American Journal of Analytical Chemistry
Vol.08 No.01(2017), Article ID:73423,9 pages
10.4236/ajac.2017.81006
Treatment of the High Concentration Nonylphenol Ethoxylates (NPEOs) Wastewater by Fenton Oxidation Process
Ruoyu Zhou, Wenqi Zhang*
College of Chemistry and Chemical Engineering, Shanghai University of Engineering Science, Shanghai, China

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: December 12, 2016; Accepted: January 9, 2017; Published: January 12, 2017
ABSTRACT
The Fenton oxidation process was applied in the treatment of an actual high concentration nonylphenol Ethoxylates (NPEOs) wastewater. The effects of H2O2 dosage, molar ratio of H2O2/Fe2+ (Fe2+ dosage), pH value and reaction time on the degradation of NPEOs were investigated. The orthogonal experiment indicated that the order of degree of influence on the COD removal was molar ratio of H2O2/Fe2+, reaction time, dosage of H2O2, and initial pH. The single-factor tests were carried out to determine the optimal conditions, and the results were H2O2 dosage of 76.32 mmol/L, molar ratio of H2O2/Fe2+ of 3, pH value of 5 and reaction time of 2 h. Under the optimum operation conditions, the COD removal efficiency was 85.6% and the effluent could be mixed with other wastewater into the large-scale biological treatment system.
Keywords:
Fenton, Nonylphenol Ethoxylates, Orthogonal Experiment, Single-Factor

1. Introduction
Nonylphenolethoxylates (NPEOs) are a group of nonionic surfactants that are most often used in detergents, emulsifiers, and dispersing agents in household, agricultural, and industrial applications [1] . As a consequence of the extensive use, discharge of NPEOs occurs in the environment via industrial effluents and domestic sewage. NPEOs can be biodegraded to generate nonylphenol (NP) and short chain NPEOs which are more toxic, more lipophilic and more persistent than the parent substance [2] [3] .
Low concentration NPEOs in water environment can be treated by biological [4] , advanced oxidation [5] [6] , photocatalytic [7] and adsorption [8] methods. However, although high concentration NPEOs wastewater is discharged by factories, very few data is available on the treatment of industrial effluent. The presence of high concentration NPEOs in biological treatment plants may cause serious problems, such as production of a large amount of foams, the loss of microorganisms in bio-reactor. In some enterprises, this wastewater is often treated by the distillation process, which is not only costly, but also produces a lot of higher concentration of NPEOs waste liquid. New environmental problems have been produced.
Fenton is a simple and effective process for industrial wastewater treatment. In this process, hydrogen peroxide is added to wastewater in presence of ferrous salt, generating species that are strongly oxidative with respect to organic compounds present. Hydroxyl radicals (・OH) are traditionally regarded as the key oxidizing species in the Fenton processes, though high valence iron species and alkoxyl radicals have also been proposed. The classical Fenton free radical mechanism in the absence of organic compounds mainly involves the sequence of reactions below. [9] [10] [11] [12]
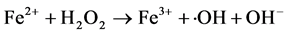 (1)
(1)
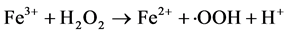 (2)
(2)
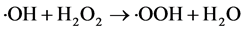 (3)
(3)
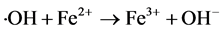 (4)
(4)
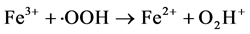 (5)
(5)
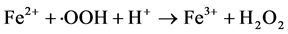 (6)
(6)
 (7)
(7)
Although Fe3+ can be reduced to Fe2+ through Equation (2), the rate is several orders of magnitude slower than that of Fe2+-Fe3+ conversion through Equation (1). And the formed Fe3+ may precipitate to iron hydroxides, particularly as pH is increased.
In this study we have evaluated reactions of Fenton’s reagent with high concentration NPEOs wastewater. Various parameters, including pH, Fe2+ and H2O2 dosages, reaction time, have been fully discussed. The optimal reaction conditions have been determined.
2. Experimental Section
2.1. Chemicals
The NPnEO [n = 10 (NP10EO)] wastewater was obtained from a chemical enterprise in China. The COD of this actual wastewater was 12,000 ± 500 mg/L and its pH was about 6.5. All chemicals were purchased from Sinopharm Chemical Reagent Co., Ltd. (China).
2.2. Experimental Procedure
The Fenton process started when the required FeSO4 dosage was added into the beaker containing 100 mL wastewater, stirred by a magnetic stirrer with a desired speed (300 rpm). In addition, the initial pH of the wastewater was adjusted by adding diluted sulfuric acid (1 mol/L) or sodium hydroxide solutions (1 mol/L), then quickly added quantitative H2O2. Samples were collected after 2 h unless otherwise noted. And COD of each sample was measured after sedimentation. All experiments were performed at ambient temperature.
2.3. Orthogonal Experimental Design
Depending on the reaction mechanism of Fenton reaction, four parameters of H2O2 dosage (A), H2O2/Fe2+ molar ratio (B), initial pH (C) and reaction time (D) were selected as influencing factors in the orthogonal experiment. Three levels were selected for each factor (shown in Table 1).
2.4. Analytical Methods
Chemical oxygen demand (COD) was determined by potassium dichromate method according to Standard Methods. The pH was measured by a PHS-2F pH meter.
3. Results and Discussion
3.1. Results of Orthogonal Experiment
The results of the orthogonal experiment were shown in Table 2.
As can be seen from the orthogonal experiment and range analysis, the order of degree of influence on the COD removal rate was molar ratio of H2O2/Fe2+, reaction time, dosage of H2O2, and the initial pH. In this orthogonal experiment, when the initial COD concentration was 12000 mg/L, the optimal reaction conditions were determined: H2O2 dosage 76.32 mmol/L, the molar ratio of 2 and initial pH of 5.0. The reaction time was 1 h.
3.2. Single Factor Experiment
In order to determine the most optimal conditions, single factor experiments were analyzed after orthogonal experiment.
3.2.1. Effect of Initial pH
According to the results of orthogonal experiment and relevant literatures [13]
Table 1. Factors and levels of orthogonal experiment.
[14] , selected H2O2 dosage of 76.32 mmol/L, molar ratio of H2O2/Fe2+ of 3, reaction time of 2 h, and the effect of initial pH on COD removal were investigated (shown in Figure 1).
As shown in Figure 1, there was a significant impact of pH on the COD removal. The optimal initial pH was 5, the COD removal reached highest with 83.5%. When the initial pH of the wastewater was increased from 6 to 7, the COD removal significantly reduced. It might be because that the reaction system generated Fe2+ and Fe3+ hydroxide, while inhibiting the Fe2+ catalysis, reducing
Table 2. Results of orthogonal experiment and range analysis.
Figure 1. Effect of initial pH on the treatment efficiencies of the Fenton process.
the production rate of ・OH. [12] When the pH was less than 3, the Fenton reagent oxidation was also inhibited. This might be due to the plenty of H+ reacted with H2O2, generation [H3O2]+ which was more stable and inhibited the production of ・OH [15] . In addition, the excess of H+ would react with ・OH, thereby reducing the oxidation Fenton reagent [16] .
In this study, the efficiency of COD removal was still high when the initial pH was 6. But the pH of all effluent after Fenton process was less than 3. This showed that the pH of the wastewater was decreasing in the reaction process which may be due to the production of some intermediate products, such as nonylphenoxy carboxylic acids (NPEC) [17] [18] . It was speculated that the optimal pH in reaction process was still in the range of 3 - 4, which was coincident with the values reported in the literatures.
3.2.2. Effect of H2O2 Dosage
Selected molar ratio of H2O2/Fe2+ of 3, reaction time of 2 h, the initial pH of 5, and the effect of H2O2 dosage on COD removal was investigated, the test results were shown in Figure 2.
It could be seen from Figure 2, with the amount of H2O2 from 9.53 mmol/L to 47.7 mmol/L, COD removal quickly increased from 5.4% to 80.1%. Increasing the dosage of H2O2 to 85.86 mmoI/L, the efficiency of COD removal grew slowed slightly. When the H2O2 concentration was higher than 85.86 mmol/L, the removal rate decreased slightly instead of increasing. This could be seen from the Equation (3), when the H2O2 dosage was large, the excess of H2O2 had quenching effect with the generation of ・OH [19] . Therefore, the optimal dosage of H2O2 was determined as 76.32 mmol/L.
3.2.3. Effect of Reaction Time
Figure 3 showed the effect of reaction time on COD removal in the condition of H2O2 dosage of 76.32 mmol/L, molar ratio of H2O2/Fe2+ of 3, the initial pH of 5.
Figure 2. Effect of H2O2 dosage on the treatment efficiencies of the Fenton process.
It could be seen form Figure 3 that in the first 1.5 h, COD removal increased significantly with the reaction time, then stabilized at 76.9% - 84.1%. The reaction time of 2 h was preferably selected.
3.2.4. Effect of Molar Ratio of H2O2/Fe2+
Fe2+ was a necessary condition to produce ・OH, since in Fenton reaction the rate of Fe3+ restoring to Fe2+ was very slow, the concentration of Fe2+ determined the amount of generation of ・OH. Selected H2O2 dosage of 76.32 mmol/L, reaction time of 2 h, the initial pH of 5, and the effect of molar ratio of H2O2/Fe2+ on COD removal was investigated, the test results shown in Figure 4.
Figure 3. Effect of reaction time on the treatment efficiencies of the Fenton process.
Figure 4. Effect of [H2O2]/[Fe2+] on the treatment efficiencies of the Fenton process.
As can be seen from Figure 4, with the molar ratio of H2O2/Fe2+ increased (the concentration of Fe2+ decreased), the efficiency of COD removal increased from 64.5% to 80.1%. Continued to reduce the dosage of Fe2+, the COD removal reduced. When the molar ratio of H2O2/Fe2+ increased to 6, the COD removal rate was only 17.6%. The phenomenon was consistent with other research results.
The appropriate molar ratio of H2O2/Fe2+ could promote Equation (1), generating ・OH quickly. When the molar ratio of H2O2/Fe2+ was too low (the Fe2+ concentration was too high), it would promote the Equation (4), resulting in the consumption of ・OH to reduce the rate of the reaction. When the molar ratio of H2O2/Fe2+ was too high (the Fe2+ concentration was too low), the generation of ・OH was very limited and the COD removal was reduced. Therefore, the optimum molar ratio of H2O2/Fe2+ was 3.
3.3. The Optimum Condition
From the orthogonal experiment and the single-factor experiment, we determined the optimum conditions: H2O2 dosage of 76.32 mmol/L, molar ratio of H2O2/Fe2+ of 3, pH value of 5 and reaction time of 2 h. Under the optimum conditions, the repeatable experiments of the NPEOs wastewater by Fenton oxidation process had been carried out 10 times. And the average COD removal rate was 85.6%.
4. Conclusions
Fenton oxidation of Nonylphenol Ethoxylate-10 (NP10EOs) in actual wastewater was investigated. Orthogonal experiment and single factor experiments were carried out to assess the optimum condition leading to the maximum removal of the surfactants. The main results obtained during the investigation were the following:
The orthogonal experiment indicated that the order of degree of influence on the COD removal rate was molar ratio of H2O2/Fe2+, reaction time, dosage of H2O2, and initial pH.
The single-factor tests were carried out to determine the optimal conditions: H2O2 dosage of 76.32 mmol/L, molar ratio of H2O2/Fe2+ of 3, pH value of 5 and reaction time of 2 h. Under the optimum operation conditions, the average COD removal rate was 85.6%.
Acknowledgements
This study was financially supported by Shanghai University of Engineering Science Innovation Fund for Graduate Students (No: E1-0903-15-01035).
Cite this paper
Zhou, R.Y. and Zhang, W.Q. (2017) Treatment of the High Concentration Nonylphenol Ethoxylates (NPEOs) Wastewater by Fenton Oxidation Process. American Journal of Analytical Che- mistry, 8, 72-80. http://dx.doi.org/10.4236/ajac.2017.81006
References
- 1. Soares, A., Guieysse, B., Jefferson, B., Cartmell, E. and Lester, J.N. (2008) Nonylphenol in the Environment: A Critical Review on Occurrence, Fate, Toxicity and Treatment in Wastewaters. Environment international, 34, 1033-1049.
https://doi.org/10.1016/j.envint.2008.01.004 - 2. Ying, G.G. (2006) Fate, Behavior and Effects of Surfactants and Their Degradation Products in the Environment. Environment International, 32, 417-431.
https://doi.org/10.1016/j.envint.2005.07.004 - 3. Sharma, V.K., Anquandah, GAK, Yngard, R.A., et al. (2009) Nonylphenol, Octylphenol, and Bisphenol—A in the Aquatic Environment: A Review on Occurrence, Fate, and Treatment. Journal of Environmental Science and Health, Part A, 44, 423-442.
https://doi.org/10.1080/10934520902719704 - 4. Lu, J., Jin, Q., He, Y. and Wu, J. (2007) Biodegradation of Nonylphenol Polyethoxylates under Fe(III)-Reducing Conditions. Chemosphere, 69, 1047-1054.
https://doi.org/10.1016/j.chemosphere.2007.04.035 - 5. Karci, A., Arslan-Alaton, I., Bekbolet, M., Ozhan, G. and Alpertunga, B. (2014) H2O2/UV-C and Photo-Fenton Treatment of a Nonylphenol Polyethoxylate in Synthetic Freshwater: Follow-Up of Degradation Products, Acute Toxicity and Genotoxicity. Chemical Engineering Journal, 241, 43-51.
https://doi.org/10.1016/j.cej.2013.12.022 - 6. Pagano, M., Lopez, A., Volpe, A., Mascolo, G. and Ciannarella, R. (2008) Oxidation of Nonionic Surfactants by Fenton and H2O2/UV Processes. Environmental Technology, 29, 423-433.
https://doi.org/10.1080/09593330801983862 - 7. Chen, L., Zhou, H.Y., Liu, L. and Deng, Q.Y. (2007) Mechanism Study on UV-Induced Photodegradation of Nonylphenol Ethoxylates by Intermediate Products Analysis. Chinese Chemical Letters, 18, 473-475.
https://doi.org/10.1016/j.cclet.2007.02.004 - 8. Liu, G., Zheng, S., Yin, D., Xu, Z., Fan, J. and Jiang, F. (2006) Adsorption of Aqueous Alkylphenol Ethoxylate Surfactants by Mesoporous Carbon CMK-3. Journal of Colloid and Interface Science, 302, 47-53.
https://doi.org/10.1016/j.jcis.2006.06.006 - 9. Tang, W.Z. and Huang, C.P. (1996) 2, 4-Dichlorophenol Oxidation Kinetics by Fenton’s Reagent. Environmental Technology, 17, 1371-1378.
https://doi.org/10.1080/09593330.1996.9618465 - 10. Jung, Y.S., Lim, W.T., Park, J.Y. and Kim, Y.H. (2009) Effect of pH on Fenton and Fenton-Like Oxidation. Environmental Technology, 30, 183-190.
https://doi.org/10.1080/09593330802468848 - 11. Pignatello, J.J., Oliveros, E. and MacKay, A. (2006) Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Critical Reviews in Environmental Science and Technology, 36, 81-84.
https://doi.org/10.1080/10643380500326564 - 12. Neyens, E. and Baeyens, J. (2003) A Review of Classic Fenton’s Peroxidation as an Advanced Oxidation Technique. Journal of Hazardous Materials, 98, 33-50.
https://doi.org/10.1016/S0304-3894(02)00282-0 - 13. Kitis, M., Adams, C.D. and Daigger, G.T. (1999) TheEffects of Fenton’s Reagent Pretreatment on the Biodegradability of Nonionic Surfactants. Water Research, 33, 2561-2568.
https://doi.org/10.1016/S0043-1354(98)00476-X - 14. Mansouri, L., Sabelfeld, M., Geissen, S.U. and Bousselmi, L. (2015) Catalysed Ozonation for Removal of an Endocrine-Disrupting Compound Using the O3/Fenton Reagents System. Environmental Technology, 36, 1721-1730.
https://doi.org/10.1080/09593330.2015.1008054 - 15. Lucas, M.S. and Peres, J.A. (2009) Removal of COD from Olive Mill Wastewater by Fenton’s Reagent: Kinetic Study. Journal of Hazardous Materials, 168, 1253-1259.
https://doi.org/10.1016/j.jhazmat.2009.03.002 - 16. Deng, Y. and Englehardt, J.D. (2006) Treatment of Landfill Leachate by the Fenton Process. Water Research, 40, 3683-3694.
https://doi.org/10.1016/j.watres.2006.08.009 - 17. Antonio, D.C. and Roberto, S. (1994) Monitoring Aromatic Surfactants and Their Biodegradation Intermediates in Raw and Treated Sewages by Solid-Phase Extraction and Liquid Chromatography. Environmental Science and Technology, 28, 850-858. https://doi.org/10.1021/es00054a016
- 18. Solea, M., Maria, J. and Loapez, D.A. (2000) Estrogenicity Determination in Sewage Treatment Plants and Surface Waters from the Catalonian Area (NE Spain). Environmental Science and Technology, 34, 5076-5083.
https://doi.org/10.1021/es991335n - 19. Sun, S.P., Li, C.J., Sun, J.H., Shi, S.H., Fan, M.H. and Zhou, Q. (2009) Decolorization of An Azo Dye Orange G in Aqueous Solution by Fenton Oxidation Process: Effect of System Parameters and Kinetic Study. Journal of Hazardous Materials, 161, 1052-1057.
https://doi.org/10.1016/j.jhazmat.2008.04.080






