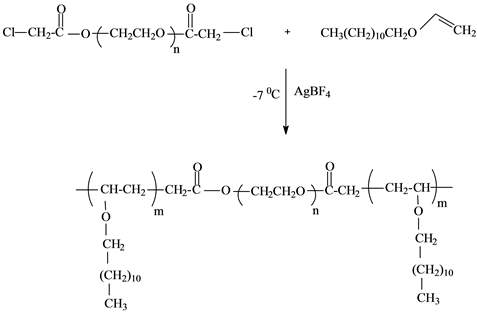American Journal of Analytical Chemistry
Vol.05 No.01(2014), Article ID:42137,5 pages
10.4236/ajac.2014.51006
Synthesis of ABA Type Block Copolymers of Poly(Ethylene Glycol) and Poly(Dodecyl Vinyl Ether) and Its Using as Surfactant in Emulsion Polymerization
Yesim Hepuzer Gursel1, Ayfer Sarac2*, Bahire Filiz Senkal1
1Department of Chemistry, Istanbul Technical University, Istanbul, Turkey
2Department of Chemistry, Yıldız Technical University, Istanbul, Turkey
Email: *ayfersarac2002@yahoo.com
Copyright © 2014 Yesim Hepuzer Gursel et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. In accordance of the Creative Commons Attribution License all Copyrights © 2014 are reserved for SCIRP and the owner of the intellectual property Yesim Hepuzer Gursel et al. All Copyright © 2014 are guarded by law and by SCIRP as a guardian.
ABSTRACT
A poly(ethylene glycol) (PEG) based macroinitiator (MI) with terminal chloride atom at both ends was prepared by the reaction of PEG-400 with chloroacetyl chloride and used for the cationic polymerization of dodecyl vinyl ether (DVE) yielding ABA type block copolymer. The block copolymer was then used as the surfactant for the emulsion polymerization of vinyl acetate and styrene in the presence of potassium persulfate as an initiator. The effects of new polymeric emulsifier on the physicochemical properties of obtained latexes were investigated de- pending on surfactant percentage in homopolymerizations.
Keywords
Cationic Polymerization; Emulsion Polymerization; Poly(Ethylene glycol); Surfactants
1. Introduction
Emulsion polymerization is a unique chemical process widely used to produce waterborne latexes with various colloidal and physicochemical properties. This heteroge- neous free radical polymerization process involves emul- sification of the relatively hydrophobic monomer in wa- ter by an oil-in-water emulsifier, followed by the initia- tion reaction with either a water insoluble initiator or an oil-soluble initiator. Typical monomers used in emulsion polymerization include vinyl acetate, butadiene, styrene, acrylonitrile, acrylate methacrylate monomers, and vinyl chloride [1-4].
Heterophase polymerizations, especially emulsion po- lymerizations, are important industrial technologies yield- ing synthetic elastomers, paints, paper coatings, adhe- sives, etc. In emulsion polymerizations, the use, or in situ production, of surfactants, is necessary in order to achieve stabilization of the latex particles produced during poly- merization and indeed later on in the derived products [5].
*Corresponding author.
Surfactants are among the most versatile products used in the chemical industry, appearing in such diverse products as motor oils, pharmaceuticals and detergents. More recently, applications have been extended to such high-technology areas as electronic printing, magnetic recording, and biotechnology [6]. There are four classes of surfactants: 1) anionic, where the head group of the molecule has a negative charge; 2) cationic, where the head group bears a positive charge; 3) zwitterionic, where both positive and negative charges are present; and 4) non-ionic, where the head group has no ionic cha- racter. Cationic surfactants, which are most relevant to the present study, usually fall into one of the following categories: long-chain amines or polyamines and their respective salts, quarternary ammonium salts (e.g. hex- adecyltrimethyl ammonium bromide), oligo (ethylene oxide) amines and their quaternized derivatives, and amine oxides.
Recently, there has been increasing interest in the synthesis of tailor-made polymeric surfactants. The sur- factants based on oligomeric and/or polymeric molecules have received wide attraction both at academic and in- dustrial scale because of their unique and tailor-made properties over the conventional low molecular weight surface active agents [7]. Although necessarily less well- defined than small-molecule surfactants, polymeric sur- factants probably offer greater opportunities in terms of flexibility, diversity and functionality [8]. This is espe- cially true in the light of recent advances in controlled/ living radical polymerization chemistry, as exemplified by atom transfer radical polymerization (ATRP) [9-15] and, to a lesser extent, reversible addition fragmentation transfer (RAFT) polymerization [16]. This new poly- mer chemistry has enabled synthetic polymer chemists to make new, well-defined amphiphilic block copoly- mers, many of which exhibit interesting surfactant be- havior.
The oligomeric/polymeric surfactants that exhibit as- sociation and self-assembly behavior in water are basi- cally amphiphilic in nature and consist of hydrophobic and hydrophilic moieties within the same molecule [17]. Hydrophilic-hydrophobic block copolymers directly pre- pared in aqueous medium without any additional surfac- tant are, therefore, of special interest as polymeric sur- factants in latex technology [18]. The most effective sta- bilization of emulsions is obtained using ABA block or ABn (or BAn) graft copolymers [19]. The B-chain(s) (the “anchor”-chain) is chosen to be highly insoluble in the aqueous medium and strongly adsorbed at (or soluble in) the oil-droplets. The A-chain is chosen to be highly so- luble in the aqueous medium and strongly solvated by its molecules [17]. ABA type block copolymers are those based on poly(ethylene oxide) (PEO) and poly(propyl- ene oxide) (PPO), which are also known as Pluronic sur- factants, associating in aqueous media into micellar ag- gregates, and the micellization can either be induced at a given temperature by increasing the concentration be- yond a critical value or at a given concentration beyond a critical micelle temperature [7,17]. The hydrophobic moieties, for example, have been either PPO, poly(buty- lene oxide) (PBO), poly(dimethyl siloxane) (PDMS), polystyrene (PS), or poly(isobutylene) (PIB) [7]. ABA type block copolymers could decrease the surface tension of both water as well as organic oils and, hence, they can be used for surfactant action in organic media, in which conventional low molecular weight surfactants as well as PEO-PPO or PEO-PPO-PEO polymeric surfactants are less effective [7]. Because of the hydrophobic structure at both ends, the ABA type block copolymer synthesized is used as surfactant in this study.
A significant difference in the stabilization mechanism of emulsions based on the Pluronic surfactants can be understood from the conformation of the two polymers at the oil-water interface. With the ABA block copolymer, the PPO B-chain produces small loops at the oil-surface leaving two tails of PEO chains dangling in the aqueous solution. In other words, this type of block copolymer would produce a “brush” at the oil-water interface. The steric repulsion in this case is due to “brush-to-brush” interaction. In contrast, the graft copolymer adsorbs with several alkyl chains in or at the oil-phase leaving strongly hydrated polyfructose loops and small tails in the solu- tion. The steric repulsion in this case is mainly due to “loop-to-loop” interaction [17].
As compared with conventional surfactants, block co- polymers in the latex technology may serve not only as a stabilizer in the polymerization process, but also as an active component in the finished product formulation, for example, as plasticizer, compatibilizer, antistatic agent, oil and/or water repellent. The major application possi- bilities of block copolymer-stabilized dispersions, as well for aqueous and nonaqueous systems, are, therefore, in the fields of coatings, inks, toners, adhesives, and func- tional packing materials for chromatography [18]. Of all the di- and tri-block copolymer surfactants with PEO- PPO, PEO-PPO-PEO have been a subject of intensive investigations, especially for their self-assembly proper- ties, phase behavior, gelation, etc. On the other hand, poly(ethylene glycol) (PEG) is one of the other common hydrophilic polymers studied especially as surfactant, which has potential for application in a variety of fields such as biological and biomedical sciences, surface che- mistry and electrochemistry [20]. In spite of some limita- tions of block copolymers in emulsion polymerization, inherent to their higher molecular weight with respect to classical surfactants, such polymeric surfactants lead, under suitable reaction conditions, to “hairy latexes” where the combination of their hydrophilic fringes and hydrophobic blocks on the particle surface are very at- tractive. For example, hydrophilic fringes of an adjusta- ble thickness enhance colloidal stability under freeze- thaw, electrolyte, and shear, reduce foaming, and im- prove the rheological characteristics [18].
In this study, a macroinitiator with PEO like chain was prepared by the reaction of PEG-400 with chloro acetyl chloride. This initiator was used for cationic polymeriza- tion of dodecyl vinyl ether to obtain ABA type block copolymer. This block copolymer was used in the emul- sion polymerizations of vinyl acetate (VAc) and styrene (St) as a surfactant, and the effects of this polymeric sur- factant quantity were investigated on the main characte- ristics of poly(vinyl acetate) (PVAc) and polystyrene (PSt) latexes.
2. Experimental
2.1. Materials
Vinyl acetate (VAc) (Fluka), styrene (St) (Fluka), potas- sium persulfate (PPS) (Fluka), chloroacetyl chloride (Fluka), poly(ethylene glycol) (PEG-400) (Aldrich), do- decyl vinyl ether (DVE) (Aldrich), AgBF4 (Aldrich) and all the chemicals used were analytical grade chemical products.
2.2. Preparation of Macroinitiator (MI)
Chloroacetyl chloride (44 mmol, 3.5 mL) in 10 mL of dry THF was added to dropwise to a stirring mixture of triethylamine, (43 mmol, 6 mL) and PEG-400 (25 mmol, 10 g) in 40 mL of dry THF at 0˚C. The reaction was con- tinued under stirring for 18 h at room temperature. The reaction mixture was filtered, solvent was evaporated and the rest remaining in the mixture was poured in cold die- thyl ether. The precipitated was filtered and dried under vacuum. Yield is 13 g.
2.3. Cationic Polymerization of Dodecyl Vinyl Ether
The polymerization was carried out under dry nitrogen atmosphere. 10 mL of DVB was introduced to a three- neck flask heated in vacuo and flushed with dry nitrogen. 1 g of MI and 1.05 g of AgBF4 in 10 mL of dichlorome- thane were added under efficient stirring at −7˚C. After 3 h, metanol was added to the reaction mixture to terminate the polymerization. The polymerization mixture was stirred at room temperature for 24 h. After centrifugation, the polymerization solution was added dropwise into cold methanol. The precipitated polymer was separated by filtration and dried under vacuum at room tempera- ture.
2.4. Emulsion Polymerization Procedure
PEG-PDVE block copolymer was used in emulsion po- lymerization of VAc and St as surfactant. A typical emulsion polymerization was carried out in a 500 mL three neck glass reactor equipped with a condenser, a mechanical stirrer having a constant speed of 400 rpm and a reflux condenser in a total batch period of 1 h. The polymerization was performed at given temperature dif- ferent time depending of the surfactant value. The condi- tions of the polymerizations were given Table 1. Ob- tained polymers were precipitated by adding salt and the polymer was filtered and washed with excess of hot wa- ter to remove salt and surfactant, and all measurements were carried out these purified latexes.
The effects of surfactant quantity on the physicoche- mical properties of polymers were investigated by mea- suring viscosity, viscosity average molecular weight, surface tension, and particle size, by using Brookfield viscometer, viscometric method, du Nouy tensiometer, and particle size analysis, respectively.
2.5. Measurements
New synthesized ABA type polymeric surfactant was
characterized by FT-IR (Nicholet 380), and 1H-NMR spectrum was measured on a Bruker Am400 instrument using CDCl3 as the solvent and TMS as internal standard. The number of average molecular weight (Mn) of the surfactant was determined by gel permeation chromato- graphy (GPC) by using an Agillant 1100 series consist- ing of a pump, a RI detector, and Waters styrogel (HR 3 and HR 4) columns. Tetrahydrofuran was used as the eluent and butylated hydroxyl toluene as reference. The flow rate was 1 mL/min.
Latexes were characterized by measuring Brookfield viscosity, viscosity average molecular weight (Mv), sur- face tension of latexes to air, and particle size. Conver- sion was monitored gravimetrically. According to the calculating results from solid contents of latexes as theo- retical and practical, conversion changed between 86% and 88%. The original viscosities of the homopolymer latexes were determined by Brookfield Programmable DV-II model viscometer with spindle number S 61 at 23˚C. Mv of polymers was determined by capillary in- trinsic viscometer at 30˚C. For this purpose, Ubbe- lohde-type viscometer, acetone and toluene used as sol- vents for PVAc and PSt respectively. Mark-Houwink constants were used as a = 0.72 and k = 1.02 × 10−3 (mL/g) and a = 0.73 and k = 1.55 × 10−4 (dL/g) for VAc and St respectively. The surface tension measurements were done with a Sigma 701 model tensiometer (KSV instruments, Helsinki, Finland) equipped with a Pt du Nouy ring at 24˚C. The particle size of the latexes was determined by Malvern Zetasizer 6.01 model instrument.
3. Results and Discussion
In this study, ABA type tree block copolymer of PEG, PDVE and PEG was prepared by cationic polymerization of dodecyl vinyl ether in the presence of PEG-400 having chlorine groups at both ends as a macroinitiator and a solution of AgBF4 in methylene chloride as catalyst at −7˚C for 3 h. The reaction path is represented below (Scheme 1).
The polymeric surfactant was characterized by FT-IR and 1H-NMR spectroscopy. According to the Figure 1, in the block copolymer, stretching vibrations occurs at 1740 cm−1 and 2922 cm−1 - 2853 cm−1 belongs to car-
Scheme 1.Synthesis of polymeric surfactant.
Table 1. Formulation used in polymerizations.
Received November 23, 2013; revised December 26, 2013; accepted January 5, 2014
Figure 1.FT-IR spectrum of the polymeric surfactant.
bonyl group and alkyl chains of dodecyl group, respec- tively.
Also, the C-O stretching vibration occurs at 1054 cm−1 belongs to etheric group. 1H-NMR spectrum of the po- lymer was given in Figure 2. Aliphatic protons of alkyl chains of dodecyl groups were observed as a multiplet at 1.20 - 1.80 ppm and etheric protons of PEG were ob- served at 3.0 - 4.0 ppm.
The number average molecular weight and polydisper- sity of the polymeric surfactant were detected as 1152 g/mol and 1.10 respectively (Figure 3). The narrow mo- lecular weight clearly show that the polymerization quan- titatively proceeds in a controlled fashion to afford a po- lymer with well-defined chain structure under the condi- tion employed.
3.1. Viscosity
Dispersion with a large number of small particles exhi- bits a higher viscosity than one with a small number of large particles [21]. Polymeric surfactant accelerated the reaction, because of increasing with the length of the hydrophobic part of the surfactant. Increasing sur- factant concentration in the polymer recipe slightly increased the latex viscosities, but it does not affect very seriously on the viscosities of VAc and St latexes
Figure 2.1H-NMR spectrum of the polymeric surfactant.
Figure 3.GPC traces of the polymeric surfactant.
(Table 2).
The main advantages of block copolymer synthesis in emulsion, over conventional bulk or solution poly- merizations, are that such polymerizations do not re- quire drastic experimental conditions such as anionic or cationic “living polymerizations”. Moreover, free rad- ical emulsion polymerizations are environmentally friendly methods for the production of polymers due to the absence of volatile organic compounds. These po- lymerizations often reach very high conversions; the heat produced by exothermic reactions is dissipated by the water and high-molecular weight polymers can be obtained in a low viscosity media. The final product can thus be directly used in its latex form, in coating applications for instance [18].
3.2. Surface Tension
The surface tension measurements were performed on a Sigma 701 model tensiometer (KSV instruments, Hel- sinki, Finland) equipped with a Pt du Nouy ring at 24˚C, and results were given in Table 2. Surface tension of homopolymer latexes increased with surfactant concen- tration. This can be explained by taking into account that the surface area stabilized by the surfactant increases with an increase of the concentration of the surfactant
Table 2. Colloidal characteristics of latexes.
resulting in smaller particles due to mass balance consid- erations. The increasing hydrophobic character into po- lymer causes to increasing emulsifier adsorption onto polymer particles. Thus the increasing of free emulsifier concentration in latex by blocking of emulsifier adsorp- tion and the decreasing of polarity differences between interfaces cause to increase the surface tension, especial- ly for VAc latexes.
Surface tension can be used as a measure for the cov- erage of particles. As can be derived from the surface tension values, the particle surfaces of the latexes are incompletely covered with surfactant molecules because the surface tensions of the latexes lie well above the val- ues of the saturated surfactant solution. The smaller the particles are, the higher the surface tension is and, there- fore, the coverage of the particles with surfactant in- creases with decreasing particle size [22].
3.3. Particle Size
The particle size of the final latexes was determined by Zetasizer, and can be seen in Table 2. It was found that the particle size mainly depends on the amount of sur- factant in both latex series. The presence of the long hy- drophobic chain of the Pluronic surfactants had a screen- ing effect on the Coulombic forces which prevents the surfactant adsorption. Consequently, the higher the chain lengths of the hydrophobic part of the surfactant, the higher the surfactant adsorption on the polymer [23]. The polymeric surfactant causes to decrease the number of particles in the volume unit, thus viscosity of latexes in- creased, and particle size decreased.
An increase of the surfactant quantity from 0.103 to 0.360 (wt) results in a decrease of the particle size and an increase viscosity. The viscosity of the dispersions measured increases drastically (Table 2). This can be explained by taking into account that the surface area stabilized by the surfactant increases with an increase of the concentration of the surfactant resulting in smaller particles due to mass balance considerations. A disper- sion with a large number of small particles exhibits a higher viscosity than one with a small number of large particles [21].
4. Conclusion
In this study, commercially available PEG-400 was mod- ified to prepare macroinitiator which was then used in the cationic polymerization of dodecyl vinyl ether. The new surfactant was used in emulsion polymerization of vinyl acetate and styrene. It was determined that when the concentration of the polymeric surfactant was increased, the resulting latex viscosity slightly increases, particle size decreases regularly, and the viscosity molecular weight of final latex increases. The surface tension and particle size of polymer latex follow different trends with the increasing of the surfactant concentration. Meanwhile, they were changed very seriously. It was found that the surfactant concentration was sensitive to such parameters as viscosity, molecular weight, surface tension, and par- ticle size.
Acknowledgements
This work were supported by the Turkish Scientific and Technological Research Council (TUBITAK) (Project Number: 108T722) and Scientific Research Projects Coordination Center of Yildiz Technical University (Project Number: 2012-01-02-KAP04).
REFERENCES
[1] F. A. Bovey, I. M. Kolthoff, A. I. Medalia and E. J. Mee- han, “Emulsion Polymerization,” Interscience Publishers, New York, 1965.
[2] D. C. Blackley, “Emulsion Polymerization. Theory and Practice,” Applied Science, London, 1975.
[3] R. M. Fitch, “Polymer Colloids: A Comprehensive Intro- duction,” Academic Press, London, 1997.
[4] C. S. Chern, “Emulsion Polymerization Mechanisms and Kinetics,” Progress in Polymer Science, Vol. 31, No. 5, 2006, pp. 443-486. http://dx.doi.org/10.1016/j.progpolymsci.2006.02.001
[5] A. Montoya-Goni, D. C. Sherrington, H. A. S. Schoon- brood and J. M. Asua, “Reactive Surfactants in Hetero- phase Polymerization. XXIV. Emulsion Polymerization of Styrene with Maleate- and Succinate-Containing Ca- tionic Surfactants,” Polymer, Vol. 40, No. 6, 1999, pp. 1359- 1366.
[6] M. J. Rosen, “Surfactants and Interfacial Phenomena,” 2nd Edition, Wiley Interscience, New York, 1989.
[7] S. S. Soni, N. V. Sastry, V. K. Aswal and P. S. Goyal, “Micellar Structure of Silicone Surfactants in Water from Surface Activity, SANS and Viscosity Studies,” The Journal of Physical Chemistry B, Vol. 106, No. 10, 2002, pp. 2606-2617. http://dx.doi.org/10.1021/jp0129434
[8] S. Liu and S. P. Armes, “Recent Advances in the Synthe- sis of Polymeric Surfactants,” Current Opinion in Colloid & Interface Science, Vol. 6, No. 3, 2001, pp. 249-256. http://dx.doi.org/10.1016/S1359-0294(01)00090-5
[9] J. S. Wang and K. Matyjaszewski, “Controlled ‘Living’ Radical Polymerization. Atom Transfer Radical Polyme- rization in the Presence of Transition-Metal Complexes,” Journal of the American Chemical Society, Vol. 117, No. 20, 1995, pp. 5614-5615. http://dx.doi.org/10.1021/ja00125a035
[10] M. Kato, M. Kamagaito, M. Sawamoto and T. Higashi- mura, “Polymerization of Methyl Methacrylate with the Carbon Tetrachloride/Dichlorotris-(triphenylphosphine) ru- thenium(II)/Methylaluminum Bis(2,6-di-tert-butyl phe- noxide) Initiating System: Possibility of Living Radical Polymerization,” Macromolecules, Vol. 28, No. 5, 1995, pp. 1721-1723. http://dx.doi.org/10.1021/ma00109a056
[11] T. E. Patten and K. Matyjaszewski, “Atom Transfer Rad- ical Polymerization and the Synthesis of Polymeric Mate- rials,” Advanced Materials, Vol. 10, No. 12, 1998, pp. 901-915. http://dx.doi.org/10.1002/(SICI)1521-4095(199808)10:12<901::AID-ADMA901>3.0.CO;2-B
[12] K. Matyjaszewski, J. Qiu, N. V. Tsarevsky and B. Charleux, “Atom Transfer Radical Polymerization of n-Butyl Methacrylate in an Aqueous Dispersed System: A Miniemulsion Approach,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 38, No. S1, 2000, pp. 4724- 4734. http://dx.doi.org/10.1002/1099-0518(200012)38:1+<4724::AID-POLA120>3.0.CO;2-Q
[13] D. H. Haddleton, S. Perrier and S. A. F. Bon, “Copper (I)-Mediated Living Radical Polymerization in the Pres- ence of Oxyethylene Groups: Online H-1 NMR Spec- troscopy to Investigate Solvent Effects,” Macromolecules, Vol. 33, No. 22, 2000, pp. 8246-8251. http://dx.doi.org/10.1021/ma001097c
[14] X. S. Wang and S. P. Armes, “Facile Atom Transfer Ra- dical Polymerization of Methoxy-Capped Oligo(Ethylene Glycol) Methacrylate in Aqueous Media at Ambient Temperature,” Macromolecules, Vol. 33, No. 18, 2000, pp. 6640-6647. http://dx.doi.org/10.1021/ma000671h
[15] F. Zeng, Y. Shen, S. Zhu and R. Pelton, “Atom Transfer Radical Polymerization of 2-(Dimethylamino)Ethyl Methacrylate in Aqueous Media,” Journal of Polymer Science Part A: Polymer Chemistry, Vol. 38, No. 20, 2000, pp. 3821-3827. http://dx.doi.org/10.1002/1099-0518(20001015)38:20<3821::AID-POLA130>3.0.CO;2-G
[16] G. Moad, J. Chiefari, Y. K. Chong, J. Krstina, R. T. A. Mayadunne, A. Postma, E. Rizzardo and S. H. Thang, “Living Free Radical Polymerization with Reversible Addition-Fragmentation Chain Transfer (the Life of RAFT),” Polymer International, Vol. 49, No. 9, 2000, pp. 993-1001. http://dx.doi.org/10.1002/1097-0126(200009)49:9<993::AID-PI506>3.0.CO;2-6
[17] D. Exerowaa, G. Gotcheva, T. Kolarova, K. Kristova, B. Leveckeb and T. Tadros, “Comparison of Oil-in-Water Emulsion Films Produced Using ABA or ABn Copoly- mers,” Colloids and Surfaces A: Physicochem Eng As- pects, Vol. 335, No. 1, 2009, pp. 50-54. http://dx.doi.org/10.1016/j.colsurfa.2008.10.025
[18] G. Riess and C. Labbe, “Block Copolymers in Emulsion and Dispersion Polymerization,” Macromol Rapid Com- mun, Vol. 25, No. 2, 2004, pp. 401-435. http://dx.doi.org/10.1002/marc.200300048
[19] J. Zhang, M. R. Dubay, C. J. Houtman and S. J. Severtson, “Sulfonated Amphiphilic Block Copolymers: Synthesis, Self-Assembly in Water, and Application as Stabilizer in Emulsion Polymerization,” Macromolecules, Vol. 42, No. 14, 2009, pp. 5080-5090. http://dx.doi.org/10.1021/ma900795f
[20] Y. J. Kim, Y. Nagasaki, K. Kataoka, M. Kato, M. Yokoyama, T. Okano and Y. Sakurai, “Heterobifunctional Poly(Ethylene Oxide)-One-Pot Synthesis of Poly(Ethy- lene Oxide) with a Primary Amino Group at One End and a Hydroxyl Group at the Other End,” Vol. 33, No. 10, 1994, pp. l-6.
[21] C. Heldmann, R. I. Cabrera, B. Momper, R. Kuropka and K. Zimmerschied, “Influence of Nonionic Emulsifiers on the Properties of Vinyl Acetate/VeoVa10 and Vinyl Ace- tate/Ethylene Emulsions and Paints,” Progress in Organic Coatings, Vol. 35, No. 1, 1999, pp. 69-77. http://dx.doi.org/10.1016/S0300-9440(99)00025-9
[22] K. Landfester, “Polyreactions in Miniemulsions,” Ma- cromolecular Rapid Communications, Vol. 22, No. 12, 2001, pp. 896-936. http://dx.doi.org/10.1002/1521-3927(20010801)22:12<896::AID-MARC896>3.0.CO;2-R
[23] D. H. Napper and A. E. Alexander, “Polymerization of Vinyl Acetate in Aqueous Media. Part II. The Kinetic Behavior in the Presence of Low Concentrations of Added Soaps,” Journal of Polymer Science, Vol. 61, No. 171, 1962, pp. 127-133. http://dx.doi.org/10.1002/pol.1962.1206117116