Modern Research in Catalysis
Vol.2 No.3(2013), Article ID:33739,7 pages DOI:10.4236/mrc.2013.23014
The Catalytic Copolymerization of Ethene with Carbon Monoxide Efficiently Carried out in Water-Dichloromethane-Sodium Dodecylsulfate Emulsion
Department of Molecular Sciences and Nanosystems, Ca’ Foscari University of Venice, Venice, Italy
Email: *vavasori@unive.it
Copyright © 2013 Andrea Vavasori et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 1, 2013; revised March 16, 2013; accepted April 18, 2013
Keywords: Palladium Catalyst; Carbonylation; Copolymerization; Polyketone
ABSTRACT
The CO-ethene copolymerization has been efficiently carried out in the water/CH2Cl2 emulsion by using water insolvable Pd(II) complexes. By using the surfactant SDS very high molecular weight copolymers have been obtained with high productivity (ca. 13,000 g/(gPd.h)).
1. Introduction
The perfectly alternated poly(1-oxo-trimethylene), commonly called polyketone (PK in reaction 1), is a thermoplastic that has peculiar chemical, physical and mechanical characteristics of considerable interest for a wide range of applications [1-5].
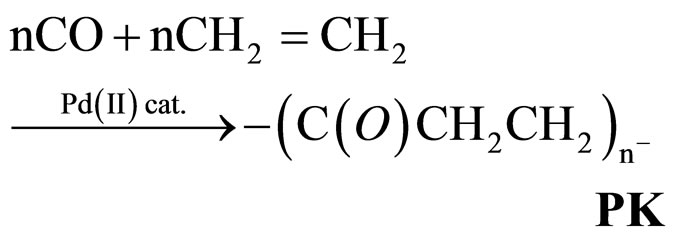 (1)
(1)
The most active catalyst are cationic Pd(II) complexes having two coordination sites occupied by a chelating diphosphine ligand [6-13]. The catalytic activity (but also the PK molecular weight), however, strongly depends also by the reaction conditions (temperature, pressure of monomers and batch time [14-20]), and by the nature of the solvent [21]. On regard to the solvent, methanol is the most widely used even though water, which represents the ideal choose in terms of sustainability, is used too. The reactions carried out in water, however, usually lead to PK with higher molecular weight in comparison to the polymer obtained in methanol as a solvent [19-22]. As matter of fact, the more active catalysts (used for the reaction in methanol) are inactive or poorly active in water, because unsolvable. Therefore, water-solvable Pd(II)- diphosphine complexes have to be used which some time shows a lower catalytic activity. These are mainly obtained by sulfonation of the diphosphine ligands (ca. 7 kg PK/(gPd∙h) [22-28]), but also by fitting the phosphorus atoms with hydroxyl alkyl groups [29,30], or generally by using tenside phosphorus ligands [31].
In some papers it is reported, however, the use of water-insolvable Pd(II) complexes in reactions carried out in emulsion copolymerization (productivity ca. 650 g/ (gPd·h) of PK) [32], or by using acetic or formic acid as a co-solvent (ca. 26,000 g/(gPd·h) of PK) [33].
As a means of implementing the emulsion CO-ethene copolymerization and with the aim to suggest new more sustainable reaction conditions to obtain high molecular weight PK, in the present paper we solubilise the water-insoluble Pd(II)-complexes [PdCl2(dppp)] (dppp = 1,3- bis(diphenylphosphino)propane) in an water/dichloromethane emulsion, formed by using the commercial sodium dodecylsulfate (SDS), as surfactant.
2. Experimental Section
2.1. Reagents
The complexes [PdX2(dppp)] X = Cl, TsO (tosilate), OAc (acetate), were prepared as reported in literature [4,7]. Carbon monoxide and ethene were supplied by SIAD Company Italy (“research grade”, purity > 99.9%). Dppp, TsOH (p-toluenesulfonic acid), and SDS were Aldrich products.
2.2. Equipments and Characterization
The catalyst precursor was weighted on a Sartorious Micro balance (precision 0.001 mg). The polymers were analyzed by FTIR and NMR spectroscopies. FTIR spectra were recorded on a Nicolet Magna 750 instrument in KBr powder. The IR spectra show typical stretching signals of CO groups at 1695 cm−1 and -CH2- groups at 2915 cm−1. All the NMR spectra were recorded on a Bruker Avance 300 spectrometer. The 1H NMR and 13C NMR spectra of the polyketone were recorded in 1,1,1,3, 3,3-hexafluoroisopropanol/CDCl3 (10/1) using the Inverse 1H-Gated Decoupling Technique. The 13C NMR spectra, show a single carbonyl absorption at 212.65 ppm (-C(O)CH2CH2-) and a single resonance for the -CH2- groups at 35.73 ppm (-C(O)CH2CH2-) in the ratio 1:2 due to the exclusive perfectly alternated structure.
The average viscosity molecular weight of polymer has been evaluated as Limit Viscosity Number (LVN). The LVN (or [η]) of a dilute PK solution was determined by using the Huggins relationship between the viscosity number and the polymer concentration by extrapolation to zero concentration [34]. The PK solution was prepared in m-cresol as a solvent and the viscosity was measured by using a Cannon-Fenske type capillary viscosimeter, thermostated at 25˚C. The average viscosity molecular weight (Mw) of the polyketone was derived from the LVN using the Mark-Houwink equation [35].
The solubility of gases was measured using an absorption technique in a high pressure stainless steel autoclave of 300 mL capacity as described in literature [36].
2.3. Copolymerization
The copolymerization reactions were carried out by using a Hastelloy C autoclave of ca. 250 mL provided with a four-blade self-aspirating turbine. In a typical experiment, 0.724 mg of [PdCl2(dppp)] (1.23 × 10−3 mmol) was added to 80 mL of water containing the dosed amount of surfactant and then placed in the autoclave. The mixture was subjected to high shear till a homogeneous liquid is obtained. The autoclave was washed by pressurizing with a 1/1 mixture of CO/C2H4 (ca. 0.5 MPa) and then depressurizing to atmospheric pressure (this cycle was repeated 5 times, at room temperature with stirring). The washed autoclave was pressurized with 0.5 MPa of the gas mixture and heated to 90˚C in ca. 10 min without stirring. At the reaction temperature the pressure was adjusted to the desired value (typically 4.5 MPa total pressure) and, while stirring, maintained constant throughout the experiment (rate stirring 700 rpm). At the end of the experiment the autoclave was quickly cooled and carefully depressurized. The polymer is insoluble in H2O and the slurry obtained was filtered, washed several times with acetone and dried under vacuum at 70˚C. The dried polymer was weighted and the productivity was calculated as gPK.(gPd∙h)−1; the reproducibility was within ca. 5%.
3. Results and Discussion
The water insolvable [PdCl2dppp] complex is used to carried out the CO-ethene copolymerization in the CH2Cl2/H2O reaction medium. The Figure 1 shows the influence on the catalytic activity of the CH2Cl2/H2O ratio and of the SDS addition.
Although CH2Cl2 readily dissolves the Pd(II) complex and is also a good solvent for both monomers (comparable to methanol), as suggested by the Henry’s law constants in the Table 1, we found that the catalyst is
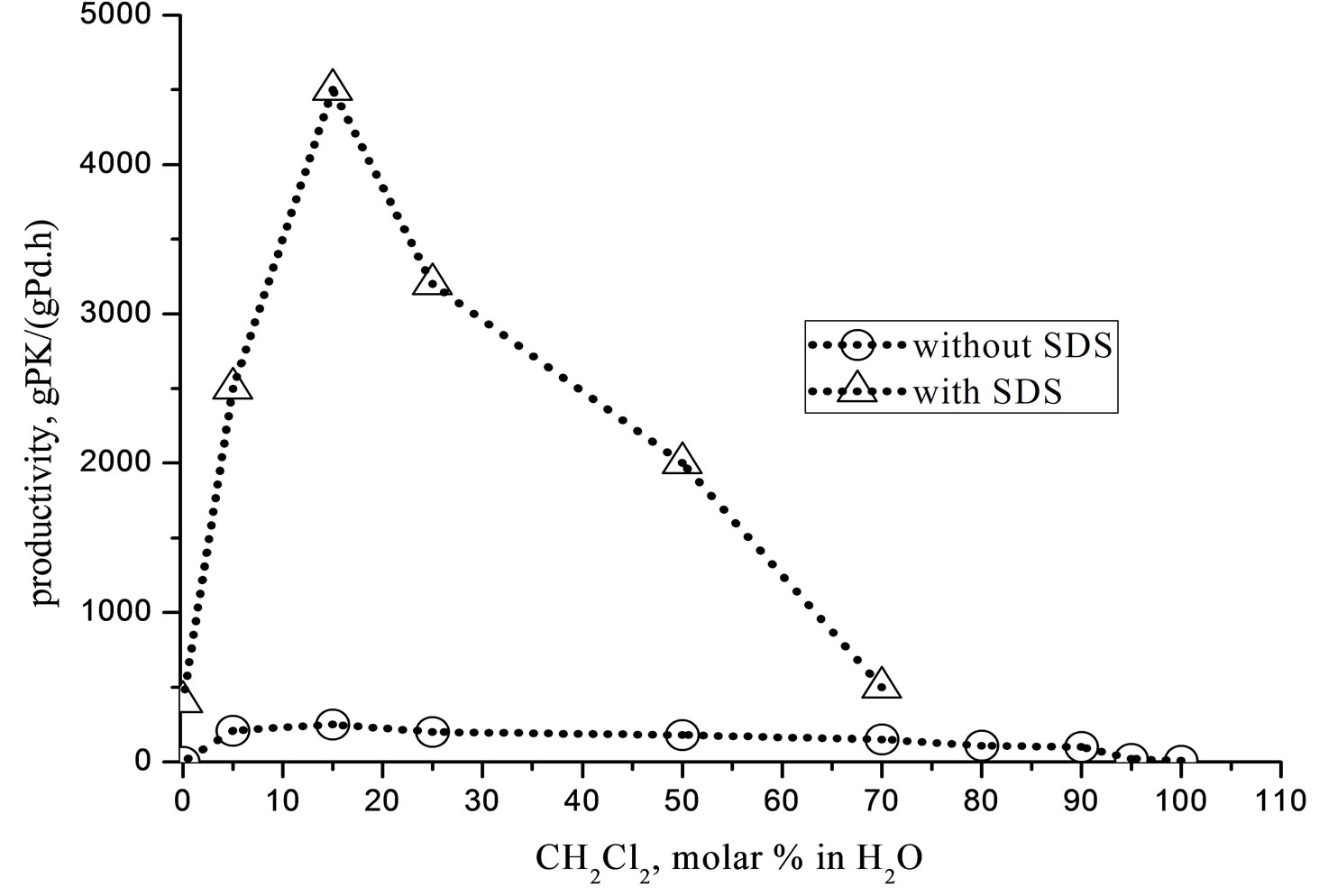
Figure 1. Influence of the CH2Cl2/H2O on the productivity. Run conditions: [PdCl2(dppp)] = 8.5 × 10−3 mmol; O: without SDS; Δ: with SDS 5 mM; volume = 80 mL; T = 90˚C; P = 4.5 MPa (CO/C2H4 = 1/1); reaction time = 1 h; stirrer = 700 rpm.
inactive, according with the suggestion that in pure CH2Cl2 the initiating Pd-H species cannot form in absence of any H-donors species (for instance H2O or alcohol, see mechanism).
On the other hand, in pure H2O the catalyst is inactive too, probably due to the poor solubility of the Pd(II) complex, which floats on the solvent. By mixing CH2Cl2- H2O together, in the absence of any surfactant, two stable immiscible phases readily form where under vigorous stirring the catalyst become active. The productivity was ca. 150 - 200 gPK/(gPd h), at CH2Cl2 concentration within the range of 15 - 20 molar %.
The addition of a dosed amount of the surface active agent SDS increases the catalytic activity, which passes through a maximum of ca. 4500 gPK/(gPd h) at CH2Cl2 20 molar %.
The surfactant favors the formation of droplets in which the monomers and the catalyst are highly partitioned (see solubility) into the CH2Cl2 phase relatively to the aqueous phase. It can form CH2Cl2 droplets in water (O/W emulsion) or vice versa H2O droplets in CH2Cl2 (W/O emulsion).
In any cases the formation of an emulsion leads to an increase of the contact area between the two phases favoring, therefore, the catalysis which occurs at the CH2Cl2/H2O interfaces (H2O or TsOH have to react on the metal center, see the Figure 2).
The Figure 1 shows that at CH2Cl2 higher than 20 molar % the productivity decreases, suggesting a correlation with the phase inversion. According to this, under W/O emulsion conditions (high CH2Cl2 concentration) the catalyst and the monomers are diluted in the organic bulk with a negative consequence on the reaction kinetic.
The addition of the acid promoter (TsOH) to the SDSCH2Cl2/H2O (20 molar %) emulsion increases the productivity up to a plateau of ca. 8000 g/(gPd·h) (Figure 3).
As widely described in literature, the addition of acid promoters avoids the catalyst deactivation to inactive or
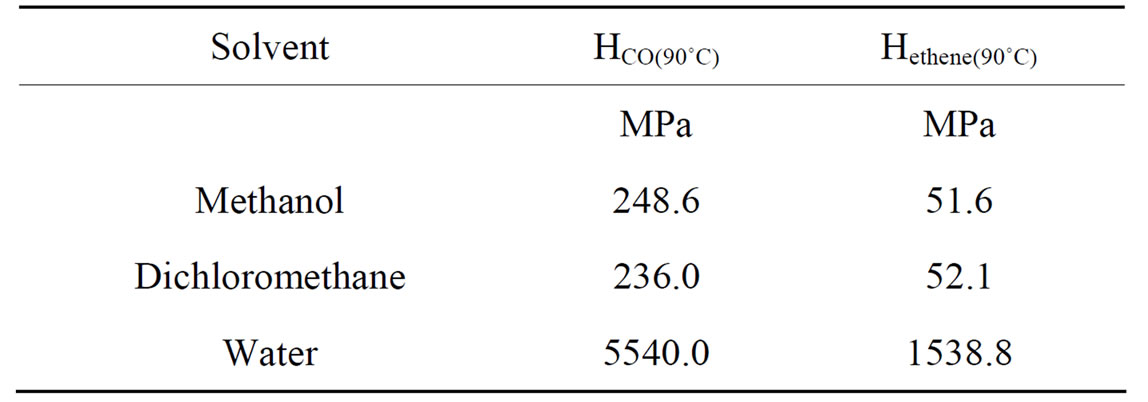
Table 1. Henry’s law constant measured at 90˚C in different solvents.
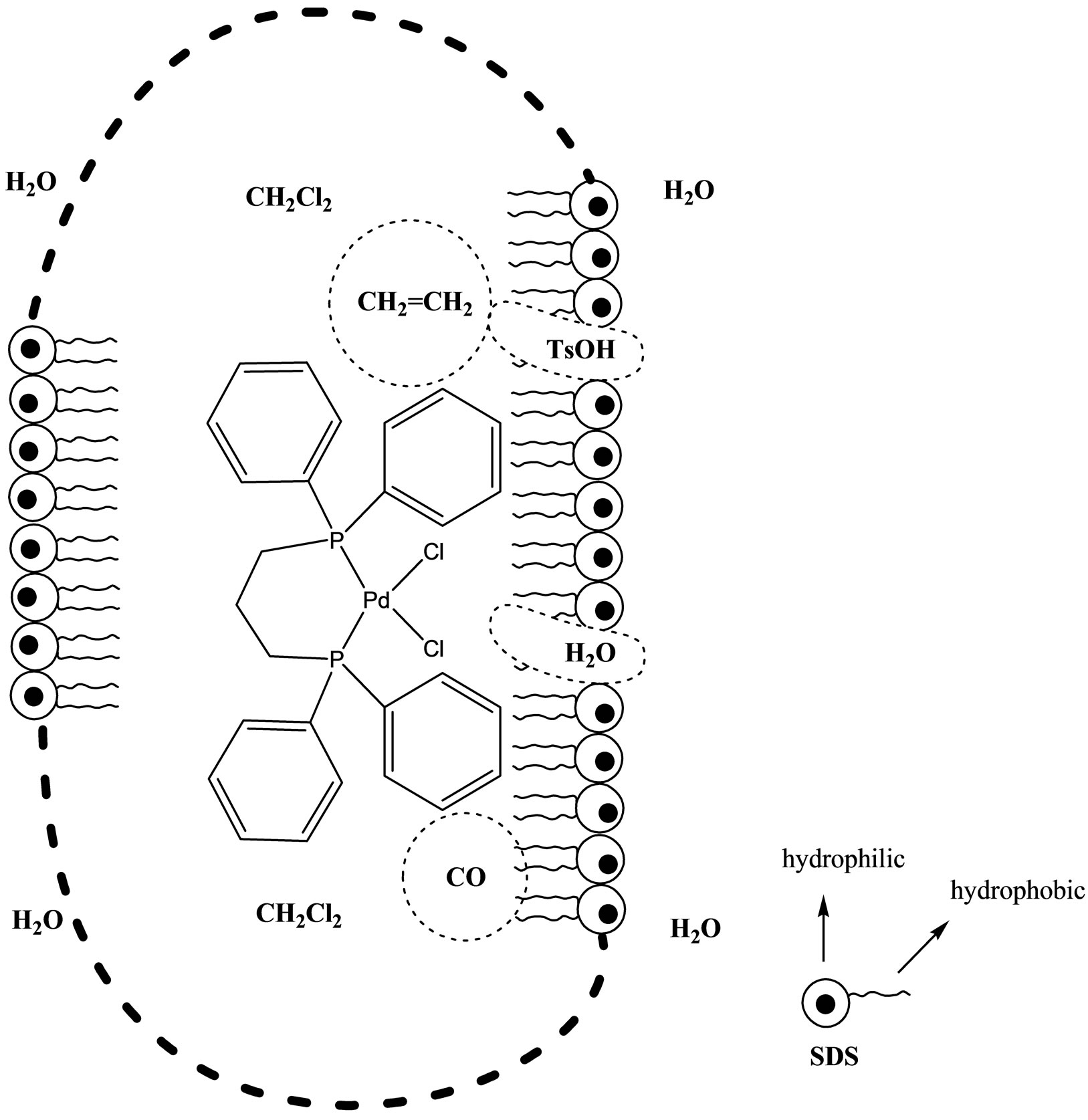
Figure 2. Schematic representation of CH2Cl2 droplet in water.
less active Pd(0) species, Pd metal included, which normally occurs under the reductive conditions of the reaction [6-12].
The Figure 4 shows that by optimizing the SDS concentration, the productivity passes through a maximum, which was ca. 13,000 g·PK/(gPd·h) at SDS concentration of ca. 10 mM.
The increase of productivity confirms that the catalysis is favored by the formation of an increasing amount of stable droplets (critical micelle concentration is 8.3 mM for SDS at 25˚C [37]. As matter of facts, the emulsion increases the O/W interface area, where the Pd(II) activation can occur more efficiently (see Figure 2).
At SDS concentration higher than ca. 10 mM, however, the decrease of productivity suggests the influence of two possible phenomena: the droplets coalescence and the formation of foams [38]. The surfactant can favor the coalescence which causes a decrease of the O/W interface area and, at the same time, can favor the formation of foams, negative for the catalysis as the reaction medium becomes inefficiently stirred and reaction rates limited by diffusion phenomena.
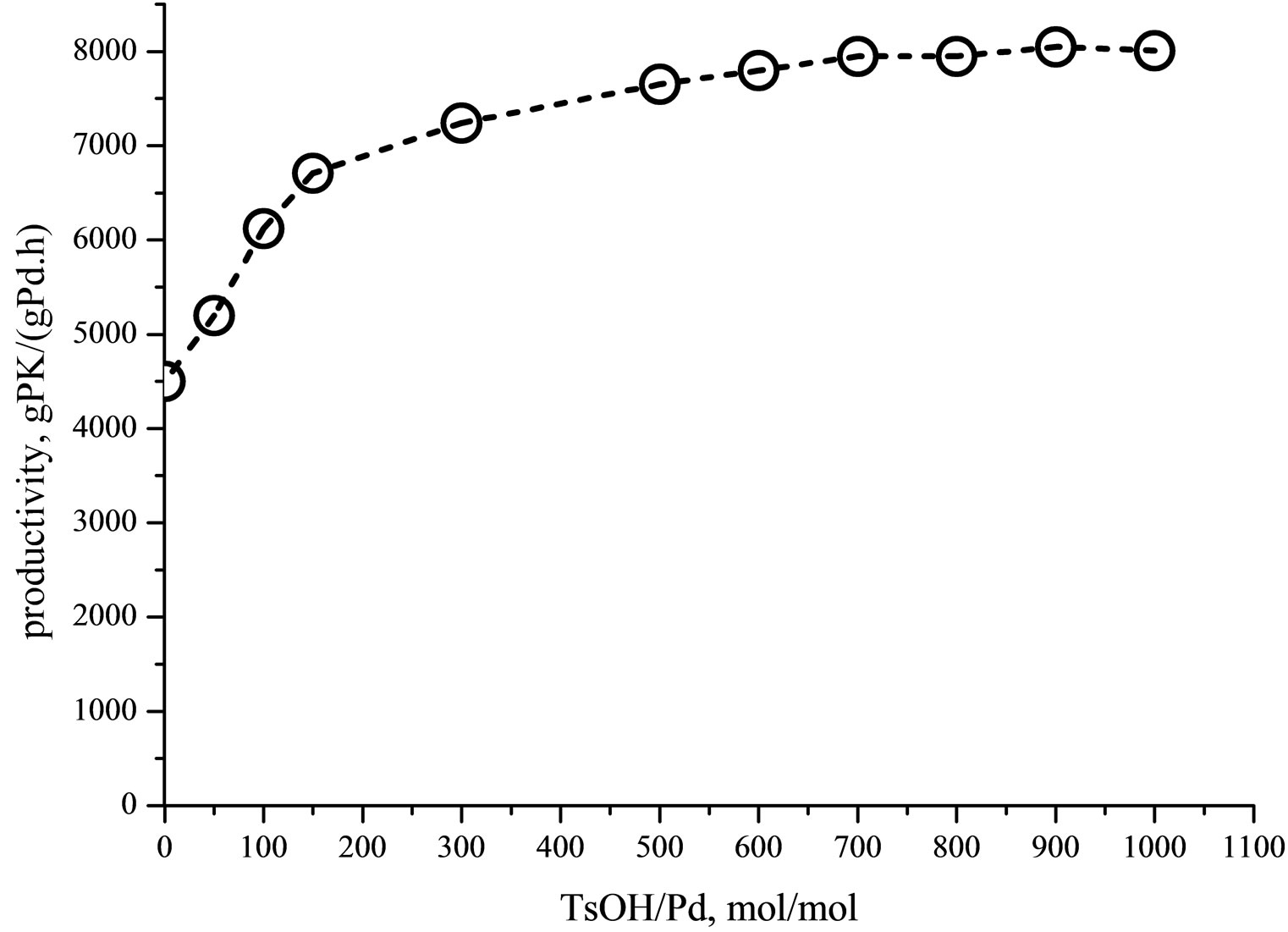
Figure 3. Influence of p-toluenesulfonic acid concentration on the productivity. Run conditions: [PdCl2(dppp)] = 8.5 × 10−3 mmol; SDS: 5 mM; SDS; volume = 80 ml; T = 90˚C; P = 4.5 MPa (CO/C2H4 = 1/1); reaction time = 1 h; stirrer = 700 rpm.
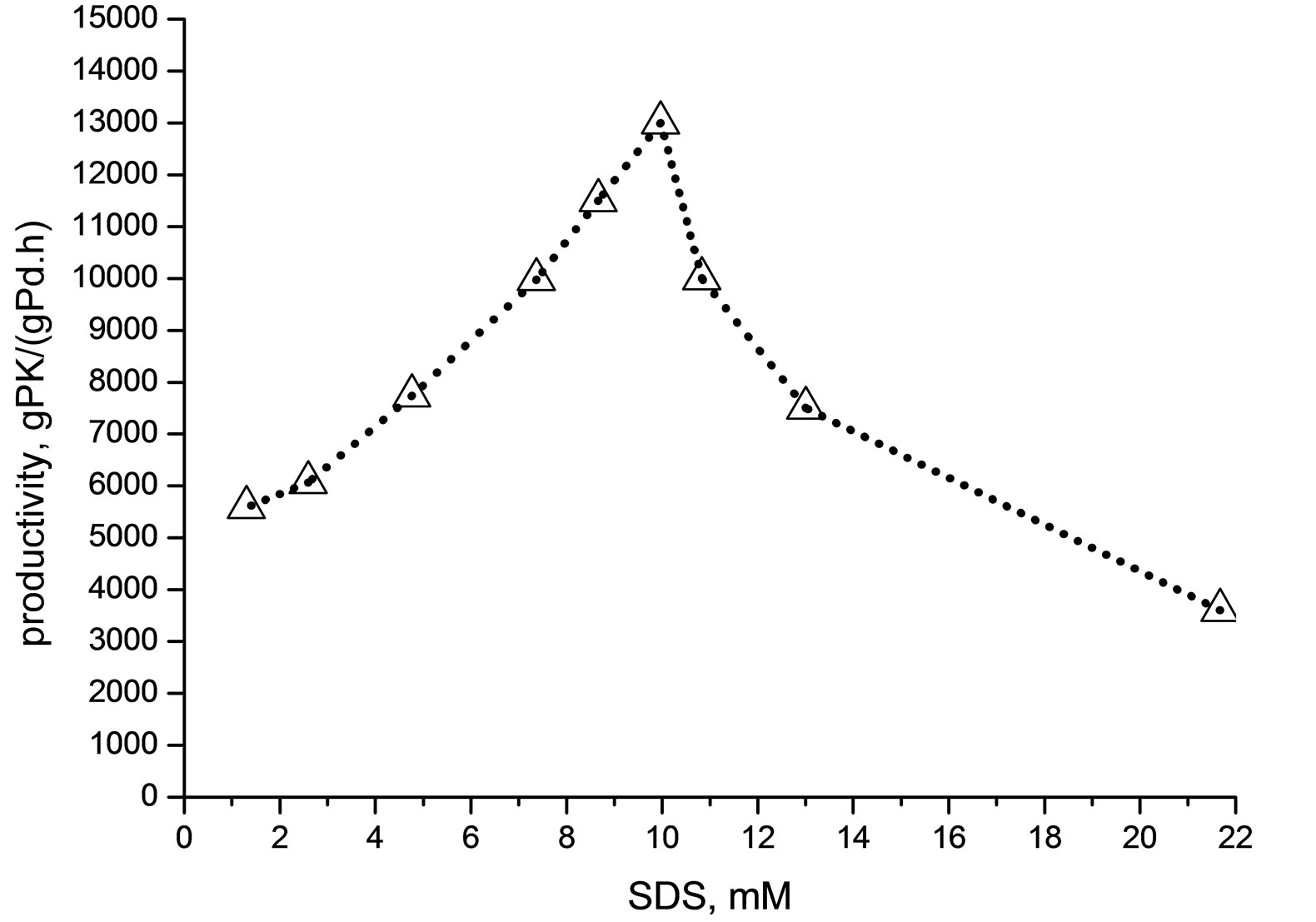
Figure 4. Influence of SDS concentration on the productivity. Run conditions: [PdCl2(dppp)] = 8.5× 10−3 mmol; O: TsOH/Pd: 800/1, mol/mol; volume = 80 ml; T = 90˚C; P = 4.5 MPa (CO/C2H4 = 1/1); reaction time = 1 h; stirrer = 700 rpm.
The PK obtained had a Limiting Viscosity Number (LVN) of 1.8 dL/g which is higher respect to the LVN obtained in methanol (0.5 dL/g). Table 2 shows the LVN and the respective viscosity average molecular weight of the PKs obtained in different solvents.
Among the solvents used in the Table 2, the SDS/ CH2Cl2/H2O emulsion copolymerization leads to the highest LVN, suggesting the following considerations on the reaction mechanism. It is accepted that in water the neutral Pd(II)-H intermediate is firstly generated from the precursor by a reaction strictly related to the water gas shift [6-12], and then converted into the catalytically active cationic Pd(II)-H+ species by a water-controlled solvolysis process [8] (Scheme 1, reactions a-b).
The fast migratory insertion of ethene into the Pd-H bond starts the copolymerization (Step c). The alternate and successive insertions of CO and ethene lead to the growth of the polymer chain (Steps d-e): the chainswollen drops quickly turn into insoluble polymer particles and the final result is a dispersion of PK particles in water.
Termination occurs, as proposed in literature [6-12], via protonolysis with H2O of the Pd-(CH2CH2-polymer)+ species (Step f), which gives the PK and the Pd-OH+ species. The latter can insert CO to form the Pd-C(O)OH+ intermediate (Step g), which gives β-hydride elimination with CO2 evolution (Step h). The active Pd-H+ species are formed again, which restart the catalytic cycle.
According with the literature and supported by the NMR analysis, which indicate the presence of only -C(O)CH2CH3 end-groups, in the SDS/CH2Cl2/H2O emulsion the termination can occur only through reaction with H2O (protonolysis) or with the acid. Protonolysis with H2O or TsOH are slower than with methanol and under emulsion condition occur mostly at the interface, being H2O and TsOH in the bulk, out of the droplets in the O/W emulsion. On the contrary, the propagation occurs inside the droplets where the monomers concentration is higher (see Introduction) in accord with the high productivity obtained. Furthermore, the high propagation rate together with the slow termination rate favor the formation of PKs with high average molecular weight.
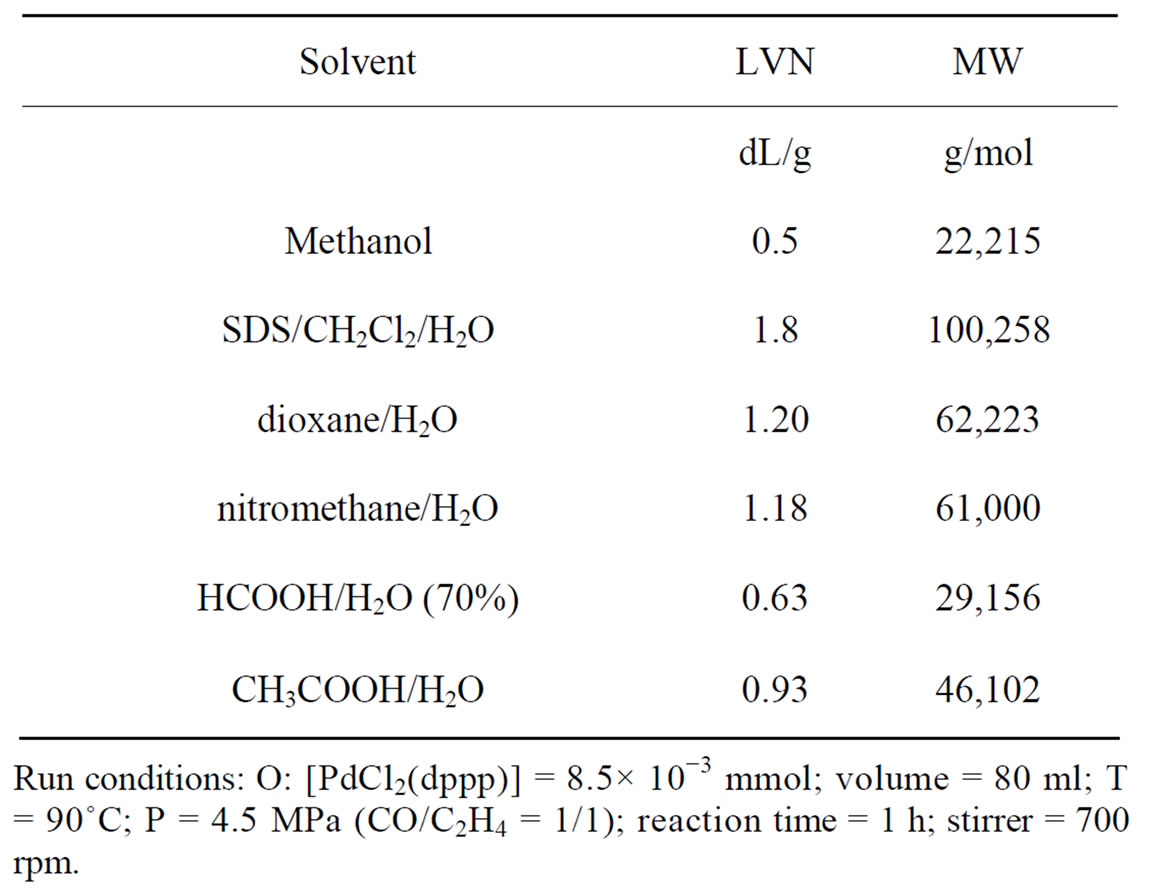
Table 2. LVN and viscosity average molecular weight of PK in different solvents.
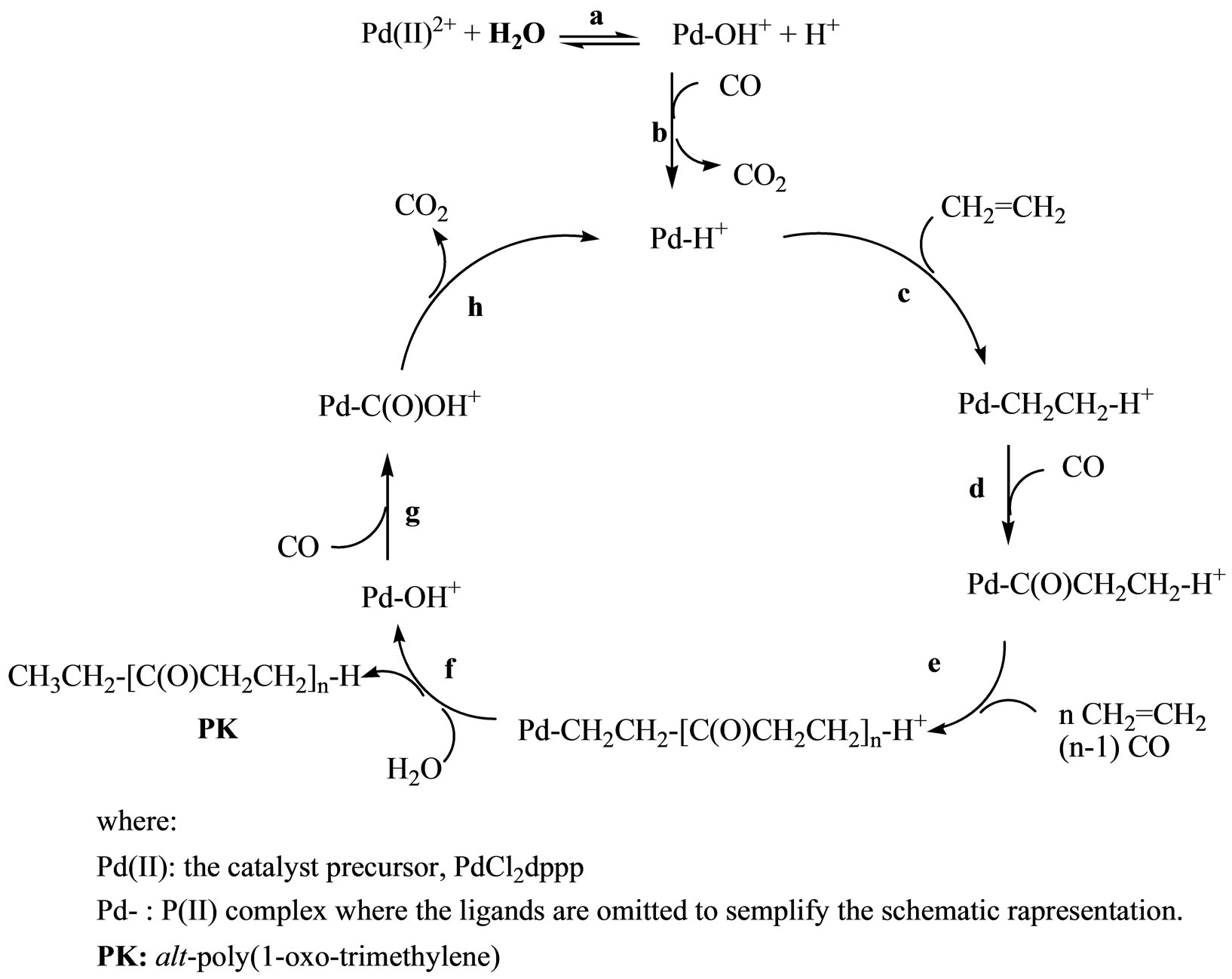
Scheme 1. Proposed reaction mechanism.
4. Conclusion
The catalyzed CO-ethene copolymerization has been efficiently performed in a SDS/H2O/CH2Cl2 emulsion by using Pd(II) water insolvable complexes. The optimization of such reaction systems shows a catalytic activity higher (ca.13,000 g/(gPd·h)) than in methanol as a solvent (ca. 8000 g/(gPd·h)). The best catalyst performance was reached with SDS 10 mM and 20 molar % of CH2Cl2 in H2O. Under conditions of the maximum productivity the PK obtained showed a molecular weight higher than in methanol (LVN was 1.8 dL/g respect to 0.5 dL/g in methanol).
REFERENCES
- A. Sommazzi and F. Garbassi, “Olefin-Carbon Monoxide Copolymers,” Progress in Polymer Science, Vol. 22, No. 8, 1997, pp. 1547-1605. doi:10.1016/S0079-6700(97)00009-9
- E. Drent, W. P. Mul and A. A. Smaardijk, “Encyclopedia of Polymer Science and Technology,” 3rd Edition, John Wiley & Sons, Hoboken, 2002.
- W. P. Mul, A. W. van der Made, A. A. Smaardijk and E. Drent, “Catalytic Synthesis of Copolymers and Terpolymers,” In: A. Sen (Ed.), Catalytic Synthesis of AlkeneCarbon Monoxide Copolymers and Oligomers, Kluwer Academic Publishers, Amsterdam, 2003, pp. 87-140. doi:10.1007/978-1-4419-9266-6_4
- R. Taniguchi, J. Kato and T. Komatsu, “Polyketone and Method for Producing the Same,” Patent US 7666975 B2, Asahi Kasei Fibers Corp., Japan, 2010.
- J.-Y. Jang, J.-I. Choi, H.-S. Cho, J.-Y. Shim, S.-K. Yoon, H.-S. Kim, L. Toniolo and A. Vavasori, “US 161531,” Hyosung Corp (Kr), Seoul, 2008.
- E. Drent, J. A. M. van Broekhoven and M. J. Doyle, “Efficient Palladium Catalysts for the Copolymerization of Carbon Monoxide with Olefins to Produce Perfectly Alternating Polyketones,” Journal of Organometallic Chemistry, Vol. 417, No. 1-2, 1991, pp. 235-251. doi:10.1016/0022-328X(91)80176-K
- E. Drent and P. H. M. Budzelaar, “Palladium-Catalyzed Alternating Copolymerization of Alkenes and Carbon Monoxide,” Chemical Reviews, Vol. 96, No. 2, 1996, pp. 663-682. doi:10.1021/cr940282j
- C. Bianchini and A. Meli, “Alternating Copolymerization of Carbon Monoxide and Olefins by Single-Site Metal Catalysis,” Coordination Chemistry Reviews, Vol. 225, No. 1-2, 2002, pp. 35-66. doi:10.1016/S0010-8545(01)00405-2
- A. Sen, “Catalytic Synthesis of Alternating Alkene-Carbon Monoxide Copolymers,” Kluwer, Amsterdam, 2003.
- G. P. Belov and E. V. Novikova, “Polyketones as Alternating Copolymers of Carbon Monoxide,” Russian Chemical Reviews, Vol. 73, No. 3, 2004, pp. 267-291. doi:10.1070/RC2004v073n03ABEH000840
- P. W. N. M. van Leeuwen, “Homogeneous Catalysis: Understanding the Art,” Kluwer Academic Publisher, Amsterdam, 2004.
- G. Cavinato, L. Toniolo and A. Vavasori, “Carbonylation of Ethene in Methanol Catalysed by Cationic Phosphine Complexes of Pd(II): from Polyketones to Monocarbonylated Products,” Topics in Organometallic Chemistry, Vol. 18, 2006, pp. 125-164. doi:10.1007/3418_019
- P. W. N. M. van Leeuwen and Z. Freixa, “Bite Angle Effects of Diphosphines in Carbonylation Reactions,” In: L. Kollár, Ed., Modern Carbonylation Methods, Wiley- VCH, 2008, pp. 1-20.
- A. Vavasori, G. Cavinato and L. Toniolo, “Carbon MoNoxide-Ethylene Copolymerization Catalyzed by a Pd(OAc)2/ Dppp/Formic Acid System [dppp=1,3-bis(diphenylphosphino)propane],” Journal of Molecular Catalysis A: Chemical, Vol. 191, No. 2, 2003, pp. 209-215. doi:10.1016/S1381-1169(02)00174-7
- A. Vavasori, L. Toniolo, G. Cavinato and F. Visentin, “Highly Active [Pd(AcO)2(dppp)] Catalyst for the COC2H4 Copolymerization in H2O-CH3COOH Solvent [dppp =1,3-bis(diphenylphosphino)propane],” Journal of Molecular Catalysis A: Chemical, Vol. 204, 2003, pp. 295-303. doi:10.1016/S1381-1169(03)00311-X
- A. Vavasori, L. Toniolo and G. Cavinato, “Carbon Monoxide-Ethylene Copolymerisation Catalysed by [PdCl2(dppp)] in Methanol-Water or in Acetic Acid-Water as Solvents (dppp = 1,3-bis(diphenylphosphine)propane),” Journal of Molecular Catalysis A: Chemical, Vol. 215, No. 1-2, 2004, pp. 63-72. doi:10.1016/j.molcata.2004.01.012
- G. Cavinato, A. Vavasori, L. Toniolo, L. Ronchin, F. Dall’Acqua and A. Dolmella, “Synthesis, Characterization and X-Ray Structure of [Pd(SO4)(dppp)]·H2O, a Catalyst for the CO-Ethene Copolymerization [dppp = 1,3-bis(diphenylphosphino)propane],” Inorganica Chimica Acta, Vol. 358, No. 15, 2005, pp. 4555-4562. doi:10.1016/j.ica.2005.09.065
- A. Vavasori, A. Bellieni, L. Ronchin, L. Toniolo, F. Dall’Acqua and G. Cavinato, “Selective Alternating Copolymerisation of Carbon Monoxide and Ethene Catalysed by [PdCl2(dppf)] in Acetic Acid-Water as a Solvent [dppf =1,1’-bis(diphenylphosphino)ferrocene],” Journal of Molecular Catalysis A: Chemical, Vol. 263, No. 1-2, 2007, pp. 9-14. doi:10.1016/j.molcata.2006.07.071
- A. Fabrello, A. Vavasori, F. Dall’Acqua and L. Toniolo, Journal of Molecular Catalysis A: Chemical, Vol. 276, No. 1-2, 2007, pp. 211-218.
- A. Vavasori, L. Ronchin and L. Toniolo, “Influence of the Operating Conditions on the Catalytic Activity of [PdCl2 (dapp)] in the CO-Ethene Copolymerization in the H2OCH3COOH as a Solvent (dapp = 1,3-bis(di(2-methoxyphenyl)phosphino)propane),” Journal of Molecular Catalysis A: Chemical, Vol. 332, No. 1-2, 2010, pp. 158-164. doi:10.1016/j.molcata.2010.09.012
- A. Vavasori, L. Ronchin and L. Toniolo, “Influence of Formic Acid and Water on the [Pd(OAc)2(dppp)] Catalyzed Ethene-Carbon Monoxide Copolymerization Carried out in Aprotic Organic Solvents,” Applied Catalysis A: General, Vol. 449, 2012, pp. 198-202. doi:10.1016/j.apcata.2012.10.005
- G. Verspui, F. Schanssema and R. A. Sheldon, “A Stable, Conspicuously Active, Water-Soluble Pd Catalyst for the Alternating Copolymerization of Ethene and CO in Water,” Angewandte Chemie International Edition, Vol. 39, No. 4, 2000, pp. 804-806. doi:10.1002/(SICI)1521-3773(20000218)39:4<804::AID-ANIE804>3.0.CO;2-Q
- E. Lindner, M. Schmid, J. Wald, J. A. Queisser, M. Geprags, P. Wegner and C. Nachtigal, “Catalytic Activity of Cationic Diphospalladium(II) Complexes in the Alkene/CO Copolymerization in Organic Solvents and Water in Dependence on the Length of the Alkyl Chain at the Phosphine Ligands,” Journal of Organometallic Chemistry, Vol. 602, No. 1-2, 2000, pp. 173-187. doi:10.1016/S0022-328X(00)00154-6
- Z. Jiang and A. Sen, “Water-Soluble Palladium(II) Compounds as Catalysts for the Alternating Copolymerization of Olefins with Carbon Monoxide in an Aqueous Medium,” Macromolecules, Vol. 27, No. 24, 1994, pp. 7215- 7216. doi:10.1021/ma00102a034
- G. Vespui, G. Papadogianakis and R. A. Sheldon, “Catalytic Conversions in Water. Part 9. High Activity of the Pd/dpppr-s/Brønsted Acid System in the Alternating Copolymerization of Ethene and Carbon Monoxide {dpppr-s = C3H6-1,3-[P(C6H4-m-SO3Na)2]2},” Chemical Communications, No. 3, 1998, pp. 401-402. doi:10.1039/a707572c
- C. Bianchini, H. M. Lee, A. Meli, S. Moneti, V. Patinec, G. Petrucci and F. Vizza, “Water-Soluble Palladium(II) Catalysts for the Alternating Coand Terpolymerization of CO and Olefins in Aqueous Phase,” Macromolecules, Vol. 32, No. 12, 1999, pp. 3859-3866. doi:10.1021/ma990419d
- G. Verspui, F. Schanssema and R. A. Sheldon, “Catalytic Conversions in Water: Part 14. The Alternating Copolymerization of Ethene and CO Catalyzed by Water-Soluble Pd Catalysts,” Applied Catalysis A: General, Vol. 198, No. 1-2, 2000, pp. 5-11. doi:10.1016/S0926-860X(00)00457-9
- E.G. Chepaikin, A. P. Bezruchenko, A. A. Leshcheva and G. N. Boiko, “Catalytic Carbonylation of Ethylene in the Presence of the Pd(acac)2-m-Ph2PC6H4SO3Na(H)-AcOH System,” Russian Chemical Bulletin, Vol. 43, No. 3, 1994, pp. 360-363. doi:10.1007/BF01169705
- C.-J. Li and T. H. Chan, “Organic Reactions in Aqueous Media,” Wiley-Interscience, New York, 1997.
- B. Cornils, W. A. Herrmann, I. T. Horváth, W. Leitner, S. Mecking and H. Olivier-Bourbigou, “Homogeneous Catalysis in the Aqueous Phase as a Special Unit Operation,” Wiley-VCH, Weinheim, 2005.
- G. Papadogianakis, “Tenside Ligands,” In: B. Cornils and W. A. Herrmann, Eds., Aqueous-Phase Organometallic Catalysis, 2nd Edition, Wiley-VCH, Weinheim, 2004, pp. 158-173.
- B. Cornils, W. A. Herrmann, I. T. Horváth, W. Leitner, S. Mecking and H. Olivier-Bourbigou, “Homogeneous Catalysis in the Aqueous Phase as a Special Unit Operation,” Wiley-VCH, Weinheim, 2005.
- A. Vavasori, L. Toniolo, G. Cavinato and F. Visentin, “Highly Active [Pd(AcO)2(dppp)] Catalyst for the COC2H4 Copolymerization in H2O-CH3COOH Solvent [dppp = 1,3-bis(diphenylphosphino)propane],” Journal of Molecular Catalysis A: Chemical, Vol. 204-205, 2003, pp. 295-303. doi:10.1016/S1381-1169(03)00311-X
- M. L. Huggins, “The Viscosity of Dilute Solutions of Long-Chain Molecules. IV. Dependence on Concentration,” Journal of the American Chemical Society, Vol. 64, No. 11, 1942, pp. 2716-2718. doi:10.1021/ja01263a056
- B. J. Lommerts and D. J. Sikkema, “Synthesis and Structure of a New Polyalcohol,” Macromolecules, Vol. 33, No. 21, 2000, pp. 7950-7954. doi:10.1021/ma991262s
- A. Vavasori, L. Toniolo and L. Ronchin, “Carbon Monoxide—Ethene Alternating Copolymerization Catalyzed by [PdCl2(dppf)] in H2O—HCOOH [dppf = 1,1’-bis(diphenylphosphino)ferrocene],” Journal of Molecular Catalysis A: Chemical, Vol. 363-364, 2012, pp. 398-403. doi:10.1016/j.molcata.2012.07.016
- K. Holmberg, B. Jonsson, B. Kronberg and B. Lindman “Surfactants and Polymers in Aqueous Solution,” John Wiley & Sons Ltd., Hoboken, 2003.
- D. O. Shah and B. M. Moudgil, “Highlights of Research on Molecular Interactions at Interfaces from the University of Florida,” In: D. O. Shah and K. L. Mittal, Eds., Adsorption and Aggregation of Surfactants in Solution, Marcel Dekker Inc., New York, 2002, 2002, pp. 1-48. doi:10.1201/9780203910573
NOTES
*Corresponding author.

