Advances in Microbiology
Vol.05 No.06(2015), Article ID:57321,7 pages
10.4236/aim.2015.56044
Microbial Fuel Cells for Nitrate Removal in Ground Water
Xiao Xiao*, Kangping Cui
Department of Resources and Environmental Engineering, Hefei University of Technology, Hefei, China
Email: *alleyxx123@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 28 May 2015; accepted 20 June 2015; published 23 June 2015
ABSTRACT
The increasing nitrate concentration in groundwater has become a serious concern all over the world. In this study, the double chamber microbial fuel cell (MFC) and single chamber MFC systems were proposed for simultaneous removal of chemical oxygen demand (COD) and nitrate (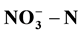 ).Transforming the various variables (cathod materials, external resistance and initial concentrations of
).Transforming the various variables (cathod materials, external resistance and initial concentrations of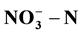 ) of double chamber MFC to determine the optimal operating parameters. Observing the treatment effect of single chamber MFC when adding an external resistance. The results showed: in the case of connecting external circuit, the double chamber MFC could reach the best degradation effect of
) of double chamber MFC to determine the optimal operating parameters. Observing the treatment effect of single chamber MFC when adding an external resistance. The results showed: in the case of connecting external circuit, the double chamber MFC could reach the best degradation effect of  and COD when cathode and anode materials are made of stainless steel velvet, the external resistance of 100 Ω and the initial concentrations of
and COD when cathode and anode materials are made of stainless steel velvet, the external resistance of 100 Ω and the initial concentrations of 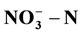 of around 250 mg/L. The best degradation rate of
of around 250 mg/L. The best degradation rate of 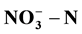 and COD reached 66.88% and 82.85% respectively. Adding an external solar power to single chamber could enhance the treatment effect; specifically,
and COD reached 66.88% and 82.85% respectively. Adding an external solar power to single chamber could enhance the treatment effect; specifically, 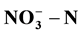 and COD removal rate reached 65.06% and 70.42% respectively, 6.14% and 9.73% higher than without external power.
and COD removal rate reached 65.06% and 70.42% respectively, 6.14% and 9.73% higher than without external power.
Keywords:
Microbial Fuel Cell, Groundwater, Nitrate

1. Introduction
Groundwater is widely used as one of the main sources of drinking water in most countries of the world [1] . In recent years, excessive use of chemical fertilizers and discharge of domestic and industrial waste water led to an elevated of nitrate 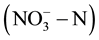 content in groundwater [2] [3] . It will cause “blue baby syndrome” or even the potential effect on cancer when people drink ground water containing high level of
content in groundwater [2] [3] . It will cause “blue baby syndrome” or even the potential effect on cancer when people drink ground water containing high level of  and nitrite
and nitrite 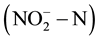 [4] -[6] . In order to reduce human health risks, the United States Environmental Protection Agency provided the maximum concentration limits for
[4] -[6] . In order to reduce human health risks, the United States Environmental Protection Agency provided the maximum concentration limits for 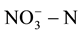 and
and 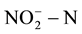 in drinking water; they are respectively 10 mg/L and 1.0 mg/L. EU and the World Health Organization provided the maximum concentration limits which are respectively 11.3 mg/L and 0.03 mg/L [7] -[9] . GB/T14848-93 in our country provided
in drinking water; they are respectively 10 mg/L and 1.0 mg/L. EU and the World Health Organization provided the maximum concentration limits which are respectively 11.3 mg/L and 0.03 mg/L [7] -[9] . GB/T14848-93 in our country provided 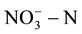 in class III groundwater which should be less than 20 mg/L. However, the groundwater in many parts far exceeded the top limit set by the agencies. Therefore, how economically and effectively remove the
in class III groundwater which should be less than 20 mg/L. However, the groundwater in many parts far exceeded the top limit set by the agencies. Therefore, how economically and effectively remove the 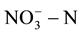 in groundwater has become focused issues of concern both at home and abroad.
in groundwater has become focused issues of concern both at home and abroad.
Although the removal of 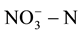 in groundwater is more efficient by physicochemical methods such as ion exchange, reverse osmosis, and electro-dialysis, these methods need to be further treated and of high treatment costs. Biological denitrification is considered to be the most economical strategy among other conventional techniques [10] . However, traditionalbiological denitrification method isdifficult to applyinpractice due to formation the secondary pollution by addition of carbon sources [11] . Overview of the previous studies, this article will explore the treatment effect of nitrate and organic compounds by using microbial fuel cell (MFC) method.
in groundwater is more efficient by physicochemical methods such as ion exchange, reverse osmosis, and electro-dialysis, these methods need to be further treated and of high treatment costs. Biological denitrification is considered to be the most economical strategy among other conventional techniques [10] . However, traditionalbiological denitrification method isdifficult to applyinpractice due to formation the secondary pollution by addition of carbon sources [11] . Overview of the previous studies, this article will explore the treatment effect of nitrate and organic compounds by using microbial fuel cell (MFC) method.
2. Materials
Strains: laboratory strains derived from sludge in reflux tank of Wang Tong sewage treatment plant in Hefei and used for experiments after acclimatization and cultivation.
Concentration of every component in microbiological culture solution: Na2HPO4, 4.0986 g/L; NaH2PO4, 2.544 g/L; NH4Cl, 0.31 g/L; KCl, 0.13g /L; C6H12O6, 0.5 g/L; Vitamin solution, 5 mL/L; Mineral solution, 12.5 mL/L.
Experimental apparatus: Ion chromatograph (CIC-100), Hash COD detector, Spectrophotometer, uv-spec- trophotometer, 250 mL Polyethylene bottles, electronic scale.
Double Chamber MFC and Single Chamber MFC
Double chamber MFC: select the polyethylene bottle of 250 mL with sealing cap as cathode and anode chamber of double chamber MFC, add 80 mL acclimated sludge into anode chamber and fill it with culture solution, cathode chamber filled with artificial simulation of nitrate-contaminated groundwater, connect anode and cathode chamber with salt bridge of 1cm in inner diameter and 25 cm in length, and series a certain resistance of resistor in external circuit. There are six groups of experiments, they are MFC0 - MFC5. The anode and cathode materials of MFC0, MFC1, MFC3, MFC4, MFC5 are stainless steel velvet as well as the anode material of MFC2, the cathode material of MFC2 is active carbon granule. Disconnect the anode and cathode of MFC0as a control group, connect the anode and cathode of MFC1 and MFC2 with copper wire and both are series a resistor of 1000 Ω in external circuit, moreover, series a resistor of 100 Ω in external circuit of MFC3, MFC4 and MFC5. The initial concentration of MFC0, MFC1, MFC2 and MFC3 is around 250 mg/L, MFC4 is around 150 mg/L, MFC5 is around 350 mg/L. Keep the anode and cathode chamber of MFC0 - 5 in the anaerobic environment and take samples for analysis every three days, from that we can ensure the best parameters of the highest removal efficiency of nitrate.
Single chamber MFC: select the glass container of 20 cm × 20 cm × 35 cm in volume as the single chamber of MFC and filled with 3 L acclimated sludge and 5 L culture solution, as well as filled with nitrogen gas to maintain the anaerobic environment. The anode and cathode materials are stainless steel velvet, series a resistor of 1000 Ω in external circuit. To explore the enhancement of nitrate and COD removal efficiency by series solar power in external circuit.
3. Results and Discussion
3.1. Degradation of  in Cathode Chamber of Double Chamber MFC
in Cathode Chamber of Double Chamber MFC
In experiments, the measured initial concentrations of 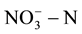 of MFC0 - 3 are respectively 253.6 mg/L, 245.1 mg/L, 246.6 mg/L and 251.8 mg/L, the change of concentrations of
of MFC0 - 3 are respectively 253.6 mg/L, 245.1 mg/L, 246.6 mg/L and 251.8 mg/L, the change of concentrations of 



Figure 1 shows that the degradation effects of MFC1, MFC2 and MFC3 are obviously better than MFC0 which is disconnected of anode and cathode. It indicates that the connection of cathode and anode can promote the transfer of electrons from the anode to the cathode, 


Figure 2 shows that different initial concentrations of 

Figure 1. The changes of concentration of 
Figure 2. Degradation curves of 
MFC3, MFC4, and MFC5 within 30 days are respectively 66.88%, 54.16%, and 47.45%. MFC3 has the highest removal rate, it indicates that under the same outside conditions, the initial concentration of 
3.2. Degradation of COD in Anode Chamber of Double Chamber MFC
Determining the concentration of 

Figure 3 shows that both 

Figure 3. The changes of concentration of COD in anode chamber from MFC0 to MFC3.
Figure 4. Degradation curves of COD under different initial concentrations of
mized the degradation of 
Figure 4 shows that the degradation curves of COD in cathode chamber differ from each other in the case of different initial concentrations of




In addition, due to the obvious effect of experiments within the first 15 days, the data of the 15th day is selected for analysis. The degradation rate of 
Figure 5 demonstrates that the experimental groups of MFC1 - 5 work better than the control group of MFC0, it indicates that connecting external circuit of anode and cathode is good for reaction. In all experimental groups, MFC3 has the highest removal rates of both 

3.3. Degradation of 
Initial concentration of 

It can be seen from Figure 6 and Figure 7 that the degradation effect of adding an extra power in external circuit is obviously better than the status of open circuit and normal circuit. Its removal rates of 



Figure 5. The comparison chart of 

Figure 6. The removal of 



Figure 7. The removal of COD with time change. (a) The concentration curves of COD; (b) the removal rate of COD.
Determine the concentrations of 


Figure 8 shows that the accumulation of 

4. Conclusions
The best degradation effect of 
Figure 8. The removal of 
the highest removal rates of 

Single chamber MFC, without proton exchange membrane, is able to remove 

References
- Tong, S., Zhang, B.G., Feng, C.P., Zhao, Y.X., Chen, N., Hao, C.B., Pu, J.Y. and Zhao. L.W. (2013) Characteristics of Heterotrophic/Biofilm-Electrode Autotrophic Denitrification for Nitrate Removal from Groundwater. Bioresource Technology, 148, 121-127. http://dx.doi.org/10.1016/j.biortech.2013.08.146
- Zhang, Y.F. and Angelidaki, I. (2013) A New Method for in Situ Nitrate Removal from Groundwater Using Submerged Microbial Desalinatione-Denitrification Cell (SMDDC). Water Research, 47, 1827-1836. http://dx.doi.org/10.1016/j.watres.2013.01.005
- Park, H.I., Kim, D.K., Choi, Y. and Pak, D. (2005) Nitrate Reduction Using an Electrode Asdirect Electron Donor in a biofilm-Electrode Reactor. Process Biochem, 40, 3383-3388. http://dx.doi.org/10.1016/j.procbio.2005.03.017
- Camargo, J.A. and Alonso, A. (2006) Ecological and Toxicological Effects of Inorganic Nitrogen Pollution in Aquaticeco Systems: Aglobal Assessment. Environment International, 32, 831-849.
- Fewtrell, L. (2004) Drinking-Water Nitrate, Methemoglobinemia, and Global Burden of Disease: A Discussion. EnvironmentalHealth Perspectives, 112, 1371-1374. http://dx.doi.org/10.1289/ehp.7216
- Suthar, S., Bishnoi, P., Singh, S., Mutiyar, P.K., Nema, A.K. and Patil, N.S. (2009) Nitrate Contamination in Groundwater of Some Rural Areas of Rajasthan. Journal of Hazardous Materials, 171, 189-199.
- United States Environmental Protection Agency. National Primary Drinking Water Standards. USEPA Office of Water, Washington DC, Doc. 810-F-94-001
- WHO (2004) Guidelines for Drinking Water Quality. World Health Organization, Geneva.
- Xia, S.Q., Zhong, F.H., Zhang, Y.H., Li, H.X. and Yang, X. (2009) Bio-Reduction of Nitrate from Groundwater Using a Hydrogen-Based Membrane Biofilm Reactor. Journal of Environmental Sciences, 22, 257-262. http://dx.doi.org/10.1016/S1001-0742(09)60102-9
- Liu, S.-J., Zhao, Z.-Y., Li, J., Wang, J. and Qi, Y. (2013) An Anaerobic Two-Layer Permeable Reactive Biobarrier for the Remediation of Nitrate Contaminated Groundwater. Water Reasearch, 47, 5977-5985. http://dx.doi.org/10.1016/j.watres.2013.06.028
- Sarina, J.E. and David, E.R. (2004) Drinking Water Denitrification Using a Membrane Bioreactor. Water Research, 38, 3225-3232. http://dx.doi.org/10.1016/j.watres.2004.04.019
- Clauwaert, P., Rabaey, K., Aelterman, P., De Schamphelaire, L., Pham, T. H., Boeckx, P., Boon, N. and Verstraete, W. (2007) Biological Denitrification in Microbial Fuel Cells. Environmental Science and Technology, 41, 3354-3360. http://dx.doi.org/10.1021/es062580r
- Rodrigo, M.A., Canizares, P., Lobato, J., Paz, R., Szea, C. and Linares, J.J. (2007) Production of Electricity from the Treatment of Urban Waste Water Using a Microbial Fuel Cell. Journal of Power Sources, 169, 198-204. http://dx.doi.org/10.1016/j.jpowsour.2007.01.054
- Wang, Y.K., Sheng, G.P., Li, W.W., Huang, Y.X., Yu, Y.Y., Zeng, R.J., et al. (2011) Development of Anovel Bioelectrochemical Membrane Reactor for Wastewater Treatment. Environmental Science & Technology, 45, 9256-9261. http://dx.doi.org/10.1021/es2019803
- Yi, T. and Harper, W. (2009) The Effect of Nitrate and Sulfate on Mediator-Less Microbial Fuel Cells with High Internal Resistance. Water Environment Research, 81, 2320-2328. http://dx.doi.org/10.2175/193864709793955816
NOTES
*Corresponding author.














