Pharmacology & Pharmacy
Vol.06 No.08(2015), Article ID:59124,9 pages
10.4236/pp.2015.68041
Nutritional and Anti-Nutritional Quantification Assessment of Cymbopopgon citratus Leaf
Anayo Joseph Uraku1*, Stanley Chukwudozie Onuoha2, Nzube Edwin1, Nkiru Ezeani1, Moses Eji Ogbanshi2, Chukwu Ezeali1, Basil Uchechukwu Nwali1, Mathias Chukwuemeka Ominyi2
1Department of Biochemistry, Ebonyi State University, Abakaliki, Nigeria
2Department of Biotechnology, Ebonyi State University, Abakaliki, Nigeria
Email: *urakuaj@yahoo.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 16 March 2015; accepted 23 August 2015; published 26 August 2015
ABSTRACT
The leaf-extract of Cymbopogon citratus was evaluated for nutritional and anti-nutritional com- positions. The results revealed that the plant leaves contained appreciable amounts of phytochemicals (alkaloids, glucosides, phenols, saponins, flavonoids and tannins), proximate compositions (proteins, carbohydrates, fats, crude fibre, ash and moisture), vitamins (A, C, E, B1, B2 and B9) and trace elements (Fe, Zn, Mn, Cu, Na, K, Ca and Co) in varying degrees. These chemical compositions obtained may be responsible for the nutritional and therapeutic uses. The proximate, vitamin and mineral compositions obtained suggested that the leaves may serve as cheap sources of vitamin A, C, E, B1, B2 and B9 as well as other macro- and micro-nutrients, and could be incorporated into human diets to meet-up with their recommended daily dietary allowances. The content of flavonoids, vitamin A, C and E in the leaf extract also suggests possible anti-oxidant effects of the plant leaves.
Keywords:
Cymbopogon citratus, Phytochemicals, Proximate and Chemical Compositions

1. Introduction
Medicinal plants are of great importance to health of individuals and communities in general. The medicinal value of plants lies in some chemical substances that produce definite physiological and biochemical actions on human body. Some of the important bioactive constituents of plants include: alkaloids, tannins, flavonoids and phenolic compounds among others. Many of the indigenous medicinal plants are used as spices, vegetables and foods. Also, they are sometimes added to foods meant for pregnant women and nursing mothers for medicinal purposes as reported by Adeniyi et al. [1] .
In addition, the use of herbal medicine for treatment of diseases and infections is as old as mankind. The World Health Organization supports use of traditional medicine provided they are proven to be efficacious and relatively safe [2] . In developing countries, a huge number of people live in extreme poverty and some are suffering and dying for want of safe water and medicine; they have no alternative for primary health care [1] . There is therefore a need to look inwards to search for herbal medicinal plants with the aim of validating the ethno- medicinal use and subsequently isolation and characterization of compounds which will be added to potential list of drugs.
In Africa, there is growing interest in exploiting plants for nutritional and therapeutic purposes. In fact, the traditional medicine of Africa constitutes an important source for ethnopharmacological investigations [3] . Locally, Cymbopogon citratus has been used tradomedically for various medicinal purposes.
Cymbopogon citrates, commonly known as lemongrass, is a tropical perennial herb belonging to the family Poaceae (true grasses). It is commonly used in traditional Indian, Chinese, and Brazilian medicines [4] . Cymbopogon citratus has been shown to be effective in treatment of fever and infections, headaches, stomach aches, and rheumatic pain [5] . It is also reported to act as sedative, antispasmodic, analgesic, anti-inflammatory, and antihypertensive agents [6] . However, the scientific evidence for its alleged therapeutic efficacy is still lacking. Furthermore, many of these reports concerning the effect of C. citratus described function of only one particular part of the plant. The leaves decoction, for example, has been shown to have antioxidant property [6] .
This paper was designed to quantify the secondary metabolite constituents, proximate, vitamins and minerals of Cymbopogon citratus used in alternative traditional medicine in South-East Nigeria.
2. Materials and Methods
2.1. Collection and Preparation of Plant Materials
Whole plant of Cymbopogon citratus was collected from Ogboji-Agoutu Ezzagu in Inyaba Development Centre of Ebonyi State, Nigeria. Dr (Mrs) Nnamani, K. of the Department of Applied Biology of Ebonyi State University, Abakaliki graciously identified the plants. Apparently, a healthy leaf of the plant was removed from plant stalk, rinsed with clean water and shade dried to a constant weight. The dried plant sample was ground to fine powder with grinding machine, packaged in glass jars and stored at 4˚C until analysis.
2.2. Quantitative Phytochemical Analysis
The method of Akubugwo et al. [7] was adopted to assay for the quantitative phytochemical analyses to determine the concentrations of alkaloids, glycosides, phenols, saponins, flavonoids and tannins in the leaves of Cymbopogon citratus.
2.3. Determination of Alkaloids [8]
Five grams of ground sample was weighed into a 250 ml beaker, and 200 ml of 20% acetic acid in ethanol was added and was covered to stand for 4 h. This was filtered and the extract was concentrated using a water bath to evaporate one-quarter of the original volume. The concentrated ammonium solution was added drop-wise to the extract until the precipitation was completed. The entire solution was allowed to settle and the precipitate was collected by filtration, after which it was weighed.
2.4. Determination of Glucosides [9]
Five grams of the ground sample was soaked with 100 ml of distilled water for 3 hours in a beaker. It was filtered using whattman filter paper and funnel. The filtrate (2 ml) was piptted into a test tube; 2 ml of 3,5-dinitro salicylic acid was added and put in a boiling water bath for 15 minutes. The test tube was removed and allowed to cool. Distilled water (5 ml) was added to serve as a dilution factor. The absorbance and concentration were read at 460 nm in a visible Spectrophotometer.
2.5. Determination of Phenols [8]
Fat free sample was prepared as follows: two grams of the sample were defatted with 100 ml of diethyl ether using a Soxhlet apparatus for 2 h. The fat free sample was boiled with a 50 ml of ether for 14 minutes. Five millilitres of the extract was pipette into a 50 ml flask, and then 10 ml of distilled water was added. Two millilitres of ammonium hydroxide solution and 5 ml of concentrated ethyl alcohol were also added. The sample was made up to mark and left to react for 30 min for colour development. The absorbance of the solution was read using a visible spectrophotometer at 505 nm wavelength.
2.6. Determination of Saponins
This was done by the method described by Obadoni and Ochuko [8] . Twenty grams of the ground plant sample was dispersed in 200 ml of 20% ethanol. The suspension was heated over a hot water bath for 4 h with continuous stirring at about 55˚C. The mixture was filtered and the residue re-extracted with another 200 ml of 20% ethanol. The combined extracts were reduced to 40 ml over water bath at about 90˚C. The concentrate was transferred into a 250 ml separating funnel and 20 ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated. 60 ml of normal butanol extracts were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation the sample were dried in the oven into a constant weight. The saponin content was calculated in percentage.
2.7. Determination of Flavonoids [9]
Five grams of the ground plant sample was weighed in a 250 ml titration flask, and 100 ml of the 80% aqueous methanol was added at room temperature and shaken for 4 h in an electric shaker. The entire solution was filtered through What man filter paper no. 1 (125 mm) and again, this process was repeated. The filtrate as a whole was later transferred into a crucible and evaporated to dryness over a water bath and weighed.
2.8. Determination of Tannins [10]
Five hundred miligrams of the ground sample was weighed into 100 ml bottle; 50 ml of distilled water was added and shaken for 1 h in a shaker. This was filtered into a 50 ml volumetric flask and made up to the mark. Then 5 ml of the filtrate was pipette out into a tube and mixed with 3 ml of 0.1 M FeCl3 in 0.1 N HCl and 0.008 M potassium ferrocyanide. The absorbance was measured in a visible spectrophotometer at 120 nm wavelength within 10 min. A blank sample was prepared and read at the same wavelength. A standard was prepared using tannin acid to get 100 ppm and measured.
2.9. Proximate Analysis
The samples were analyzed for proximate compositions which include moisture content, fat/oil, ash, protein, fiber and carbohydrate contents. Official methods of AOAC [11] were used in carrying out the proximate analysis.
2.10. Determination of Carbohydrate
The carbohydrate content was determined by calculation (by difference) according to Akubugwo et al. [7] .
Calculation:
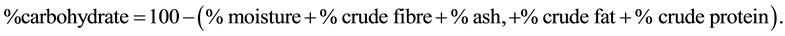
2.11. Determination of Crude Protein (Micro-Kjedahl Method)
Ten grams of the sample was weighed and transferred into a Kjedahl flask. Four tablets of Kjedahl catalysts (tablet contain 1 g of Na2SO4 and 0.5 g of selenium) were added. Concentrated H2SO4 (20 ml) and glass beads were introduced to avoid bumping on heating. The flask was set in the fume cupboard; heated gently immediately and then continue heating until a slight charring begin to clear and the mixture become colourless. The heating process was approximately one hour. The flask was allowed to cool to room temperature and slowly washed the long neck flask with 20 ml of distilled water into 500 ml distillation flask.
Distillation: Pieces of hot cleaps were added into the flask and connected up to the splash head and water cooled condenser. NaOH solution (5%, 4 ml) was added in the dropping funnel and 50 ml of 2% boric acid into the 250 ml receiving flask with methyl red indicator. The dropping funnel tap was opened slowing to allow the 5% NaOH to enter the boiling flask. The distillation flask was heated to boiling with water passing through the condenser. Distillation continued until about 150 ml was collected in the receiving flask. The content of the flask was titrated with 0.1 M HCl until pink end point. The reading was recorded and blank was ran along the same treatment.
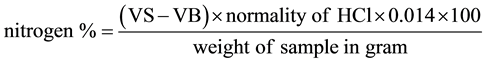
VS = Volume of acid used to titrate sample.
VB = Volume of acid used to titrate blank.
N = 0.1 M of acid.
% crude protein = N% × conversion factor (6.25).
2.12. Determination of Fat/Oil
Ten grams of the ground sample was weighed and transferred into thimbles of a soxhlet extractor containing 250 ml of petroleum ether. The thimble and the contents were placed in a 100 ml beaker and dried in an oven for 30 minutes at 105˚C - 110˚C to expel traces of moisture. The beaker was rinsed with the extractant and added to the soxhlet extractor. The sample was extracted for 7 hours at a condensation rate of 240 drops per minute. After the extraction, the sample was transferred to an already weighed evaporating dish and rinsed 2 - 3 with the extractant. The dish was placed in a fume chamber to cause solvent to evaporate. The sample was dried in an oven for an hour at 105˚C - 110˚C and then cooled in a desicator and weighed.
The percentage oil content was calculated as:
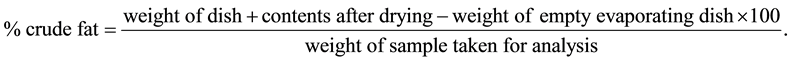
2.13. Determination of Crude Fiber
Two grams of the ground sample was weighed and placed into a conical flask. The sample was extracted by stirring with petroleum ether. 200 ml of 1.25% H2SO4 solution was heated to boiling and transferred to the dried sample. The sample was allowed to settle. The flask was connected immediately to a water-cooled reflux condenser and heated. The flask was boiled gently for 30 minutes and mixed. The flask was removed and filtered using a filter paper held in the funnel and washed with boiing water until no longer acidic to litmus paper. 200 ml of 1.25% NaOH was brought to boiling under a reflux condenser. This alkaline solution was used to wash the sample back into the initial flask and then boiled for 30 minutes under condenser. Again, the flask was removed and immediately filtered. All the insoluble matter was then transferred to the sintered crucible using boiling water. The residue was washed first with boiling water, 1% HCl and boiling water to render the insoluble matter free of acid. The residue was washed three times with alcohol and diethyether and then dried in an oven at 150˚C to a constant weight. The dried sample was also ashed by incineration in a muffle furnace at 560˚C for an hour. The crucible was cooled in desicators and then weighed.
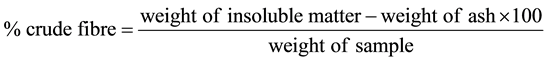
2.14. Determination of Moisture [11]
Three dried dishes with lids were weighed and into each of the dishes, 2.0 g of the each ground samples was weighed and placed in an oven at 100˚C for 3 hours without the lids. The samples were removed from the oven after drying and the lids were replaced. The samples were transferred to the desicator containing a suitable moisture absorbing material and weighed repeatedly after they have reached room temperature until constant weight were obtained.
The moisture content was calculated as:
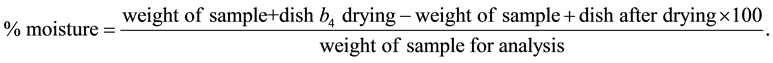
2.15. Determination of Ash
Clean dry porcelain dishes and two grams of the ground samples were weighed. The dishes were dried in an oven at 100˚C - 110˚C for 3 hours and then removed. The dishes were heated over a burnsen flame to initiate the destruction of carbon until the contents turn black. The dishes were placed into a muffle furnace and heated at 560˚C for 2 hours or until grayish-white residues were formed. The hot dishes were removed from the furnace using tongs and moistened with some drops of distilled water to expose any unashed still present. The dishes were again placed into muffle furnace and heated at 560˚C for 3 hours. They were removed and palced in a desicator to cool. Each dish and the content were weighed and ash level determined.
The percentage ash content or the sample was calculated using the formular:
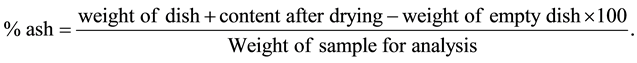
2.16. Determination of Vitamin Content
The leaf extracts of Cymbopogon citratus were assayed to determine the amount of vitamin A, C, E, B1, B2 and B9 using Spectrophotometric method.
2.17. Determination of Vitamin A
This was determined spectrophotometrically using a modified standard method of AOAC [11] .
The leaf extract (0.5 g) was homogenized and saponified with 2.5 ml of 12% alcoholic potassium hydroxide in a water bath at 60˚C for 30 minutes. The saponified extract was transferred to a separating funnel containing 10 - 15 ml of petroleum ether and mixed well. The lower aqueous layer was then transferred to another separating funnel and the upper petroleum ether layer containing the carotenoids was collected. The extraction was repeated until the aqueous layer became colourless. A small amount of anhydrous sodium sulphate was added to the petroleum ether extract to remove excess moisture. The final volume of the petroleum ether extract was noted. The absorbance of the yellow colour was read in a visible Spectrophotomete at 450 nm using petroleum ether as blank.
2.18. Determination of Vitamin C (Ascorbic Acid)
The method of Hussian et al. [12] was used. 1g of each ground sample was weighed in a 25 ml conical flask. Then 10 ml of the oxalic acid (0.05 M)-EDTA (0.02 M) solution was added and the mixture allowed standing for 24 h, to provide the required reaction time. After 24 h, the samples were filtered through 0.45 μm Whatman filter paper No.1. Then 2.5 ml of each sample was transferred to a separate 25 ml volumetric brown flask, after which 2.5 ml of the oxalic acid (0.05 M)-EDTA (0.02 M) solution was added. Subsequently, meta phosphoric acid was added separately with acetic acid (0.5 ml), sulphuric acid (5% v/v) solution (1 ml) and ammonium molybdate solution (2 ml) in each volumetric brown flask and the volume was made up to 25 ml with distilled water. The absorbance was measured at 760 nm in a visible Spectrophotometer.
2.19. Determination of Vitamin E (Tocopherol)
This was determined spectrophotometrically using a modified standard method of AOAC [11] .
Into 3 stoppered centrifuge tubes, 1.5 ml of plant extract, 1.5 ml of the standard and 1.5 ml of water were pipetted out separately. To all the tubes, 1.5 ml of ethanol and 1.5 ml of xylene were added, mixed well and centrifuged at 300 rpm. Xylene (1.0 ml) layer was transferred into another stoppered tube. To each tube, 1.0 ml of dipyridyl reagent was added and mixed well. The mixture (1.5 ml) was pipetted out into a cuvette and the extinction was read at 460 nm (A460). Ferric chloride solution (0.33 ml) was added to all the tubes and mixed well. The red colour developed was read exactly after 15 minutes at 520 nm (A520) in a visible Spectrophotometer.
2.20. Determination of Vitamin B1 (Thiamin) [13]
Five grams of the sample was homogenized with Ethanol sodium hydroxide (50 ml) it was filtered into a 100 ml flask. 10 ml of the filtrate was pipette and the colour developed by addition of 10 ml of potassium dichromate and read at 360 nm in a spectrophotometer. A blank sample was prepared and the colour also developed and read at the same wavelength.
2.21. Determination of Vitamin B2 (Riboflavin) [14]
Five grams of the ground sample was extracted with 100 ml of 50% ethanol solution and shaken for 1 h. This was filtered into a 100 ml of the extract that was pipette into 50 ml volumetric flask. Ten millilitres of 5% potassium permanganate and 10 ml of 30% H2O2 were added and allowed to stand over a hot water bath for about 30 min. two milliliters of 40% sodium sulphate was added. This was made up to 50 ml mark and the absorbance measured at 510 nm in a spectrophotometer.
2.22. Determination of Vitamin B9 (Folic Acid)
This was determined spectrophotometrically using a modified standard method of AOAC ([11] . Homogenized leaves powder (500 mg) was weighed into 100 ml volumetric flask. It was dissolved with 50 ml, 3% of disodium hydrogen orthorphosphate and shaked for 20 minutes, and make up to mark with the same solution, and then filter. The standard folic acid powder (28 mg) was weighed and treated as above. The filtrate (40 ml) was taken from the test and 3 ml of the filtrate was taken from the standard and each into separate 100 ml volumetric flask and was made up to mark with 3% disodium hydrogen orthorphosphate. Five millilires of both standard and test each was added into 50 ml volumetric flask and was treated for colour development as follows;
1) Two millilitres of KMNO4 (0.4%) was added and allow to stand for one minute.
2) Two millilitres of sodium nitrate (2%) was added.
3) Two millilitres of 5 M HCl was also added and both standard and test was shaken.
4) Two millilitres of sulphuric acid (5%).
5) 2 ml of sodium edadate/EDTA (5%) was added, shake and allow to stand for 10 minutes.
6) Two millilitres of Azodye (0.1%w/v) was also added and allowed to stand for 10 minutes and absorbance read at 550 nm.
Potency = AT/AS ´ WS/WT ´ 3/100 ´100/40 ´ Average fill weight.
AT = Absorbance of test.
AS = Absorbance of standard.
WS = Weight of standard.
WT = Weight of test.
Average fill weight = 480 mg.
2.23. Determination of Trace Elements/Macronutrient Status
Mineral was estimated by the used of an Atomic Absorption Spectrophotometer. The sample solutions in the sample bottles were analyzed for the concentration of the individual elements. Each element has specific cathode discharge lamp and this lamp was used to determine a particular element. Discharge lamp emits radiation at a wavelength specific for each element being assayed. This specificity can be obtained only from a pure sample of the element that is excited electrically to produce an arc spectrum on that element.
3. Statistical Analysis
The data was analyzed by ANOVA and results expressed as means and standard deviation.
4. Results
The results of phytochemica analysis showed that the leaf extracts of Cymbopogon citratus had varying (mg/100g) of phytochemical constituents.
The results of proximate analysis showed that the plant leaves contained variable amount (%) of proximate composition.
The results of trace elements/macronutrients analysis showed that the leaf extracts of Cymbopogon citratus had varying (mg/100g) of trace elements/macronutrients composition.
5. Discussion
Higher contents of tannins, flavonoids and phenolics were observed than alkaloids, saponins and glycosides in Cymbopogon citratus (Table 1). Tannins are known anti-nutritional factor that reduce the uptake of blood glucose by binding with calcium which is needed to stabilize amylase activity. They can equally bind with starch to influence its degree of gelatinization or its accessibility to the digestive enzymes. Tannins can suppress hyperglycemia by inhibiting glucose transport across the intestine through inhibiting sodium-glucose co-transporter-1 [14] . Tannins were reported as one of the most important of the bioactive constituents of plants. The phytoconstituents which includes saponins and other bioactive compounds are antibiotic principles of plants [15] . Tannins are plant polyphenols, which have ability to form complexes with metal ions and with macro-molecules such as proteins and polysaccharides [16] . Dietary tannins are said to reduce feed efficiency and weight gain in chicks [17] . Soaking and boiling of plant materials in water is said to improve their utilization in terms of feed intake and protein digestibility [18] . Environmental factors and the method of preparation of the plant samples may influence the concentration of tannins present. Saponins are glycosides, which include steroid saponins and triterpenoid saponins [19] . High levels of saponins in feed affect feed intake and growth rate in poultry [17] . Reduction in feed intake was ascribed to the bitter or irritating taste of saponins [18] . Saponins in excess cause hypocholestrolaemia because it binds to cholesterol making it unavailable for absorption [20] . Saponins also have haemolytic activity against RBC [17] . Saponins-protein complex formation can reduce protein digestibility [21] . Saponins decrease the uptake of certain nutrients such as glucose and cholesterol at the gut through intra- lumenal physicochemical interaction [20] . Hence, it has been reported to have hypocholesterolemic effects and thus may aid in lessening the metabolic burden that would have been placed on the liver. Though, the toxic effect of saponins has been highlighted by many researches [18] [21] [22] . Glycosides are toxic when ingested by monogastric animals in large quantity. These quantities of glycosides and tannins present in these leaves are still lower than the 10 mg HCN/kg recommended by FAO/WHO [23] , for glycosides and the lethal value above 5% for tannins [22] . These are not high enough to constitute human poison.
Alkaloids are beneficial chemicals to plants with predator and parasite repelling effects. However, they inhibit certain mammalian enzymic activities such as those of phosphodiesterase, prolonging the action of cyclic AMP. They also affect glucagons and thyroid stimulating hormones, while some forms have been reported to be carcinogenic [24] . It is noteworthy that at the concentration of these chemicals in this plant, they are usually non- toxic. Further, steaming or boiling reduces their leaves in plant extracts [25] . The plant also contains flavonoids which are phenolic compounds that serve as flavoring ingredients of plant leaves. They have been found to possess an anti-oxidant potential in animals.
Proximate composition of leaves of Cymbopogon citratus is presented in Table 2. The values showed that the leaves contain high percentage composition of crude fibre, protein, carbohydrate and moisture with low level of fat and ash. The good distribution of nutrients in the leaves may explain the popular use of the plant by local users in treatment of diseases. When compare with some other common use medicinal plants, Cymbopogon citratus leaves contain good quantities of crude fibre (37.53%), protein (22.59%), carbohydrate (19.64%) and moisture (11.35%) with low level of fat (2.43%) and ash (7.15%) than Boerhavia diffusa; 2.40%, 2.26%, 10.56%, 82.22%, 1.44% and 0.96% respectively and Commelina nudiflora; 1.50%, 1.69%, 5.67%, 88.63%, 1.44% and 1.07% respectively [20] . The fibre, carbohydrate and crude protein content of the leaves of C. citratus observed in this study are similar to reported values for both the leaves and seeds of S. nigrum as well as number of tropical plants [26] .
The ash content of the leaves (7.15%) is similar to the values reported for some commonly consumed leafy vegetable in Nigeria, including Ocinum graticinum, Hibiscus esculenta and Ipomea batata. It is however; lower than the reported value of 20.05% for Talinum triangulare [27] [28] . This value compares favourably with a reported value of 7.18% for S. nigrum from Congo Brazzavile [29] . The lipid content observed for Cymbopogon citratus leaves is similar to those reported for calchorus Africa, Amaranthus hybidus, Talinum triangulare but about half the value for Bacsilla alba leaves [27] .
The vitamin content is shown in Table 3. The order of magnitude of the studied vitamins is vitamin C > B1 > B2 > A > E > B9. Though, the vitamin content of the leaves are low, consumption of this plant material will contribute in meeting the daily vitamin requirement as stipulated for healthy adults [30] . The result obtained in Cymbopogon citratus is in agreement with the report of Okwu and Josiah [31] . Vitamins play physiological roles in the normal functioning of the body. Thus, these plants may be considered as good since they have the potential for the management of cardoivascular diseases and oxidative stress due their vitamin A, E and C contents [32] .
The mineral element analysis as shown in Table 4 indicate that Cymbopogon citratus contains high levels of manganese and calcium, fairly adequate concentration of potassium, sodium, copper, cobalt but relatively low level of zinc and iron. The mineral content of this plant is low when compared to the previously cited common medicinal plants but the low content of zinc and iron are in comparison with those reported for Boerhavia diffusa and Commelina nudiflora leaf extracts [20] .
Table 1. Phytochemical composition (mg/100g) of Cymbopogon citratus leaves.
Values are mean ± standard deviation of triplicate determination.
Table 2. Proximate composition (%) of Cymbopogon citratus leaves.
Values are mean ± standard deviation of triplicate determination The results of vitamin analysis showed that the plant leaves contained appreciable quantity of vitamin content.
Table 3. Vitamin content (mg/100g) of Cymbopogon citratus leaves.
Values are mean ± standard deviation of triplicate determination.
Table 4. Mineral composition (mg/100g) of Cymbopogon citratus leaves.
Values are mean ± standard deviation of triplicate determination.
6. Conclusion
In conclusion, this study has revealed that leaves of C. citratus are potential sources of nutrients and some essential macro and micronutrients needed by man. The importance of these nutrients cannot be over emphasized for effective and proper metabolism as well as the maintenance of good physiological state of man and animals.
Acknowledgements
Authors are grateful to Director of Research, Innovation and Commercialization (DRIC), EBSU and TET Fund for financial support.
Cite this paper
Anayo JosephUraku,Stanley ChukwudozieOnuoha,NzubeEdwin,NkiruEzeani,Moses EjiOgbanshi,ChukwuEzeali,Basil UchechukwuNwali,Mathias ChukwuemekaOminyi, (2015) Nutritional and Anti-Nutritional Quantification Assessment of Cymbopopgon citratus Leaf. Pharmacology & Pharmacy,06,401-410. doi: 10.4236/pp.2015.68041
References
- 1. Adeniyi, S.A., Ehiagbonare, J.E. and Nwangwu, S.C.O. (2012) Nutritional Evaluation of Some Staple Leaf Vegetables in Southern Nigeria. International Journal of Agricultural and Food Science, 2, 37-43.
- 2. Amin, M.M., Sawhney, S.S. and Jassal, M.M.S. (2013) Qualitative and Quantitative Analysis of Phytochemicals of Taraxacum Officinale. Wudpecker Journal of Pharmacy and Pharmocology, 2, 1-5.
- 3. Dame, Z.T.D., Petros, B. and Mekonnen, Y. (2013) Evaluation of Antiplasmodium berghei Activity of Crude and Column Fractions of Extracts from Withania somnifera. Turkish Journal of Biology, 37, 147-150.
- 4. Negrelle, R.R.B. and Gomes, E.C. (2007) Cymbopogon citratus (Dc) Stapf: Chemical Composition and Biological Activities. Revolution of Brasilian Plant Medical Botucatu, 9, 80-92.
- 5. Agbafor, K.N. and Akubuogwo, E.I. (2007) Hypocholesterolemia Effect of Ethanolic Extract of Fresh Leaves of Cymbopogon citratus (Lemongrass). African Journal of Biotechnology, 6, 596-598.
- 6. Vanisha, S.N. and Hema, M. (2012) Potential Functions of Lemon Grass (Cymbopogon citratus) in Health and Disease. International Journal of Pharmaceutical and Biological Archives, 3, 1035-1043.
- 7. Obadoni, B.O. and Ochuko, P.O. (2001) Phytochemical Studies and Comparative Efficacy of the Crude Extracts of Some Homeostatic Plants in Edo and Delta States of Nigeria. Global Journal Pure Applied Sciences, 8, 203-208.
- 8. Harborne, J.B. (1998) Textbook of Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 5th Edition, Chapman and Hall Ltd, London, 21-72.
- 9. Boham, A.B. and Kocipai, A.C. (1994) Flavonoid and Condensed Tannins from Leaves of Hawaiian vaccininum and Vicalycinium vaticulum. Pacific Science, 48, 458-463.
- 10. Van-Burden, T.P. and Robinton, W.C. (1981) Formation of Complexes between Protein and Tannin Acid. Journal of Agriculture and Food Chemistry, 1, 77-82.
- 11. AOAC (2000) Official Method of Analysis of Association of Analytical Chemists International. 17th Edition, Horowitz, Maryland.
- 12. Hussian, I., Saleem, M., Iqbal, Y. and Khalil, S.J. (2006) Comparison of Vitamin C Contents in Commercial Tea Brands and Fresh Tea Leaves. Journal of the Chemical Society of Pakistan, 28, 421-425.
- 13. Poornima, G.N. and Ravishankar, R.V. (2009) Evaluation of Phytonutrients and Vitamin Contents in a Wild Yam, Dioscorea belophylla (Prain) Haines. African Journal of Biotechnology, 8, 971-973.
- 14. Uraku, A.J., Okala, A.N.C and Ibiam, U.A. (2014) Effect of Spilanthes uliginosa (sw), Ocimum basilicum, Hyptis spicigera and Cymbopogon citratus Leaf Extracts on Biochemical and Histological Parameters of Mice Exposed to Plasmodium berghei. PhD Thesis Submitted to the Department of Bichemistry, Ebonyi State University, Abakaliki Nigeria.
- 15. Ajayi, I.A., Ajibade, O. and Oderinde, R.A. (2011) Preliminary Phytochemical Analysis of Some Plant Seeds. Research Journal of Chemical Sciences, 1, 58-62.
- 16. Akubugwo, I.E. and Ugbogu, A.E. (2007) Physicochemical Studies on Oils from Five Selected Nigerian Plant Seeds. Pakistan Journal of Nutrition, 6, 275-278.
- 17. Ogbe, A.O. and George, G.A.L. (2012) Nutritional and Anti-Nutrient Composition of Melon Husks: Potential as Feed Ingredient in Poultry Diet. Research Journal of Chemical Sciences, 2, 35-39.
- 18. Igwe, C.O., Onyeze, G.O.C., Onwuliri, V.A., Osuagwu, C.G. and Ojiako, A.O. (2010) Evaluation of the Chemical Composition of the Leaf of Spondias mombin Linn from Nigeria. Australian Journal of Basic and Applied Sciences, 4, 706-710.
- 19. George, M.D. (2001) Encyclopedia of Medicinal Plants. Part 1, Editorial Safelize, Madrid, 78-93.
- 20. Ujowundu, C.O., Igwe, C.U., Enemor, V.H.A., Nwaogu, L.A. and Okafor, O.E. (2008) Nutritive and Antinutritive Properties of Boerhaviar diffuse and Commelina nudiflora Leaves. Parkistan Journal of Nutrition, 7, 90-92.
http://dx.doi.org/10.3923/pjn.2008.90.92 - 21. Njoku, P.C. and Akumefula, M.I. (2007) Phytochemical and Nutrient Evaluation of Spondias mombin Leaves. Parkistan Journal of Nutrition, 6, 613-615.
http://dx.doi.org/10.3923/pjn.2007.613.615 - 22. Ndie, E.C. and Okaka, J.C. (2009) Evaluation of Dietary and Anti-Nutrient Intake of Plant Food in Selected States of South Eastern Nigeria. PhD Thesis Submitted to the Department of Food Science and Technology, Enugu State of University of Science and Technology, Enugu.
- 23. FAO/WHO (1991) Joint FAO/WHO Food Standard Programme, Codex Alimentaries Commission. XII, Supplement 4, FAO/WHO, Rome.
- 24. Okaka, J.C., Akobundu, E.N.T. and Okaka, A.N.C. (2006) Food and Human Nutrition an Integrated Approach. OCJ Academic Publishers, Enugu, 135-368.
- 25. Mohammad, A., Dangoggo, S.M., Tsafe, A.I., Itodo, A.U. and Atiku, F.A. (2011) Proximate, Minerals and Antinutritional Factors of Gardenia aqualla (Gauden dutse) Fruit Pulp. Pakistan Journal of Nutrition, 10, 577-581.
http://dx.doi.org/10.3923/pjn.2011.577.581 - 26. Akubugwo, I.E., Obasi, A.N. and Ginika, S.C. (2008) Nutritional Potential of the Leaves and Seeds of Black Nightshade—Solanum nigrum L. Var virginicum from Afikpo-Nigeria. Pakistan Journal of Nutrition, 6, 323-326.
http://dx.doi.org/10.3923/pjn.2007.323.326 - 27. Akindahunsi, A.A. and Salawu, S.O. (2005) Phytochemical Screening and Nutrient-Anti Nutrient Composition of Selected Tropical Green Leafy Vegetables. African Journal of Biotechnology, 4, 497-501.
- 28. Antia, B.S., Akpan, E.J., Okon, P.A. and Umoren, I.U. (2006) Nutritive and Anti. Nutritive Evaluation of Sweet Potatoes (Ipomoea batatas) Leaves. Pakistan Journal of Nutrition, 5, 166-168.
http://dx.doi.org/10.3923/pjn.2006.166.168 - 29. Dhellot, J.R., Matouba, E., Maloumbi, M.G., Nzikou, J.M., Dzondo, M.G., Linder, M., Parmentier, M. and Desobry, S. (2006) Extraction and Nutritional Properties of Solanum nigrum L. Seed Oil. African Journal of Biotechnology, 5, 987-991.
- 30. National Academy of Science (2004) Recommended Dietary Allowance. The National Academies Press, New York, 218-220.
http://www.nap.edu - 31. Okwu, D.E. and Josiah, C. (2006) Evaluation of the Chemical Composition of Two Nigerian Medicinal Plants. African Journal of Biotechnology, 5, 357-336.
- 32. Somer, E. (1995) Essential Guide to Vitamins and Minerals. Health Media of America, 1, 32-33.
NOTES
*Corresponding author.



