garden of the Department of Botany, University of Kalyani, Kalyani, India. Explants were washed thoroughly under running tap water and then treated with 5% (m/v) Teepol (Qualigen, Mumbai, India) for 15 min, followed by rinsing three to five times in sterile distilled water. The nodal segments were then surface disinfested with 70% alcohol for 1 min followed by immersion in 0.1% (m/v) aqueous mercuric chloride (HgCl2) solution for 5 - 6 min and finally rinsed with autoclaved sterile distilled water (four to five times) in a flow chamber. The surface sterilized explants were trimmed at cut ends and about 1 - 1.2 cm prior to inoculation on culture media.
2.2. Culture Media and Conditions
Surface sterilized nodal segments (1 - 1.2 cm) were cultured on MS [26] basal medium containing 3% (w/v) sucrose (Himedia, Mumbai, India) for culture initiation and served as explant sources for subsequent experiments. The pH of the medium (Supplemented with respective growth regulators) was adjusted to 5.7 with 1N NaOH or 1N HCl before gelling with 0.8% (w/v) agar (Himedia, Mumbai, India). In all the experiments, the chemicals used were of analytical grade. The explants initially were implanted vertically on the culture medium in test tube (150 × 25 nm) and plugged tightly with non-absorbent cotton. All the cultures were kept under cool fluorescent light (16 h photo period 40 µmol·m–2·s–1, Philips, India at 25˚C ± 2˚C) and 60% - 70 % relative humidity (RH).
2.3. Shoot Induction and Multiplication
For shoot induction, the nodal explants were cultured on MS medium supplemented with various concentrations of BA/KN/2-iP (0.5 - 3.0mg/l), either individually or in combination with NAA (0.1 - 2.0 mg/l), IBA (0.1 - 2.0 mg/l). The regenerated shoots were excised, cut into single nodal segments and further multiplied for five to six subcultures to fresh medium of the same composition for induction of multiple shoots at an interval of 4 weeks. The frequency with which explants produced shoots, the number of shoots per explant and the shoot length were recorded after 4 weeks of culture.
2.4. Histological Observation
For histological studies, the explants were fixed in FAA (formaldehyde: acetic acid: ethanol; 100:50:50) solution for 7 days. The fixed samples were washed for 30 min, thrice with double distilled water. After washing, the fixed samples were dehydrated through the ethanol series (30%, 50%, 70%, 80%, and 90%) for 30 min at each stage. The samples were embedded in paraffin wax (melting point 58˚C) and section longitudinally at 10µM thickness on a rotary microtome (Leica). The sections were mounted onto slides and allowed to dry for at least 10 min before staining. The specimens were stained with hematoxylin-eosin and counter stained with fast green blue solutions. The sections were examined under an optical microscope (CARL ZEISS Microimaging GmbH, Model no: 10001076, Germany).
2.5. Rooting of Shoots
For root induction, excised microshoots (3 - 4 cm length) with three to four fully expanded leaves from in vitro grown plants were transferred to full or half strength basal MS medium supplemented with different concentrations of NAA, IBA, IAA (0.5 - 2.0 mg/l) and 2% (w/v) sucrose. IAA was filter sterilized and added to the medium after autoclaving. Data were recorded on the percentage of rooting, the mean number of roots per shoot and the root length after four weeks of transfer onto the rooting medium.
2.6. Chromosome Number Analysis
Healthy root tips were excised from 15 randomly selected in vitro culture regenerants, washed with distilled water and pretreated with chilled saturated solution of Para-dichloro benzene (PDB) with aesculine for 3 - 4 hrs at 4˚C. Pretreated material was washed distilled water and then fixed in ethanol: acetic acid (2:1) for 24 hrs. After hydrolysis with root tips was performed with 1N HCl at 60˚C for 10 - 12 min. Hydrolyzed root tips were stained in 2% aceto-orcein for 2½ - 3 hrs and squashed in 45% acetic acid to obtained somatic metaphase plates on the glass slides. Photomicrographs were taken under Zeiss photomicroscope and chromosome numbers were counted manually.
2.7. Acclimatization of Regenerated Plants
The complete rooted plantlets with 5 - 7 fully expanded leaves were removed from the culture medium and the roots were washed gently under running tap water to remove agar. The plantlets were transferred to plastic pots (5 cm diameter) containing a mixture of sterilized garden soil and vermiculite in the ratio 1:1 and covered with transparent plastic bags to ensure high humidity. Each was irrigated with 1/8 MS basal salt solution devoid of sucrose and inositol every 4 days for 2 weeks. The growth chamber was maintained at 26˚C ± 1˚C, 80% - 85% relative humidity with light intensity of 50 µmol·m–2·s–1 on a 16 h photoperiod inside the culture room conditions. The relative humidity was reduced gradually and after 30 days the plantlets were transferred to pots (25 cm diameter) containing garden soil and kept under green house for another 2 weeks for further growth and development. Well acclimatized in vitro raised plants were transferred finally to its original habitat for its survivalability. The morphological characteristics, growth characteristics and floral features were examined.
2.8. DNA Extraction and PCR Amplification
Genomic DNA was extracted from young leaves of in vitro-raised field grown plants of Ocimum gratissimum L. and mother plant by Cytl trimethyl ammonium bromide (CTAB) procedure [27] with minor modifications. Quality and quantity of DNA was checked on 0.8% agarose gel and also from values obtained by 260/280 nm UV absorbance ratio [28]. Twelve arbitrary decamer RAPD primers (Bengalore Genni Pvt. Ltd., India) were used for polymerase chain reaction (PCR) for DNA amplification. DNA finger printing profiles were compared to evaluate clonal fidelity and genetic stability. Amplification was performed in 25 µL using PCR mixture of consisting of 2.5 µL Taq buffer, 1 µL dNTPs, 0.5 µL Taq polymerase, 2 µL DNA (approximate 50 ng/µL), 1.0 µL primer (10 pmol), 2.5 µL MgCl2, 1 µL oil and 14.5 µL MiliQ water. The PCR reaction conditions were: preheating for 4 min at 94˚C; 40 cycles of 15 sec at 94˚C, 15 sec at 40˚C and 1.15 min at 72˚C and elongation was completed by a final extension of 7 min at 72˚C. After amplification, the PCR product was resolved by electrophoresis in 1.4% agarose gel (Himedia, Mumbai, India) and stained with ethidium bromide (0.5 µg/ml). 2.0 - 23.1 kb λ DNA digested Hind III was used as the DNA marker, and bands were visualized under UV light and photographed using the Gel Doc equipment (Bio Rad). All the PCR reaction was repeated twice.
2.9. Statistical Analysis
Experiments were set up in completely randomized block design. Each experiment was repeated three times with 10 - 12 replicates. Data were analyzed by one way analysis of variance (ANOVA) and the difference between means were scored using Duncan’s Multiple Range Test P £ 0.05 [29] on the statistical package of SPSS (Version 10).
3. Results and Discussion
3.1. In Vitro Establishment
For the primary establishment of in vitro culture from field-grown plants surface sterilization of the explants was essential because of the microorganism can live or survive in the vascular tissue of the plants, therefore contamination attached to the surface of the explants. The main contaminants that affect tissue cultured plants are bacteria and fungi. Particularly hairy plants are a problem because bubble of air became entrapped in the explants and prevent good contact with the disinfesting agent. Evenly storage water and its included microorganism can be a source of some of the internal (endogenous) contamination observed in vitro [30]. The duration of exposure of the explants of the sterilizing agent is most important for any tissue culture studies. Therefore, to overcome contamination problem, surface sterilization of explants was done with 0.1% Mercuric chloride (HgCl2) for 1, 2, 4, 6, 8, 10 and 12 min. Mercuric chloride (HgCl2) is a very strong sterilant [31], when the explants sterilization with 0.1% HgCl2 for treatment duration less than 4 min resulted for contamination of 50% explants (Table 1). Most of the cultured explants showed fungal contamination within 5 to 12 days of inoculation. Whereas,
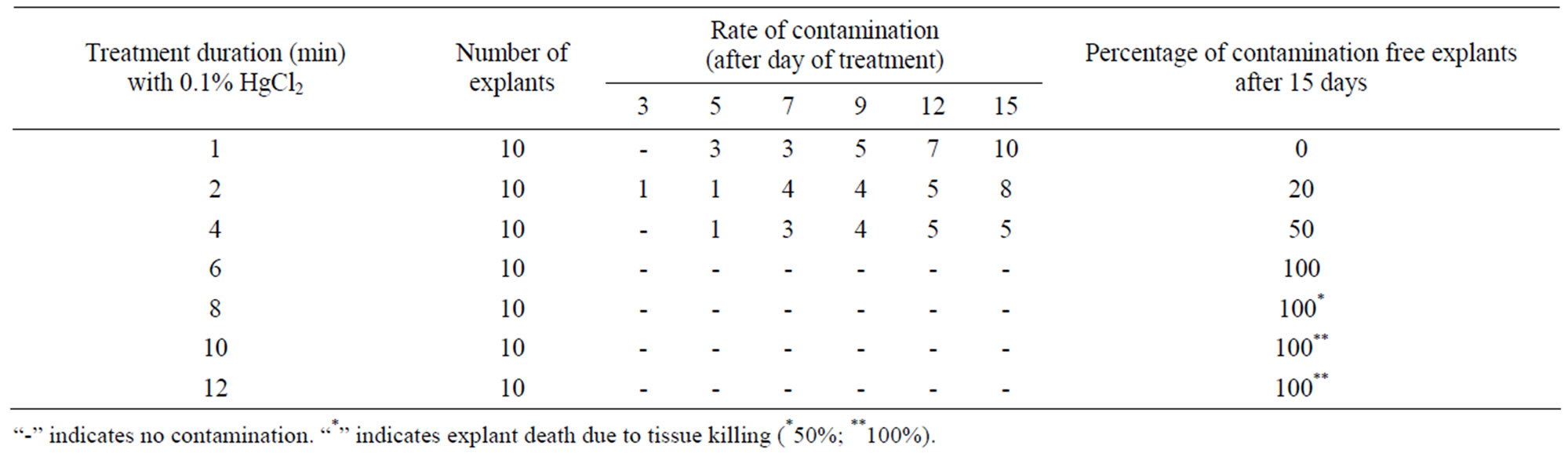
Table 1. Standardization of HgCl2 treatment period for surface sterilization of the explants.
when the duration of exposure was above 4 to 6 min contamination frequency was significantly reduce 100%. These explants remained green and showed healthy growth and proliferation of auxillary shoots. But when the duration of exposure was above 6 - 8 min significant increase in death rates (50%) of explants. On the other hand 100% explants died when the explants were treated with 1% HgCl2 for 10 - 12 min.
3.2. Effect of Cytokinins
The morphogenetic responses of nodal segment explants to various cytokinins (BA/KN/2-iP) are summarized in Table 2. When Explants cultured on MS medium without cytokinins failed to produce shoots even after 4 weeks of inoculation, but the explants when cultured on MS medium supplemented with different cytokinins at different concentrations showed variation in the regeneration percentage and number of shoots formed. Nodes cultured on half strength MS basal medium showed no visible signs of tissue differentiation. This was possibly due to a greater demand of nitrogen and potassium-containing compounds, which induce greater amount of new proteins [32]. These components are lower in half strength MS basal medium compared to full strength MS basal medium. Initial induction of shoots was noted after 10 - 12 days of inoculation (Figure 1(a)). Data on different growth parameters from different treatments were recorded after 4 weeks of culture initiation following one transfer to the new medium. Among the three cytokinins tested, the best response (91.10%) was obtained in the presence of 1.0 mg/l BA and was found to be significantly higher than shoots induced per nodal explant in other concentrations of cytokinins (KN/2-iP) in the present study (Figure 1(b)). Nodes cultured on MS medium with different concentrations of KN and 2-iP showed lower induction of axillary shoot bud proliferation. The maximum number of multiple shoots was obtained (5.17 ± 0.04) in the medium containing 1.0 mg/l of BA. The shoots developed in this medium also attained maximum
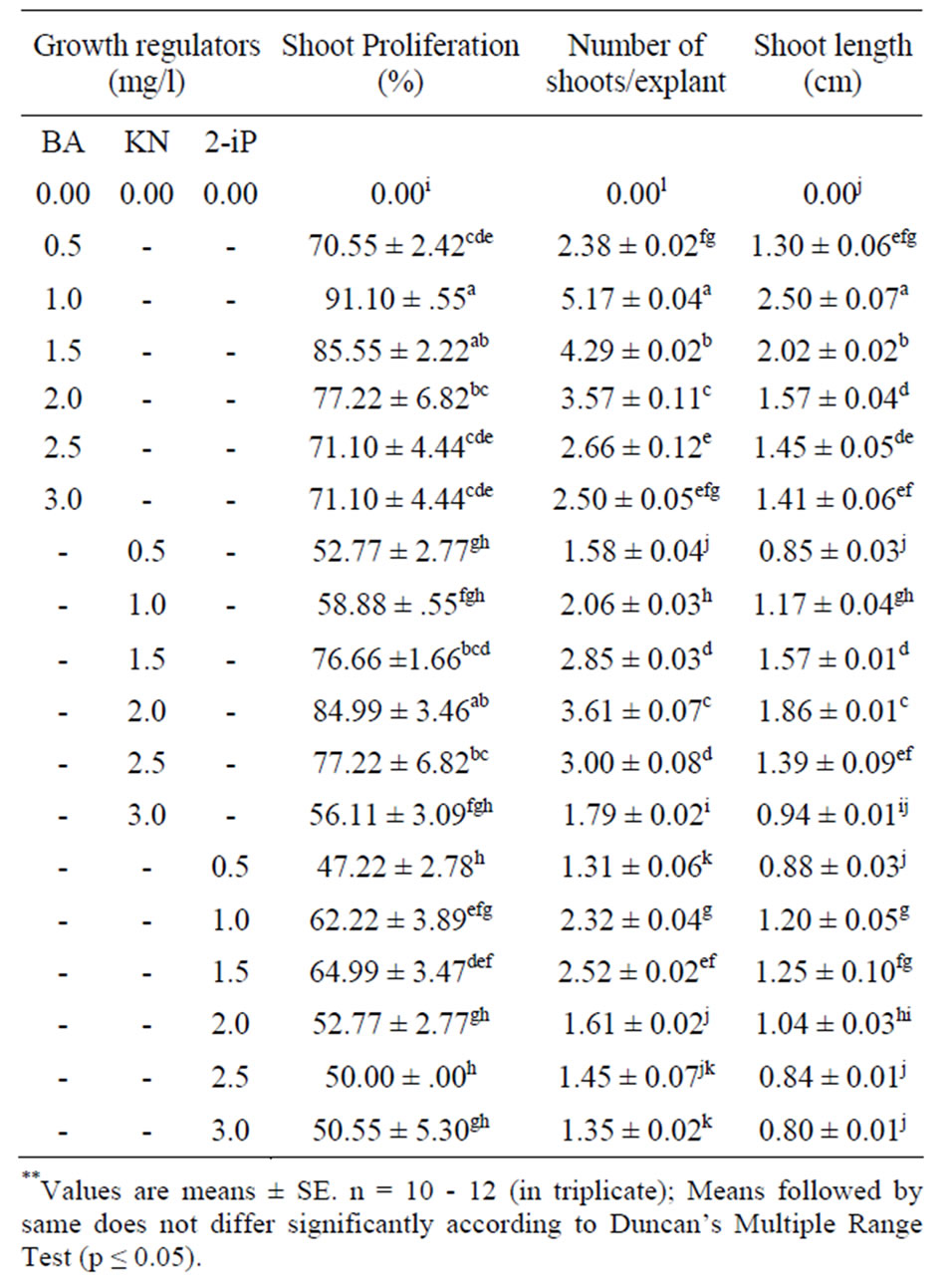
Table 2. Effect of different concentrations of BA, KN and 2- iP in MS medium on multiple shoot induction from nodal explants of O. gratissimum L. after 4 weeks of culture.
height of 2.50 ± 0.07 cm after 4 weeks. The BA concentrations higher than 1.0 mg/l, the number of shoots as well as percent response was reduced (Table 2). Reduction in the number of shoots generated from each node at BA concentration higher than the optimal level was also reported for several medicinal plants [22,33]. Of the three cytokinin (BA, KN and 2-iP) tested, BA was most effective in inducing multiple shoot formation especially BA were reported to overcome apical dominance, release

Figure 1. In vitro clonal propagation of Ocimum gratissimum L. (a) Shoot proliferation from nodal explant on MS medium supplemented with 1.0 mg/l BA after 10 - 12 days of culture (bar 0.7 cm); (b) Shoot multiplication on MS medium supplemented with 1 mg/l BA after four weeks of culture (bar 1.0 cm); (c) High rate of shoot multiplication from subculture nodal segment on MS medium supplemented with 1 mg/l BA (bar 1.5 cm); (d) Formation of roots from the regenerated shoots cultured on 1/2 MS medium supplemented with 1.5 mg/l IBA (bar 1.8 cm); (e) Well developed root system and complete plant (bar 1.2 cm); (f) Plantlets developed In vitro, transferred to pot (bar 1.8 cm); (g) In vitro raised plant after 3 months of transplantation in field (bar 15 cm).
lateral buds from dormancy and promote shoot formation [34]. The stimulating effective of BA on multiple shoot formation has been reported earlier for several medicinal plant species including Ocimum basilicum L. [24,35-37], Feronia limonia (L.) [38], Mentha piperita L. [39].
3.3. Effect of Auxin and Cytokinin
The interactions of the optimal concentration of BA (1.0 mg/l) with various concentrations (0.1 - 2.0 mg/l) of auxin (NAA and IBA) were also evaluated statistically on the rate of multiple shoot induction (Table 3). BA in
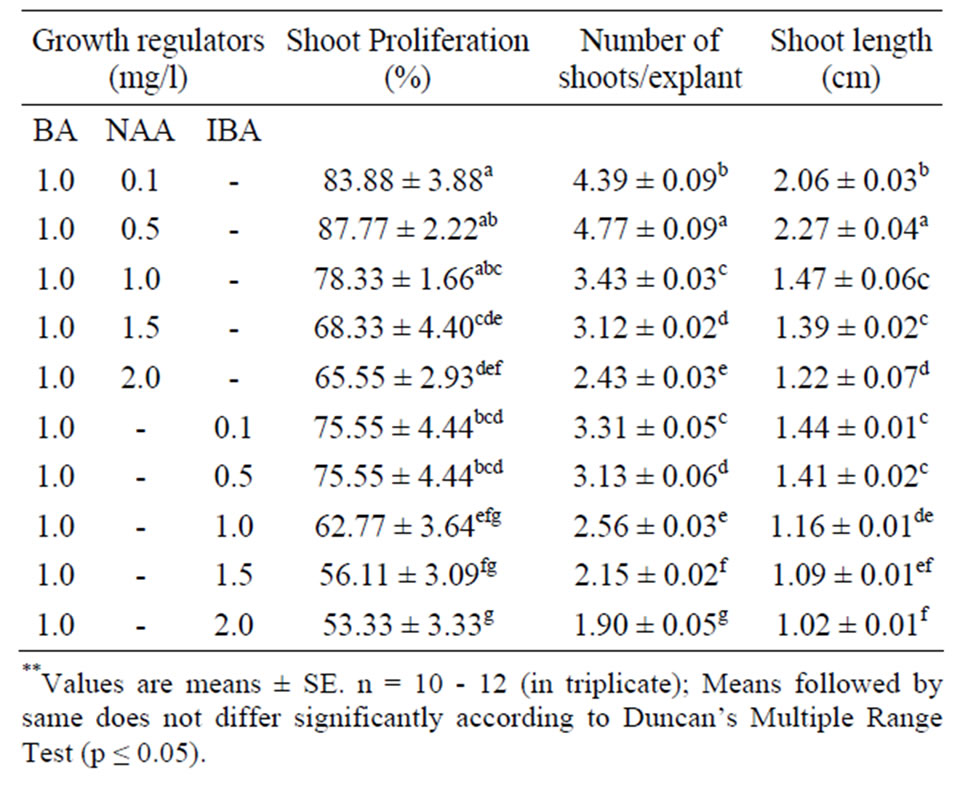
Table 3. Effect of auxins with the optimal concentration of BA on shoot regeneration form nodal segments of O. gratissimum L. in MS medium after 4 weeks of culture.
combination with NAA markedly enhanced the percent regeneration, number of shoots and shoot length whereas IBA did not improve the parameters evaluated. Nodal explants cultured on MS medium supplemented with 1.0 mg/l BA and 0.5 mg/l NAA exhibited 87.77% shoot regeneration. Upon increasing the concentration of NAA up to 2.0 mg/l, a gradual decrease in regeneration frequency and the number of shoots per explants and also induction of callus at the base and failed to induced further shoot regeneration. Among the various concentration of IBA with optimal concentration of BA used, the highest shoot regeneration frequency 75.55% and number of shoots per explants 3.31 ± 0.05 along with the maximum shoot length 1.44 ± 0.01 were recorded on MS medium after 4 weeks of inoculation (Table 3). Among the combination of hormones tried, only 4.77 ± 0.09 shoots per explants induced. This result shows that, in Ocimum gratissimum L. combination of hormones do not produced synchronized effect towards large scale multiple shoot formation. The similar result was observed in other plant species viz. M. pruriens [40,41]. These differential responses, revealed that by different type and concentrations of the hormones used indicate possible existence of genotype specific optimum responses within large group. Similar results have also been reported in Morus cultivars [42]. It is established fact that, in the cultured tissues, the requirement for exogenous hormone depends on the endogenous level of the plant tissue, which varies with organ, plant genotype, and the phase of the growth [43]. Thus, the result establishes the need for independent standardization and examination of micropropagation protocols.
3.4. Effect of Subculture
The effect of subculture passages was also evaluated on shoot induction medium (MS supplemented with 1.0 mg/l BA) after harvesting newly formed shoot. The shoot generation ability was maintained up to sixth subcultures on shoot induction medium by regular subculturing. Therefore, average number of 14.21 ± 0.29 (Figure 3) shoots could be obtained from single nodal explants after 20 weeks of culture (Figure 1(c)). The multiplication rate is higher than the earlier reports [44,45]. After the fourth subcultures the shoot multiplication rate declined with the explants. A similar result was recorded in Gardenia jaminoides [34] Vitex trifolia [38]. Sub-culture effects on the multiplication rate of cultures are known to differ from one species to another. In Bacopa monniera, increase in shoot induction and multiplication has been reported upto third subculture passage beyond which the frequency and number of shoots decreased [46] whereas in Cassia angustifolia, an increase in shoot production has been reported during subculturing which become stable in fifth passage [47]. In many plants species [48] it was reported that micropropagation requires two media i.e. a propagation medium and a shoot elongation medium, which makes the micropropagation procedures cumbersome and uneconomical. However, in the present studies shoot multiplication and subsequent elongation was achieved on the same medium. After 19 - 20 weeks old in vitro regenerated shoots were transferred in rooting medium and roots were obtained successfully after 12 - 15 days.
3.5. Histological Observation
Structural analysis in an important step in the study of the organization and changes in the plant body, and it is an extremely useful approach in the study of plant morphogenesis [49]. Therefore; Histological studies were conducted on responding nodal segments to trace the origin of multiple shoot buds. Because, Direct formation of multiple shoots from fresh plant tissue without passing stage makes it possible to raise many cloned rapidly and these clone have genetic stability [50]. Longitudinal sections of the nodal explant showing development of multiple shoots with leaf primordia from the basal region without callus phase (Figures 2(a) and (b)). However, direct de novo origin of the adventitious shoot buds was observed in 15-d-old longitudinal sections.
3.6. Effect of Auxin
Healthy elongated shoots (3 - 4 cm in length) were excised and placed on full and half strength MS basal medium supplemented with different concentrations of auxins (NAA, IBA and IAA) at the range of 0.5 - 2.0 mg /l for induction of roots (Table 4). The effects of these auxins on root induction as well as the length of the roots were examined after one months of culture. In the pre-
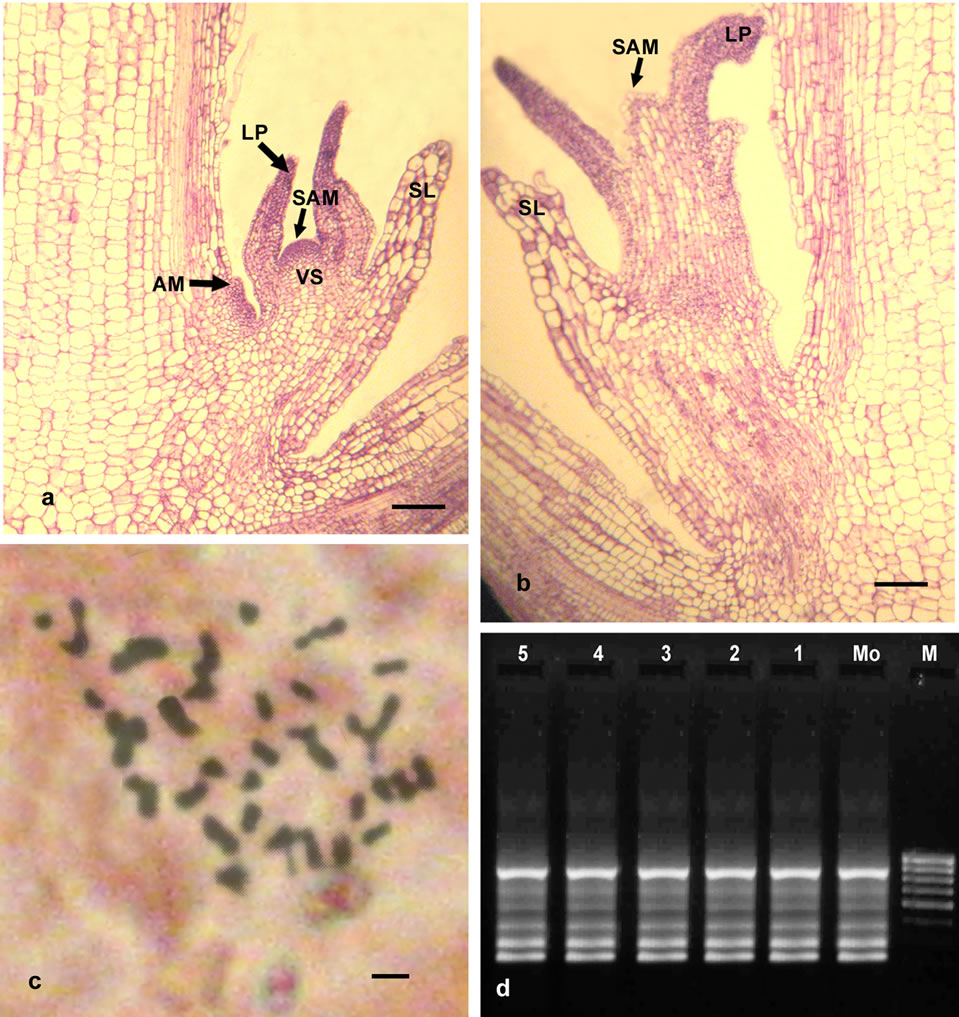
Figure 2. (a), (b) Microphotographs of Longitudinal section showing developmental sequence of induction of adventitious buds on the basal region (bar 0.7 mm) Note: SAM = Shoot apical meristem, LP = Leaf Primordium, AM = Axillary meristem, SL = Sheath leaves, VS = Provascular stand; (c) Somatic metaphase plate showing 2n = 40 in regenerated plant (bar 5 m); (d) RAPD marker analysis of in vitro raised field-grown plants and donor plant; lane M corresponds to λ DNA digested Hind III as molecular weight marker (2.0 - 23.1 kb), lane Mo-DNA from mother plant, lanes 1-5-DNA from randomly selected regenerated plantlets.
liminary experiments conducted, no rooting was observed when the shoots were culture on hormone free (Control) MS medium. Full strength MS medium containing auxins showed very poor response in rooting, but well developed roots were achieved on half strength MS medium supplemented with NAA, IBA & IAA and with reduce sucrose concentration (2%) gave us well developed roots within 12 - 15 days. In many species such as Lavandula vera [51)], Ocimum kilimandscharicum [52], rooting frequency was higher when shoots were rooted on low strength MS medium. The rational behind the favorable effect of reduced macronutrient concentration is that the concentration of nitrogen ions needed for root formation is much lower than for shoot formation and growth [53]. Of the three types of auxins, IBA was found to be comparatively more effective for root induction in both (half & full strength MS) types to media than IAA and NAA (Table 4, Figure 1(d)). The possible reason could be that IBA is more stable than IAA to chemical degradation in tissue culture media, both during autoclaving and at room temperature [54]. However, NAA and IAA formed slender roots in both type of media.
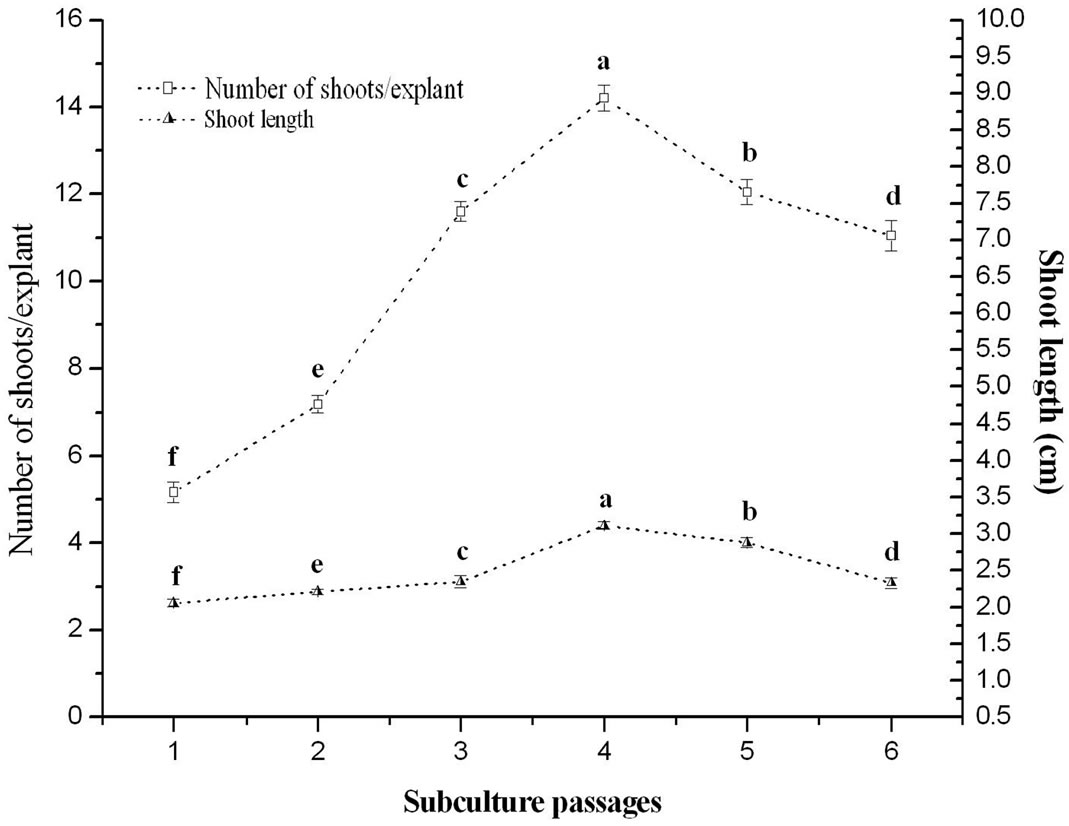
Figure 3. Morphogenic response of in vitro raised nodal explants of O. gratissimum L. grown on BA (1.0 mg/l) supplemented with MS media during six subculture passages.
Similar response was observed by other species [55,56]. Among various concentrations tested IBA 1.5 mg /1 regardless of the kind of auxin, proved to be the best in eliciting the highest frequency of root formation. Shoot formed roots at a high frequency (87.77%) on medium containing 1.5 mg/l. In this medium a maximum number of 5.09 ± 0.05 roots attaining a length of 2.11 ± 0.05 cm were obtained. Further increase in the IBA concentration to 1.5 mg/l reduces root initiation. Although shoot grown roots formed callus at their cut end. According to [57], shoots contain high level of endogenous auxins and the addition of exogenous auxin caused the inhibition of root development, thus resulted in callusing at the base of the shoots. The stimulatory effect of IBA for root formation has also been reported in many medicinal plant species, including Ocimum basilicum L. [37], Mentha piperita L. [39], Tylophora indica [58].
3.7. Acclimatization and Field Establishment
The ultimate success of in vitro propagation lies in the successful establishment of plants in soil. The well-developed rooted plantlets were taken out gently from the test tubes and thoroughly washed with sterile water to remove adhered agar and traces of the medium to avoid contamination. 40 plantlets were transferred to plastic pots containing potting a mixture of (1:1) soil and vermiculite (Figure 1(f)). In the first week of transplantation, plantlets kept covered in a polythene tent for providing the condition of high humidity and sufficient light. The polythene cover was removed periodically and progressively whenever leaves appeared water soaked. Polythene covers were completely withdrawn after 2 - 3 weeks of hardening. After 4 weeks plants were then transferred to larger potted filled soil with organic manure kept under green house for further growth and development. Finally the acclimated plants were then shifted to the field conditions having 82.5% survived. The growth characteristics of in vitro raised plants did not show any significant morphological variations from those of the natural habitat. All in vitro regenerated plants grew normally and produced multiple branching twelve weeks after their transfer to the original habit (Figure 1(g)).
3.8. Chromosome Analysis
High levels of both morphological and chemical variability exist within the genus Ocimum due to interspecific hybridization, polyploidy and the existence of chemotypes or chemical races that do not differ significantly in morphology [59]. In this aspect initial studies were carried out to determine if any major genetic changes were detectable within a population Ocimum gratissimum L. plants regenerated through the above morphogenic system. Somatic chromosome number analysis revealed 2n = 40 (Figure 2(c)). No variation was noted at the chromosomal level. Therefore, it was essential to evaluate genetical stability through molecular techniques.
3.9. Molecular Analysis
Genetic uniformity is one of the most important prerequisites for the successful micro-propagation of any crop species. Nevertheless, a major problem encountered in cells grown in vitro is the occurrence of genetic variation due to change in either DNA sequences (point mutation, activation of transposons), in chromosome structure (duplications, translocations) or in chromosome number (leading to polyploidy) [60]. Furthermore, abnormalities in tissue culture particularly growth regulators (in par-
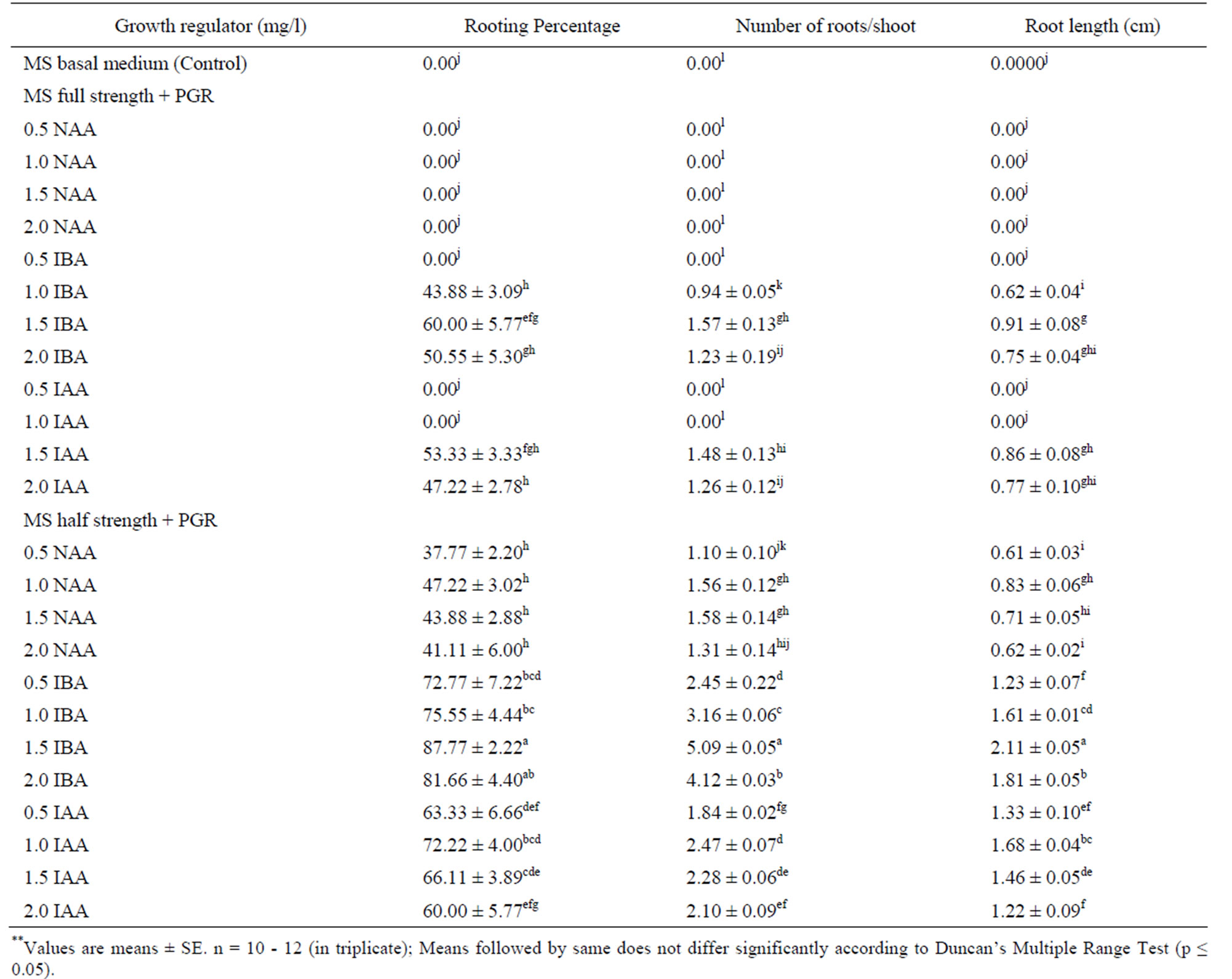
Table 4. Effect of different concentrations of NAA, IBA and IAA in full and half-strength MS medium on root induction from regenerated shoots of O. gratissimum L. after 4 weeks of culture.
ticular 2, 4-D) [61], and in the plants produce from them often increase in frequency with increasing culture passages [62].
The PCR based RAPD technique does not require DNA sequence information and species specificity and hence it is being conveniently used for assessing genetic stability and clonal fidelity of micropropagated plants in a number of genera. There are many reports on molecular characterization of micropropagated plants by the RAPD technique especially to confirm the clonal fidelity and genetic stability among tissue culture grown plants and donor [52,63-66]. Because RAPD analysis is particularly well suited to high output system required for plant breeding, it is easy to perform, fast, reliable and of relatively low cost [67]. Genetic fidelity of micropropagated plants has immense practical utility and commercial implications. Keeping this perspective in mind, we assessed the genetic integrity of in vitro regenerated from nodal stem segment explants and respective donor plant of Ocimum gratissimum L.
Total 12 primers were initially screened and finally 9 primers produce clear and scorable amplified bands ranging from 3 - 6 bands per primer (Table 5). Each primer produced a unique set of amplification products ranging in size from 2.0 - 23.1 kb (Figure 2(d) with primer MS10G6). All 9 primers produced a total of 37 bands with an average of 4.11 fragments. All the 37 scorable bands were monomorphic in nature, indicating homogeneity among the culture regenerates and genetic uniformity with that of the donor plants. The possible reason may be multiple shoot bud differentiation without intervening callus phase is least vulnerable to genetic changes. However, no differences were observed between mother plant and plantlets regenerated from nodal stem segments by any nine primers tested in present RAPD study.
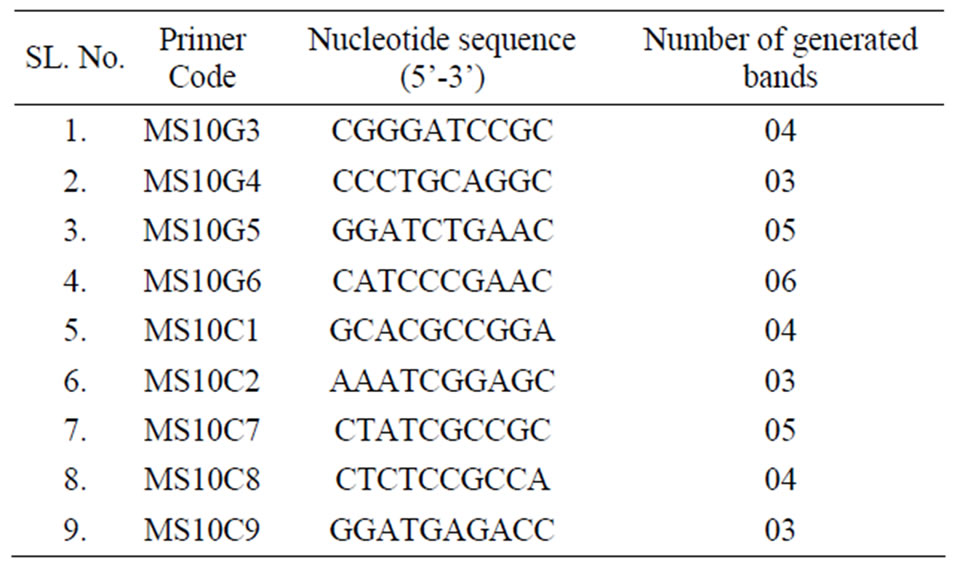
Table 5. Number of amplification products generated with the use of RAPD primers in the analyses of genetic fidelity of O. gratissimum L. plants propagated in vitro.
4. Conclusion
In conclusion, the present study, we established an efficient and reliable micropropagation protocol for in vitro regeneration of Ocimum gratissimum L. from nodal explant, which can ensure large scale propagation, as well as protocol can also be used for raising genetically uniform plants, which is important for the sustainable supply of plant materials to the pharmaceutical industries and for conservation of elite germplasm. Our results also indicate that multiple shoot bud induction and regeneration in Ocimum gratissimum L. regulated by appropriate cytokinin and auxin concentration, combination of hormones do not influence towards large scale multiple shoot formation. Further, our results demonstrate that RAPD markers can be applied to evaluate the genetic stability of regenerants for the ex situ conservation of this important aromatic and medicinal herb.
5. Acknowledgements
We would like to thank Dr. U.C. Luvania CIMAP, Lucknow for supplying plants material. Authors acknowledge financial support from University Grant Commission (UGC), New Delhi, Govt. of India. Authors are also grateful to DST-FIST programme, Govt. of India, &, Department of Botany, University of Kalyani for Instrumental facilities.
REFERENCES
- S. Pei, “Ethnobotanical Approaches of Traditional Medicine Studies: Some Experiences from Asia,” Pharmaceutical Biology, Vol. 39, 2001, pp. 74-79.
- S. J. Murch, S. KrishnaRaj and P. K. Saxena, “PhytoPharmaceuticals: Mass-Production, Standardization, and Conservation,” Herbal Medicine, Vol. 4, No. 2, 2000, pp. 39-43.
- K. C. Wen, “The Turnover Rate of Marker Constituents in Chinese Herbal Medicine,” Journal of Food and Drug Analysis, Vol. 8, No. 4, 2000, pp. 270-277. www.fda.gov.tw/files/publish_periodical/8-4-6.PDF
- A. B. Cunningham, “African Medicinal Plants: Setting Priorities at the Interface between Conservation and Primary Healthcare,” People and Plants Working Paper 1, UNESCO, Paris, 1993, p. 92.
- M. W. Flower, “Commercial Application and Economic Aspects of Mass Cell Cultures,” In: H. S. Mantel and H. Smith, Eds., Plant Biotechnology, Cambridge University Press, Cambridge, 1983, pp. 3-37.
- R. Arora and S. S. Bhojwani, “In Vitro Propagation and Low Temperature Storage of Saussurea lappa C.B. Clarke—An Endangered, Medicinal Plant,” Plant Cell Reports, Vol. 8, No. 1, 1989, pp. 44-47.
- G. C. Sudha and S. Seeni, “In Vitro Propagation and Field Establishment of Adhatoda beddomei C.B. Clarke, a Rare Medicinal Plant,” Plant Cell Reports, Vol. 13, No. 3-4, 1993, pp. 203-207. doi:10.1007/BF00239893
- D. L. Sulistiarini, “Ocimum gratissimum L,” In: A. P. L. Oyen and D. X. Nguyen, Eds., Plant Resources of SouthEast Asia No. 19: Essential Oils Plants, Prosea Foundation, Bogor, 1999, pp. 140-142.
- T. T. Adebolu and S. A. Oladimeji, “Antimicrobial Activity of Leaf Extracts of Ocimum gratissimum on Selected Diarrhea Causing Bacteria in Southwestern Nigeria,” African Journal of Biotechnology, Vol. 4, No. 7, 2005, pp. 682-684.
- J. A. Lemos, X. S. Passos, O. F. L. Fernandes, J. R. Paula, P. H. Ferri, L. K. H. Souza, A. A. Lemos and M. R. R. Silva, “Antifungal Activity from Ocimum gratissimum L. towards Cryptococcus Neoformans,” Memorias do Instituto Oswaldo Cruz, Vol. 100, No. 1, 2005, pp. 55-58. doi:10.1590/S0074-02762005000100011
- C. V. Nakamura, T. U. Nakamura, E. Bando, A. F. N. Melo, D. A. G. Cortez and B. P. Diaz Filho, “Antibacterial Activity of Ocimum gratissimum L. Essential Oil,” Memorias do Instituto Oswaldo Cruz, Vol. 94, No. 5, pp. 675-678. doi:10.1590/S0074-02761999000500022
- C. N. Ezekwesili, K. A. Obiora and O. P. Ugwu, “Evaluation of Anti-Diarrhoeal Property of Crude Aqueous Extract of Ocimum gratissimum L. (Labiatae) in Rats,” Biokemistri, Vol. 16, No. 2, 2004, pp. 122-131.
- F. B. Holetz, T. U. Nakamura, B. P. D. Filho, D. A. G. Cortez, J. A. M. Diaz and C. V. Nakamura, “Effect of Essential Oil of Ocimum gratissimum on Herpetomonas Samuelpessoai,” Acta Protozoologica, Vol. 42, 2003, pp. 269-276.
- L. M. Pessoa, S. M. Morais, C. M. L. Bevilaqua and J. H. S. Luciano, “Anthelmintic Activity of Essential Oil of Ocimum gratissimum Linn. and Eugenol against Haemonchus contortus,” Veterinary Parasitology, Vol. 109, No. 1-2, 2003, pp. 59-63. doi:10.1016/S0304-4017(02)00253.4
- D. J. Chitwood, “Phytochemical Based Strategies for Nematode Control,” Annual Review of Phytopathology, Vol. 40, 2002, pp. 221-249. doi:10.1146/annurev.phyto.40.032602.130045
- R. E. Veira, R. Fahoer and S. K. Raina, “Genetic Diversity of Ocimum gratissimum L. Based on Volatile Oil Constituents, Flavonoids and RAPD Markers,” Biochemical Systematics and Ecology, Vol. 29. No. 3, 2001, pp. 287-304.
- Wealth of India, “A Dictionary of India Raw Materials and Industrial Products Vol. VII (N-Pe),” CSRI Publication, New Delhi, 1966, pp. 79-89.
- V. H. Heywood, “Flowering Plants of the World,” Oxford University Press, Oxford, 1978, pp. 239-240.
- D. Ajitkumar and S. Seeni, “Rapid Clonal Multiplication through in Vitro Axillary Shoot Proliferation of Aegle marmelos (L.) Corr., a Medicinal Tree,” Plant Cell Reports, Vol. 17, No. 5, 1998, pp. 422-426. doi:10.1007/s002990050418
- E. Prakash, P. S. Sha Valli Khan, P. S. Reddy and K. R. Rao, “Regeneration of Plants from Seed-Derived Callus of Hybanthus Enneaspermus L. Muell., a Rare Ethnobotanical Herb,” Plant Cell Reports, Vol. 18, No. 10, 1999, pp. 873-878. doi:10.1007/s002990050677.
- C. Hu and P. J. Wang, “Meristem Shoot Tip and Bud Culture,” In: A. D. Evans, R. W. Sharp, V. P. Ammirato and Y. Yamada, Eds., Handbook of Plant Cell Culture, Macmillan, New York, 1983, pp. 177-227.
- K. A. Vincent, K. M. Mathew and M. Hariharan, “Micropropagation of Kaemferia galanga L.—A Medicinal Plant,” Plant Cell Tissue and Organ Culture, Vol. 28, No. 2, 1992, pp. 229-230. doi:10.1007/BF00055522
- S. R. Bhat, K. P. S. Chandel and S. K. Malik, “Plant Regeneration from Various Explants of Cultivated Piper Species,” Plant Cell Reports, Vol. 14, No. 6, 1995, pp. 398-402. doi:10.1007/BF00238605
- Y. Sahoo, S. K. Pattnaik and P. K. Chand, “In Vitro Clonal Propagation of an Aromatic Medicinal Herb Ocimum basilicum L. (Sweet Basil) by Axillary Shoot Proliferation,” In Vitro Cellular and Developmental Biology—Plant, Vol. 33, No. 4, 1997, pp. 293-296. doi:10.1007/s11627-997-0053-3
- C. Gopi, Y. S. Nataraja and P. Ponmurugan, “In Vitro Multiplication of Ocimum gratissimum L. through Direct regeneration,” African Journal of Biotechnology, Vol. 5, No. 9, 2006, pp. 723-726.
- T. Murashige and F. Skoog, “A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures,” Physiologia Plantarum, Vol. 15, No. 3, 1962, pp. 473-497. doi:10.111/j.1399-3054.1962.tb08051.x
- M. G. Murray and W. F. Thompson, “Rapid Isolation of High Molecular Weight Plant DNA,” Nucleic Acids Research, Vo. 8, No. 19, 1980, pp. 4321-4325. doi:10.1093/nar/8.19.4321
- J. Smbrook and D. W. Russed, “Molecular Cloning. A Laboratory Manual,” 3rd Edition, Spring Harbor Laboratory Press, Cold Spring Harbor, 2001.
- D. B. Duncan, “Multiple Range and Multiple F Test,” Biometrics, Vol. 11, No. 1, 1955, pp. 1-42. doi:10.2307/3001478
- R. M. Skirvin, O. McMeans and W. L. Wang, “Storage Water Is a Source of Latent Bacterial Contamination in Vitro,” Trends of Agricultural Science, Vol. 1, 1993, pp. 63-63.
- J. Gopal, J. L. Minocha and H. S. Dhaliwal, “Microtuberization in Potato (Solanum tuberosum L.),” Plant Cell Reports, Vol. 17, No. 10, 1998, pp. 794-798. doi:10.1007/s002990050485
- S. K. Guru, R. Chandra, S. Khetrapal, A. Raj and R. Palisetty, “Protein Pattern in Differentiating Explants of Chick Pea (Cicer arietinum L.),” Indian Journal of Plant Physiology, Vol. 4, 1999, pp. 147-151.
- J. Sen and A. K. Sharma, “Micropropagation of Withania somnifera from Germinating Seeds and Shoot Tips,” Plant Cell Tissue and Organ Culture, Vol. 26, No. 2, 1991, pp.71-73. doi:10.1007/BF00036108
- P. S. George, G. A. Ravishankar and L. V. Venkataraman, “Clonal Multiplication of Gardenia jaminoides Elllis through Axillary Bud Culture,” Plant Cell Reports, Vol. 13, No. 1, 1993, pp. 59-62. doi:10.1007/BF0023237
- A. Ahuja, M. Verma and S. Grewal, “Clonal Propagation of Ocimum Species by Tissue Culture,” Indian Journal of Experimental Biology, Vol. 20, 1982, pp. 455-458.
- F. Begum, N. Amin and M. A. K. Azad, “In Vitro Rapid Clonal Propagation of Ocimum basilicum L.,” Plant Tissue Culture and Biotechnology, Vol. 12, No. 1, 2002, pp. 27-35.
- S. Saha and P. D. Ghosh and C. Sengupta, “An Efficient Method for Micropropagation of Ocimum basilicum L.,” Indian Journal of Plant Physiology, Vol. 15, No. 2, 2010. pp. 168-172.
- L. V. Hiregoudar, H. N. Murthy, J. G. Bhat, A. Nayeem, B. P. Hema, E. J. Hahn and K. Y. Paek, “Rapid Clonal Propagation of Vitex trifolia,” Biologia Plantarum, Vol. 50, No. 2, 2006, pp. 291-294. doi:10.1007/s10535-006-0023-3
- S. Saha and P. D. Ghosh and C. Sengupta, “In Vitro Multiple Shoot Regeneration of Mentha piperita,” Journal of Tropical Medicinal Plants, Vol. 11. No. 1, 2010, pp. 89- 92.
- S. Chattopadhyay, S. K. Datta and S. B. Mahato, “Rapid Micropropagation for Mucuna pruriens f. Pruriens L.,” Plant Cell Reports, Vol. 15, No. 3-4, 1995, pp. 271-273. doi:10.1007/BF00193734
- M. Faisal, I. Siddique and M. Anis, “An Efficient Plant Regeneration System for Mucuna Pruriens L. (DC.) Using Cotyledonary Node Explants,” In Vitro Cellular and Developmental Biology—Plant, Vol. 42, No. 1, 2006, pp. 59-64. doi:10.1079/IVP2005717
- P. K. Tewary, M. K. Raghunath, M. Venkateshwarulu and A. Sarkar, “Genotypic Difference in Response to in Vitro Shoot Development of Mulberry (Morus Spp.),” Indian Journal of Sericulture, Vol. 35, No. 2, 1996, pp. 104-106.
- C. Suresh and K. S. Ajay, “In Vitro Shoot Regeneration from Cotyledonary Node Explants of a Multipurpose Leguminous Tree, Pterocarpus marsupium Roxb,” In Vitro Cellular and Developmental Biology-Plant, Vol. 40, No. 2, 2004, pp. 167-170. doi:10.1079/IVP2003488
- M. Hossain, B. K. Biswas, M. R. Karim, S. Rahman, R. Islam and O. I. Jorder, “In Vitro Organogenesis of Elephant Apple (Feronia limonia),” Plant Cell Tissue and Organ Culture, Vol. 39, No. 3, 1994, pp. 265-268. doi:10.1007/BF00035980
- L. V. Hiregoudar, H. N. Murthy, B. P. Hema, E. J. Hahn and K. Y. Peak, “Multiple Shoot Induction and Plant Regeneration of Feronia limonia (L.) Swingle,” Scientia Horticulturae, Vol. 98, No. 4, 2003, pp. 357-364. doi:10.1016/S0304-4238(03)00018-9
- V. Tiwari, K. N. Tiwari and B. D. Singh, “Comparative Studies of cytokinins on in Vitro Propagation of Bacopa monniera,” Plant Cell Tissue and Organ Culture, Vol. 66, No. 1, 2001, pp. 9-16. doi:10.1023/A:1010652006417
- I. Siddique and M. Anis, “In Vitro Shoot Multiplication and Plantlet Regeneration from Nodal Explants of Cassia angustifolia (Vahl): A Medicinal Plant,” Acta Physiologiae Plantarum, Vol. 29, No. 3, 2007, pp. 233-238. doi:10.1007/s11738-007-0029-2
- P. C. Debergh and L. J. Maene, “A Scheme for Commercial Propagation of Ornamental Plants by Tissue Culture,” Scientia Horticulturae, Vol. 14, 1981, pp. 335-345.
- R. H. Wetmore and C. W. Wardlaw, “Experimental Morphogenesis in Vascular Plants,” Annual Review of Plant Physiology, Vol. 2, 1951, pp. 269-292. doi:10.1146/annurev.pp.02.060151.001413
- C. Detrez, R. S. Sangwan and B. S. Sangwan-Norreel, “Phenotypic and Karyotypic Status of Beta Vulgaris Plants Regenerated from Direct Organogenesis in Petiole Culture,” Theoretical and Applied Genetics, Vol. 77, No. 4, 1989, pp. 462-468. doi:10.1007/BF00274264
- L. B. Andrade, S. Echeverrugaray, F. Fracaro, G. F. Pauletti and L. Rota, “The Effect of Growth Regulators on Shoot Propagation and Rooting of Common Lavender (Lavandula vera DC),” Plant Cell Tissue and Organ Culture, Vol. 56, No. 2, 1999, pp. 79-83. doi:10.1023/A:1006299410052
- S. Saha, T. Dey and P. D. Ghosh, “Micropropagation of Ocimum Kilimandscharicum Guerke (Labiatae),” Acta Biologica Cracoviensia Series Botanica, Vol. 52, No. 2, 2010, pp. 50-58. doi:10.2478/v10182-010-0023-7
- J. A. Driver and G. R. Suttle, “Nursery Handling of Propagules,” In: J. M. Bonga and D. J. Durzan, Eds., Cell and Tissue Culture in Forestry, Dordrecht, Netherlands, 1987, pp. 320-335. doi:10.1007/978-94-009-4484-8_17
- S. Cuenca, J. B. Amo-Marco and R. Parra, “Micropropagation from Inflorescence Stems of the Spanish Endemic Plant Centaurea Paui Loscos ex Willk. (Compositae),” Plant Cell Reports, Vol. 18, No. 7-8, 1999, pp. 674-679. doi:10.1007/s002990050641
- Y. Sahoo and P. K. Chand, “Micropropagation of Vitex negundo L., a Woody Aromatic Medicinal Shrub, through High-Frequency Axillary Shoot Proliferation,” Plant Cell Reports, Vol. 18, No. 3-4, 1998, pp. 301-307. doi:10.1007/s002990050576
- N. Komalavalli and M. V. Rao, “In Vitro Micropropagation of Gymnema Sylvestre—A Multipurpose Medicinal Plant,” Plant Cell Tissue and Organ Culture, Vol. 61, No. 2, 2000, pp. 97-105. doi:10.1023/A:1006421228598
- H. R. Juliani Jr., A. R. Koroch, H. R. Juliani and V. S. Trippi, “Micropropagation of Lippia junelliana (Mold.) Trone,” Plant Cell Reports, Vol. 59, No. 3, 1999, pp. 175-179. doi:10.1023/A:1006396531647
- M. Faisal and M. Anis, “Rapid Mass Propagation of Tylophora Indica Merrill via Leaf Callus Culture,” Plant Cell Tissue and Organ Culture, Vol. 75, No. 2, 2003, pp. 125-129. doi:10.1023/A:1025084203958
- J. E. Simon, J. Quinn and R. G. Murray, “Basil: A Source of Essential Oils,” In: J. Janick and J. E. Simon, Eds., Advances in New Crops, Timber, Portland, 1990, pp 484- 989.
- G. J. De Klerk, “How to Measure Somaclonal Variation,” Acta Botanica Neerlandica, Vol. 39, 1990, pp. 129-144.
- M. K. Smith, “A Review of Factors Influencing the Genetic Stability of Micropropagated Bananas,” Fruits, Vol. 43, No. 4, 1988, pp. 219-223.
- Y. R. Dutta, G. Gangopadhyay, S. Das, B. K. Dutta and K. K. Mukherjee, “Esterase as a Marker to Study the Genetic Fidelity of Micropropagated Banana,” Biologia Plantarum, Vol. 47, No. 3, 2003, pp. 421-424. doi:10.1023/B:BJOP.0000023886.9365.b4
- M. K. U. Chawdhury and J. K. Vasil, “Molecular Analysis of Plants Regenerated from Embryogenic Cultures of Hybrid Sugarcane Cultivars (Saccharum spp.),” Theoretical and Applied Genetics, Vol. 86, No. 2-3, 1993, pp. 181-188. doi:10.1007/BF00222077
- V. Rani, A. Parida and S. N. Raina, “Random Amplified Polymorphic DNA (RAPD) Markers for Genetic Analysis in Micropropagated Plants of Populus Deltoides Marsh,” Plant Cell Reports, Vol. 14, No. 7, 1994, pp. 459-462. doi:10.1007/BF00234055
- R. K. Chaudhuri, A. Pal and T. B. Jha, “Production of Genetically Uniform Plants from Nodal Explants of Swertia Chirata Buch. Ham. Ex Wall—A Critically Endangered Medicinal Herb,” In Vitro Cellular and Developmental Biology, Vol. 43, No. 5, 2007, pp. 467-472. doi:10.1007/s11627-007-9095-9
- S. Bhattacharya, T. K. Bondopadhyay and P. D. Ghosh, “Somatic Embryogenesis in Cymbopogon Pendulus and Evaluation of Clonal Fidelity of Regenerants Using ISSR Marker,” Scientia Horticulturae, Vol. 123, No. 4, 2010, pp. 505-513. doi:10.1016/j.scienta.2009.10.011
- J. G. K. Williams, A. R. Kubelik, K. J. Livak, J. A. Rafalski and S. V. Tingey, “DNA Polymorphisms Amplified by Arbitrary Primers Are Useful as Genetic Markers,” Nucleic Acids Research, Vol. 18, No. 22, 1990, pp. 6531- 6535. doi:10.1093/nar/18.22.6531

