Surgical Science
Vol. 3 No. 6 (2012) , Article ID: 19967 , 4 pages DOI:10.4236/ss.2012.36062
Lung Transplant and Outcomes: A Single-Center Experience
Department of Surgery, Division of Cardiothoracic Surgery, King Abdulaziz University Hospital, Jeddah, Saudi Arabia
Email: algithmi@hotmail.com
Received April 10, 2012; revised April 19, 2012; accepted May 18, 2012
Keywords: Lung; Transplant; Outcomes
ABSTRACT
Introduction: Lung transplant is the preferred treatment for several end-stage pulmonary diseases. The first successful human lung transplant was performed by the Toronto Group in 1983 [1]. Objectives: This article discusses our initial experience with single and double lung transplant. Study Design: A retrospective analysis was done on 11 consecutive lung transplants for end-stage pulmonary diseases performed at our institution between 2008 and 2010. Materials and Methods: Major indications were idiopathic pulmonary fibrosis (n = 6), bronchiectasis (n = 2), primary pulmonary hypertension (n = 1), lymphangioleiomyomatosis (n = 1), and scleroderma (n = 1). Results: Two patients (18.2%) died within 30 days of surgery. Oneand 2-year survival rates for the recipients were 81.8% and 72.7%. Sepsis caused the deaths of 2 recipients. Conclusions: Although sepsis and chronic rejection limit the benefits, lung transplant gives many patients with end-stage pulmonary disease the ability for a better quality of life.
1. Introduction
Lung transplant has been the preferred treatment for several end-stage pulmonary diseases for more than 40 years, since the first human lung transplant was performed by James Hardy at the University of Mississippi in 1963 [2], for a patient with bronchogenic carcinoma. The patient died of renal failure on day18 after lung transplantation. During the following two decades, 40 lung transplants were performed, but only 1 patient discharged home 8 months after the transplant and died shortly from sepsis. In 1983, Cooper, Patterson and colleague from Toronto General Hospital at the University of Toronto performed the first successful isolated singlelung transplantation [1].
The agency for health care policy and research in the United States concluded: “Lung transplant has evolved as a clinical procedure achieving a favorable risk-benefit ratio and acceptable 1- and 2-year survival rates”. Indications for lung transplant have widened over the years, with selection criteria becoming less restrictive. Unfortunately, a wider donor pool has limited application of this treatment, but this is being addressed through donor management protocols, refinement of the technique of lung preservation, and development of Toronto ex-vivo perfusion system to recondition suboptimal donor lungs. Bronchiolitis obliterans, infection, and primary organ dysfunction are major impediments to long-term survival. Here, we analyze our early experience with single and double lung transplant.
2. Materials and Methods
Between November 2008 and October 2010, 11 patients (8 women, 3 men; mean age, 43 years; range, 25 - 63 years) with end-stage pulmonary diseases underwent single lung transplants (n = 8) and double lung transplants (n = 3) at our institution. Preoperative patient demographic data are presented in (Table 1).
2.1. Recipient Selection
Recipients were selected according to the guidelines outlined by the International Society for Heart and Lung Transplant (Tables 2 and 3). Single lung transplants were performed in patients with pulmonary fibrosis (n = 6), scleroderma (n = 1), and lymphangioleiomyomatosis (n = 1). Double lung transplants were performed in patients with bronchiectasis (n = 2) and pulmonary hypertension (n = 1). Organs were allocated to recipients based on blood group, size match, and patient status.
2.2. Lung Preservation
Donor lungs were preserved with ice-cold low-potassium
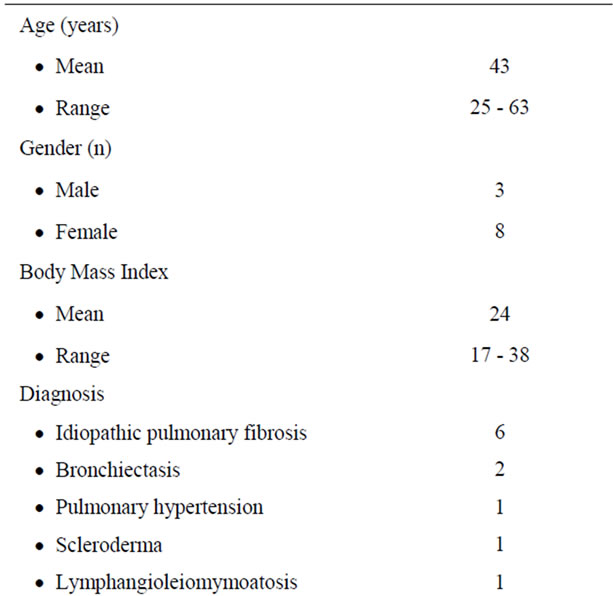
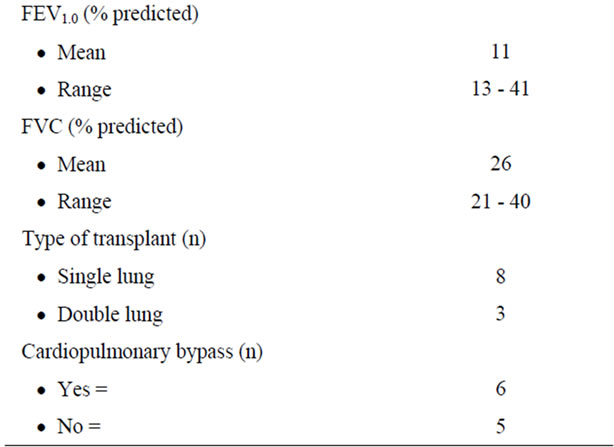
Table 1. Preoperative patients demographic data.

Table 2. Recipients selection—general guidelines.

Table 3. Recipients selection—contraindications.
Dextran (50 mL/kg, Perfadex, Vitrolife, Goteborg, Sweden) mixed with buffer solution tromethamine (THAM), prostaglandin E1 (500 µg), and calcium gluconate (10%). A bolus of prostaglandin E1 (500 µg) is administered directly into the pulmonary artery just before antegrade pulmonary artery flush. In addition, we added an in situ retrograde flush with 1.5 L of Perfadex.
2.3. Lung Transplant Technique
Single lung transplants are done through an anterolateral thoracotomy. Double lung transplants are done through a bilateral anterolateral thoracotomy in the fourth intercostal space with transverse division of the sternum. The lung with the least amount of preoperative ventilation perfusion is removed first. The donor lung is prepared at the back table, taking care to preserve peribronchial collateral circulation to the donor lungs. The donor bronchus is shorten up to 1 cartilage proximal to the upper lobe of the bronchus. A bronchial anastomosis is done with continuous 4-0 Prolene suture for the membranous part, and interrupted 4-0 Prolene suture for the cartilaginous part. The pulmonary artery anastomosis is performed with 5 - 0 Prolene, and the venous anastomosis is performed with 5-0 Prolene continuous suture. Postoperatively, we administer low-dose heparin (100 U) and Rheomacrodex (10% Dextran 40) as an intravenous infusion for 7 days to improve bronchial microcirculation.
Cardiopulmonary bypass was used in 6 patients (55%). Indications were primary pulmonary hypertension (n = 1) and secondary pulmonary hypertension (n = 5).
2.4. Infection Prophylaxis
Infection prophylaxis includes broad-spectrum antibiotics and antiviral therapy. Antiviral therapy consists of intravenous ganciclovir if the donor or recipient has Cytomegalovirus-positive serology, which is limited for 2 weeks after by oral ganciclovir for 12 weeks. Acyclovir is administered if the donor or recipient has Cytomegalovirus-negative serology as prophylaxis for herpes simplex. Pneumocystis carinii pneumonia prophylaxis consists of trimethoprim/sulfamethoxazole, double-strength, 3 times per week.
2.5. Immunosuppressive Management
Immunosuppression with tacrolimus, mycophenolate mofetil or azathioprine, and prednisone were administered to all patients postoperatively. One oral dose of cyclosporine (5 mg/kg) was introduced immediately before transplant. Tacrolimus level of 14 - 18 ng/mL is targeted for the first 3 months, and the dosage adjusted to a trough level of 8 - 10 ng/mL. Methylprednisolone is administered intravenously at a dose of 500 mg during the transplant procedure before reperfusion of the allograft, and then methylprednisolone is given at a dosage of 0.5 mg/kg daily for 3 days followed by 0.5 mg/kg daily. Azathioprine is administered at oral dosage of 2 mg/kg/d or mycophenolate mofetil at oral dosage of 1 g twice daily.
3. Results
Eleven recipients (8 women [72.7%] and 3 men [27.3%]) underwent 14 lung transplants. There were 8 patients with single lung transplants and 3 patients with double lung transplants. There was no perioperative mortality. Two patients (18.2%) died within 30 days of surgery and both were due to multiorgan failure as a result of sepsis. The overall 1- and 2-year survival for recipients were 81.8% and 72.7%. Recipients’ body mass index, age, and use of cardiopulmonary bypass had no significant effect on the length of mechanical ventilation, length of stay in the intensive care unit, and advantage on recipient survival. Three patients (27.3%) developed acute rejection within the first year. At 1 year after surgery, a significant improvement was observed in pulmonary function.
4. Discussion
Indications for lung transplant have rapidly widened and have been extended from patients with noninfectious to infectious parenchymal lung diseases [3]. At our institution, 54.5% of lung transplants are performed for idiopathic pulmonary fibrosis. This is a higher proportion than worldwide experience, as reported recently by the International Society for Heart and Lung Transplant registry [4]. Frequent indications for lung transplant are chronic obstructive pulmonary disease, alpha 1-antitrypsin deficiency, idiopathic pulmonary fibrosis, cystic fibrosis, bronchiectasis, and primary or secondary pulmonary hypertension [5]. Our results show that overall survival rates are comparable with international experience. Owing to a severe limitation of available donor organs, we perform single lung transplant in patients with pulmonary fibrosis, so that more patients can receive a lung transplant and shorten the waiting list time. Bilateral lung transplant achieves better functional recovery when compared with single lung transplant [6]. Yet this procedure at our institution is performed only for patients with endstage bronchiectasis and pulmonary hypertension.
The use of cardiopulmonary bypass is a matter of active discussion and institutional experience. Several centers prefer the routine use of cardiopulmonary bypass for bilateral lung transplants [7]. In our experience with 11 patients, we used cardiopulmonary bypass in more than half because of severe primary or secondary pulmonary hypertension. Infection represents the major cause of mortality during the first 6 months after surgery, whereas chronic rejection is the main cause of mortality after 6 months [8]. Rejection monitoring by routine surveillance lung biopsies has not been helpful in managing asymptomatic patients [9-11]. Pulmonary function tests are performed routinely after transplant as persistent decline in forced expiratory volume 1 second (FEV1.0) of 20% or more of the baseline value in the absence of infection; acute rejection is a useful clinical surrogate.
Stanford University has demonstrated a decline in the forced expiratory flow (FEF25%-75%) to less than 70% of the predicted values occurring 4 months earlier than the 20% decline in FEV1.0. This appears to be a sensitive marker for detecting bronchiolitis obliterans. The methacholine challenge test at 3 months after the transplant has predicted the early detection of bronchiolitis obliterans with a positive predictive value of 72% [12].
Our single-center experience in lung transplant confirms satisfactory results. Moreover, our results demonstrate frequent use of cardiopulmonary bypass. This may reflect liberal acceptance for transplant in patients with severe illness. Limitations of this study include it being retrospective, and a having a small number of patients.
REFERENCES
- Toronto Lung Transplant Group, “Unilateral Lung Transplantation for Pulmonary Fibrosis,” New England Journal of Medicine, Vol. 314, 1986, pp. 1140-1145. doi:10.1056/NEJM198605013141802
- H. D. Hardy, “The First Lung Transplant in Man (1963) and the First Heart Transplant in Man (1964),” Transplant Proceedings, Vol. 31, No. 1, 1999, pp. 25-29. doi:10.1016/S0041-1345(98)02059-4
- C. Aigner and W. Klepetko, “Lung Transplantation: State of the Art,” Journal of Cardiovascular Surgery, Vol. 6, 2002, pp. 22-28.
- J. D. Hosenpud, L. E. Bennet, M. K. Berkeley, et al., “The Registry of International Society for Heat and Lung Transplantation: Eighteen Official Report-2001,” Journal of Heart and Lung Transplantation, Vol. 20, No. 8, 2001, pp. 805-815. doi:10.1016/S1053-2498(01)00323-0
- S. M. Arcasoy and R. M. Kotloff, “Lung Transplantation,” New England Journal of Medicine, Vol. 340, 1999, pp. 1081-1091. doi:10.1056/NEJM199904083401406
- K. Bando, J. M. Armitage, I. L. Paradis, et al., “Indications for and Results of Single, Bilateral and Heart-Lung Transplantation for Pulmonary Hypertension,” Journal of Thoracic and Cardiovascular Surgery, Vol. 108, 1994, pp. 1056-1065.
- J. N. Rao, A. Hasan, G. Parry, et al., “Routine Cardiopulmonary Bypass (CPB) for Bilateral Lung Transplantation,” Abstract Presented at 14th Annual Meeting of EACTS, Frankfurt, 7-11 October 2000.
- M. de Perrot, C. Chaparro, K. McRae, et al., “Twenty Year Experience of Lung Transplantation at a Single Center: Influence of Recipient Diagnosis on Long-Term Survival,” Journal of Thoracic and Cardiovascular Surgery, Vol. 127, No. 5, 2004, pp. 1493-1501. doi:10.1016/j.jtcvs.2003.11.047
- E. .P Trulock, “Lung Transplantation,” American Journal of Respiratory and Critical Care Medicine, Vol. 155, No. 3, 1997, pp. 789-818.
- R. E. Girgis, H. Reichenspmer, R. C. Robbins, et al., “Utility of Annual Surveillance Bronchoscopy in HeartLung Transplant Recipients,” Transplantation, Vol. 160, 1995, pp. 1458-1461. doi:10.1097/00007890-199560120-00015
- S. Kesten, D. Chamberlain and J. Maurer, “Yield of Surveillance Trans-Bronchial Biopsies Performed beyond Two Years after Lung Transplantation,” Journal of Heart and Lung Transplant, Vol. 15, 1996, pp. 384-388.
- I. Al-Githmi, N. Batawil, N. Shigemura, et al., “Bronchiolitis Obliterans Following Lung Transplantation,” European Journal Cardio-Thoracic Surgery, Vol. 30, No. 6, 2006, pp. 846-851. doi:10.1016/j.ejcts.2006.09.027

