Surgical Science
Vol.4 No.5(2013), Article ID:30958,4 pages DOI:10.4236/ss.2013.45053
Efficacy of Vagus Nerve Stimulation (VNS) after Multiple Subpial Transections (MST) for Extra-Temporal Seizure Foci
Division of Neurosurgery, University of Nebraska Medical Center, Omaha, USA
Email: apatil@unmc.edu
Copyright © 2013 Arun Angelo Patil et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received March 20, 2013; revised April 21, 2013; accepted April 30, 2013
Keywords: Vagus Nerve Stimulation; Multiple Subpial Transaction; Intractable Epilepsy
ABSTRACT
Background: The effect of Vagus nerve stimulation (VNS) therapy following major resective surgeries has been reported. However, the effect of VNS therapy following multiple-subpial-transections (MST) has not been reported. The objective of this paper is to examine the beneficial effect of VNS therapy following MST. Methods: There are 22 patients aged 10 - 55 years. Male/female distribution is 11/11 and follow-up is 24 - 148 months (median of 120 months). Seizure foci were bilateral in 9 patients, multi-lobar (unilateral) in 12 patients and single-lobar in 1 patient. MST was performed over broad areas in and around the seizure foci. VNS implantation was done when the response to MST procedure was poor (1 patients), or there was recurrence of seizures (21 patients). Interval between MST and VNS implanttation varied from one month to three years (median of 2 years). Results: Thirteen patients (59%) are seizure free (Engel’ Class I), 8 (36.5%) have greater than 90% reduction in seizure frequency (Class II), and 1 (4.5%) has between 50% - 90% reduction in seizure frequency (Class III). Conclusion: The results show that VNS therapy produced meaningful improvement in seizure outcome in all patients with extra-temporal seizures that had inadequate response to MST.
1. Introduction
Treatment of medically intractable epilepsy is challenging [1]. Many surgical approaches have been devised to treat patients with intractable epilepsy. Among them anterior temporal lobectomy for temporal lobe onset seizures, and lesionectomy for seizures originating from focal lesions have been the most the successful procedures [2]. Surgical treatment of extra-temporal onset seizures remains a challenge. This is especially true for multifocal multi-lobar seizure onset seizures [1,3,4]. Multiple subpial transections (MST) is useful in these cases, but a small percentage of patients have suboptimal outcomes [5,6]. These patients could, potentially, benefit from VNS therapy.
Vagus nerve stimulation (VNS) was approved in 1997 as an adjunctive therapy for patients with medically intractable partial-onset seizures. It has been used as a primary surgical method to treat epilepsy, and there are several reports of its use as a supplementary procedure when results of resective procedures proved unsatisfactory [7-9]. There are, however, no reports of its use following MST for extra-temporal onset seizures. The authors have therefore examined the results of VNS therapy following MST in patients with extra-temporal onset seizures which were medically refractory [10] to determine if its addition would improve seizure outcome in this group of patients.
2. Materials and Methods
Case records were examined of all patients with medically intractable epilepsy who had extra-temporal seizure foci who underwent MST as the primary procedure, and then had VNS implantation either because the outcome from MST was unsatisfactory or there was recurrence of seizures. Medical intractability was determined based the criteria described by Kwan et al. [10]. A total of 22 patients were followed for 24 - 148 months (median of 120 months) following VNS implantation. Male/female distribution is 11/11 and ages are between 10 - 55 years. The seizure frequency before surgery varied between 1 - 2/week to several/day. Five patients had abnormal preoperative Magnetic resonance scans of the brain. The abnormalities included: cortical dysplasia, arachnoid cyst, tuberous sclerosis, subdural calcification and cortical atrophy.
The areas of the brain with seizure foci are documented in Table 1. Thirteen patients had unilateral seizure foci over large areas involving 2 or more lobes; and 9 patients had seizure foci involving both hemispheres in 2 or more lobes on each side.
Patients underwent standard pre-operative evaluations, including magnetic resonance imaging of the brain, neuropsychological evaluation, positron emission tomography in selected cases, and electro-clinical seizure localization using scalp and subdural electrodes. In all 22 patients MST was performed over broad areas that showed epileptogenic activity during long term video EEG recording using subdural electrodes. Two to three topectomies (measuring 0.5 - 1 centimeter in diameter) were done in areas in which intra-operative EEG recording showed persistent epileptiform activity after 2 - 3 MST passes in 7 patients. In addition 4 patients had radiofrequency ablation of the amygdala hippocampus complex (amygdale hippocampotomy) because it was involved in seizure generation. VNS implantation was done when the response from surgery was poor (Engel’s Class IV) (1 patients) or there was recurrence of seizures (21 patients).
During the follow up period the epileptologist was free to alter the seizure medication regimen as needed because of seizure recurrence or adverse effects. VNS parameters were adjusted at the discretion of the epileptologist. Increments of 0.25 mA current output were added every several weeks, depending upon response and tolerance. The amplitude is progressively increased. The stimulation parameters after final adjustment were:
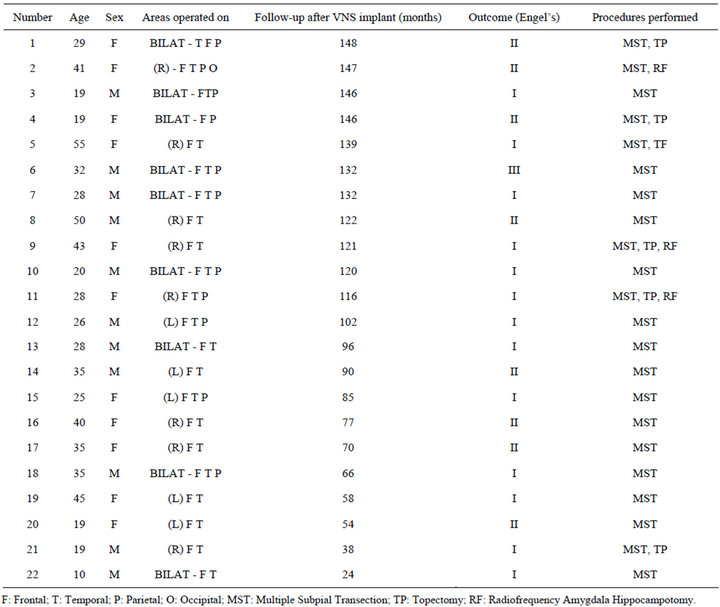
Table 1. Patient data.
current and magnet amplitudes 0.5 - 2.75 mA, frequency 20 - 30 Hz , pulse width 250 - 500 micro seconds, on time 14 - 30 seconds and off time 0.2 to 1.1 minutes.
Patients were requested, as a matter of course, to keep detailed seizure calendars. Details regarding the seizure frequency were obtained during clinic visits or through telephone interviews. Engel’s classification outcome was utilized to classify groups. Class I is seizure free or auras only; Class II is greater than 90% reduction in seizure frequency; Class III is 50% - 90% reduction is seizure frequency; and Class IV is less than 50% reduction in seizure frequency.
3. Results
Seizure onset was bilateral in 9 patients, and unilateral in 13 patients. Twelve patients with unilateral seizure foci had more than one lobe involved. Bilaterality of the seizure onset was based on long term video-EEG recordings using intracranial electrodes. The interval between MST and VNS implantation ranged from one month to three years (median of 2 years). There was no neurological complication from VNS implantation. There were no lasting side effects from VNS activation.
The seizure outcome after VNS implant is (see Table 2): thirteen out of 22 patients (59%) are seizure free (Class I); 8/22 (36.5%) have Class II outcome; and, 1/22 (4.5%) has Class III outcome. None of those in Class I were Class IA (free of seizure since surgery); as it took several months to progressively adjust the parameters of the VNS until they became seizure free. There was no significant difference between those who had unilateral surgery and those who had bilateral surgery, and between those who had MST alone and those who had MST plus additional procedures (Table 3). On an average the need for antiepileptic drugs (AED) decreased from a preopera-
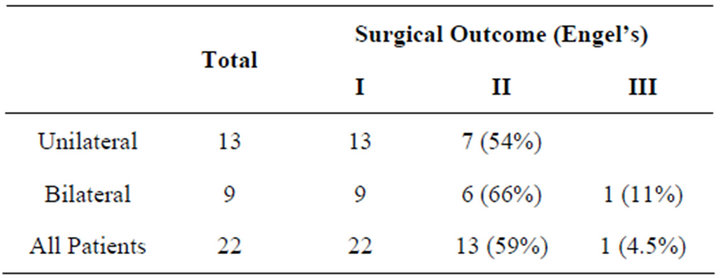
Table 2. Comparison between unilateral and bilateral surgery.

Table 3. Procedures prior to VNS implant.
tive 3 AEDs to 2 AEDs after VNS implant. Zonisamide, valproic acid, felbamate, levetiracetam, lamotrigine and Topamax were some of the AED used during pre and post-operative periods. No other adjunctive therapy was used.
4. Discussion
Results of resective surgery for extra-temporal seizures are generally less optimal [9,11]. One large study revealed that at 2 years follow-up, 50.3% were reported to have complete seizure freedom (or auras only) (Engel’s Class I), 18.3% have Class II outcome, 17.7% have Class III outcome. This is greater than 50% reduction in seizures in 86% of the patients at 2 years follow-up [12]. Similar results were seen in patients who had MST as the primary procedure for extra-temporal onset seizures [1, 3-6].
Effectiveness of VNS therapy as a primary surgical intervention for intractable epilepsy has been reported in several publications. Meta-analysis of results for 2634 patients showed that 4.6% are seizure free (Engle’s Class I); 7.6% have 90% - 99% reduction in seizures (Engel’s Class II); and 38.4% have between 50% - 90% reduction in seizures (Engel’s Class III). Thus, 50% have a 50% or greater reduction in seizures [13].
There are several reports of seizure outcome after VNS implantation following major resective epilepsy surgery. One study group showed 18.5% of 16 patients with temporal and frontal lobectomy showed 50% or more reduction in seizure frequency after placement of VNS. The type or extent of resective surgery had no effect on the outcome [7]. Vale et al reported on patients who had either lobectomy or corpus callosotomy, followed by VNS implantation; 64.9% had <30% reduction in seizures and 10.8% of the patients had a >60% reduction in seizures [9]. Amar et al analyzed data from a VNS therapy outcome registry; 921 patients had prior lobectomy or corpus callosotomy. Median reduction of seizure frequency was 45.7% within 12 months of VNS therapy onset, and 50.5% reduction after 24 months of VNS therapy [14]. A study published more recently reported on 110 patients with VNS and prior craniotomy, most of whom had major resective surgery; 24.5% showed 90% or greater improvement (Engel’s Classes I & II) and 70% showed 50% or greater improvement in seizures (Engel’s Classes I, II & III) [15].
In this series, patients had MST for extra-temporal onset seizures, and then underwent VNS implantation. All showed greater than 50% improvement in seizure frequency, and 59% of patients are seizure free. Minimum follow-up is 24 months and median follow-up is 120 months. These results indicate that VNS does improve seizure outcome in patients who have undergone MST with suboptimal results on seizure frequency. Furthermore, the outcome is better than those who had MST alone, resection alone, or VNS therapy alone for extra-temporal onset epilepsy. The result of patients who had MST alone at our center has been described in our previous publications [1,3,4]. In addition, the improvement with VNS therapy after MST as the primary operation is significantly better that those who had VNS therapy following major resective surgery for extra-temporal seizures.
There are many theories about the mechanism by which VNS therapy reduces seizure frequency. A recently published study suggested that it could be due to slowing of re-hyperpolarization of cortical neurons [16]. It is therefore possible that transecting horizontal fibers makes VNS induced impulses reaching the cortical neurons more intense (and therefore more effective) by reducing peripheral spread. Furthermore, MST is performed over a fairly large cortical surface. Satellite foci in somewhat peripheral areas, thus, also get treated. This may reduce seizure activation from the surrounding areas, making VNS therapy more effective.
In conclusion, this study indicates that there is an excellent outcome with VNS therapy in patients who previously had MST as their primary surgery for extra-temporal onset seizures. Outcomes are distinctly better than with other interventions. Follow-up in this series is adequate. However, validation of these results still requires a much larger study population.
REFERENCES
- A. Patil and R. Andrews, “Is Epilepsy Surgery on both Hemisphere Effective?” Stereotactic and Functional Neurosurgery, Vol. 82, No. 5-6, 2004, pp. 214-221. doi:10.1159/000082769
- A. A. Cohen-Gadol, B. G. Wilhelmi, F. Collignon, et al., “Long-Term Outcome of Epilepsy Surgery among 399 Patients with Nonlesional Seizure Foci Including Mesial Temporal Lobe Sclerosis,” Journal of Neurosurgery, Vol. 104, No. 4, 2006, pp. 513-524. doi:10.3171/jns.2006.104.4.513
- A. Patil, R. Andrews and R. Torkelson, “Treatment of Intractable Seizures with Multilobar and BIHEMISPHERIC Seizure Foci,” Surgical Neurology, Vol. 47, No. 1, 1997, pp. 72-78. doi:10.1016/S0090-3019(96)00389-8
- A. Patil, R. Andrews and R. Torkelson, “Multiple subpial Transection (MST) in the Treatment of Extensive Seizure Foci,” Journal of Epilepsy, Vol. 10, No. 4, 1997, pp. 203- 207. doi:10.1016/S0896-6974(97)84200-4
- S. Spencer and L. Huh, “Outcomes of Epilepsy Surgery in Adults and Children,” Lancet Neurology, Vol. 7, No. 6, 2008, pp. 525-537. doi:10.1016/S1474-4422(08)70109-1
- S. S. Spencer, J. Schramm, A. Wyler, M. O’Connor, D. Orbach, G. Krauss, M. Sperling, O. Devinsky, C. Elger, R. Lesser, L. Mulligan and M. Westerveld, “Multiple Subpial Transection for Intractable Partial Epilepsy: An International Meta-Analysis,” Epilepsia, Vol. 43, No. 2, 2002, pp. 141-145. doi:10.1046/j.1528-1157.2002.28101.x
- M. Koutroumanidis, C. D. Binnie, M. J. Hennessy, G. Alarcon, R. D. Elwes, B. K. Toone, C. Chandler, R. Selway, C. E. Polkey and S. A. O’Connor, “VNS in Patients with Previous Unsuccessful Resective Epilepsy Surgery: Antiepileptic and Psychotropic Effects,” Acta Neurologica Scandinavica,” Vol. 107, No. 2, 2003, pp. 117-121. doi:10.1034/j.1600-0404.2003.01211.x
- H. O. Lee, E. J. Koh, Y. M. Oh, S. S. Park, K. H. Kwon and H. Y. Choi, “Effect of Vagus Nerve Stimulation in Post-Traumatic Epilepsy and Failed Epilepsy Surgery: Preliminary Report,” Journal of Korean Neurosurgical Society, Vol. 44, No. 4, 2008, pp. 196-198. doi:10.3340/jkns.2008.44.4.196
- F. L. Vale, A. Ahmadian, A. S. Youssef, W. O. Tatum and S. R. Benbadis, “Long-Term Outcome of Vagus Nerve Stimulation Therapy after Failed Epilepsy Surgery,” Seizure: The Journal of the British Epilepsy Association, Vol. 20, No. 3, 2011, pp. 244-248. doi:10.1016/j.seizure.2010.12.003
- P. Kwan, A. Arzimanoglou, A. T. Berg, M. J. Brodie, W. Allen Hauser, G. Mathern, S. L. Moshé, E. Perucca, S. Wiebe and J. French, “Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies,” Epilepsia, Vol. 51, No. 6, 2010, pp. 1069-1077. doi:10.1111/j.1528-1167.2009.02397.x
- A. R. Wyler, B. P. Hermann and E. T. Richey, “Results of Reoperation for Failed Epilepsy Surgery,” Journal of Neurosurgery, Vol. 71, No. 6, 1989, pp. 815-819. doi:10.3171/jns.1989.71.6.0815
- A. E. Elsharkawy, F. Behne, F. Oppel, H. Pannek, R. Schulz, M. Hoppe, G. Pahs, C. Gyimesi, M. Nayel, A. Issa and A. Ebner, “Long-Term Outcome of Extratemporal Epilepsy Surgery among 154 Adult Patients,” Journal of Neurosurgery, Vol. 108, No. 4, 2008, pp. 676-686.
- D. J. Englot, E. F. Chang and K. I. Auguste, “Vagus Nerve Stimulation for Epilepsy: A Meta-Analysis of Efficacy and Predictors of Response,” Journal of neurosurgery, Vol. 115, No. 6, 2011, pp. 1248-1255.
- A. P. Amar, M. L. Apuzzo and C. Y. Liu, “Vagus Nerve Stimulation Therapy after Failed Cranial Surgery for Intractable Epilepsy: Results from the Vagus Nerve Stimulation Therapy Patient Outcome Registry,” Neurosurgery, Vol. 62, Suppl. 2, 2002, pp. 506-513.
- R. E. Elliott, A. Morsi, E. B. Geller, C. C. Carlson, O. Devinsky and W. K. Doyle, “Impact of Failed Intracranial Epilepsy Surgery on the Effectiveness of Subsequent Vagus Nerve Stimulation,” Neurosurgery, Vol. 69, No. 6, 2011, pp. 1210-1217. doi:10.1227/NEU.0b013e3182230ae3
- A. Zagon and A. A. Kemeny, “Slow Hyperpolarization in Cortical Neurons: A Possible Mechanism behind Vagus Nerve Sim. Ulation Therapy for Refractory Epilepsy?” Epilepsia, Vol. 41, No. 11, 2000, pp. 1382-1389. doi:10.1111/j.1528-1157.2000.tb00113.x

