American Journal of Analytical Chemistry
Vol.4 No.5(2013), Article ID:31705,7 pages DOI:10.4236/ajac.2013.45032
Promethazine-Tetraphenyl Boron(III) Modified Carbon Paste Electrode for the Determination of Promethazine Hydrochloride
Department of Chemistry, Faculty of Science, Cairo University, Cairo, Egypt
Email: *ssbadawy@yahoo.com
Copyright © 2013 Sayed Sayed Badawy, Sherif Abo El Seoud El Said. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 21, 2013; revised March 25, 2013; accepted April 28, 2013
Keywords: Carbon Paste Electrode; Ion Pair; Promethazine Hydrochloride; Phenergan (Syrup)
ABSTRACT
Promethazine modified carbon paste ion selective electrode has been prepared, based on the ion pair of promethazine hydrochloride Pm.Cl with sodium tetraphenyl boron(III) Pm-TPB dissolved in dioctyl phthalate DOP as a pasting liquid. The electrode showed the linear response with mean calibration graph slope of 58 mV/decade at 25˚C; at a concentration range and a lower detection limit of 1 × 10−5 - 6 × 10−2 M and 8 × 10−6 M promethazine hydrochloride, respectively. The change of pH of the tested solution within the range 4.2 - 6.8 did not affect the electrode performance. The electrode showed a very good selectivity toward Pm+ cations with respect to a large number of organic and inorganic cations and compounds. The standard addition and potentiometric titration methods were applied for the determination of promethazine hydrochloride in pure solution and in pharmaceutical preparations with an average recovery range of 97.3% - 101.4% and a mean relative standard deviation (RSD) of 0.35% - 1.83% which were of comparable accuracy and precision to the previously reported works.
1. Introduction
Promethazine hydrochloride [N,N-dimethyl-1-(10H-phenothiazine-10-yl)propan-2-amine] hydrochloride, belonging to phenothiazine group (Scheme 1), is widely used as antihistaminic, sedative, antiemetic, antipsychotic, analgesic and anticholinergic drug.
Several methods have been reported for the quantitative determination of promethazine in pure solution and/ or in pharmaceutical preparations. These methods were mainly chromatographic which, in spite of their good sensitivities, are very expensive, time consuming and require special training. They include LC-MS [1], HPLC [2-7], GLC [8-10], turbidimetry [11], high performance capillary electrophoresis [12,13], nephelometric and conductmetric titrations [14,15], chemometric calibration [16], (square wave adsorptive [17]; anodic striping [18]; differential pulse adsorptive [19,20]; cyclic [21,22]) voltamertic methods of analysis, PVC membrane potentiometry [23,24] and UV-visible spectrophotometry [25-31].
In the present work, a new chemically modified carbon paste electrode for Promethazine cations was constructed and its performance characteristics were studied. The electrode was successfully used for the determination of Pm.Cl in pure solution and in pharmaceutical preparations.
2. Experimental
2.1. Apparatus
Potentiometric and pH measurements were carried out by using HANNA pH-mV meter model 8519, a Techne circulator thermostat Model C-100 to control the tempera-

Scheme 1. Promethazine hydrochloride.
ture of the test solution and an Ag/AgCl/3M KCl reference electrode model Metrohm 6.0733.100. The electrochemical system of the measurements may be represented as follows: CMCPE/test solution//reference electrode.
2.2. Materials and Reagents
All reagents were of analytical reagent grade. Doublydistilled water was used throughout all experiments. Promethazine hydrochloride was provided by Alexandria Pharmaceutical Industries Co., Alexandria, Egypt. The Pharmaceutical preparations [Phenergan syrup, Promethazine hydrochloride 1 mg/ml] and [Phenergan syrup, Expectorant, (Promethazine hydrochloride1mg/ml, thicol 45 mg/ml and Ipecacuanha extract 3 mg/ml)] were obtained from local drug stores. Graphite powder (Fluka). Sodium tetraphenyl borate NaTPB, dioctyl phthalate DOP, dibutyl phthalate DBP, o-nitrophenyl octyl ether ONPOE, diisononyl phthalate DINP and paraffin oil PO were obtained from Aldrich chemical company.
2.3. Preparation of the Ion Exchanger
The ion exchanger, Promethazine tetraphenyl borate PmTPB, was prepared by adding 50 ml 0.01 M of promethazine hydrochloride to 50 ml 0.01 M of NaTPB. The formed white precipitate was filtered off, washed thoroughly with doubly-distilled water, dried at room temperature and ground to fine powder. This ion exchanger was used as a modifier for the carbon paste electrode which was prepared as described elsewhere [32]. The composition of the ion exchanger was confirmed by elemental analysis at the Micro-Analytical Center, Faculty of Science , Cairo university, to be 1:1 [Pm:TPB]. The calculated percentages of C, H, N and S were 81.4%, 6.7%, 4.6% and 5.2% and the found ones were 80.5%, 6.5%, 5.1% and 4.9%, respectively.
2.4. Construction of the Electrode [32]
A Teflon holder (12 cm length) with a hole at one end (7 mm diameter, 3.5 mm depth) for the paste filling served as the electrode body. Electrical contact was made with a stainless steel rod through the center of the holder. This rod can move up and down by screw movement to press the paste down when renewal of the electrode surface is needed. The modified paste was prepared by mixing the weighed amount of Pm-TPB ion pair and high purity graphite with acetone. The mixture was homogenized, and the weighed amount of DOP was added to the mixture. Very intimate homogenization is then achieved by careful mixing with glass rod in agate mortar. The readyprepared paste was packed into the hole of the electrode body. The carbon paste was smoothed onto paper until it had a shiny appearance and the electrode was used directly for potentiometric measurements without preconditioning requirements.
2.5. Recommended Procedures
2.5.1. Construction of the Calibration Graphs
Suitable increments of standard Pm.Cl solution were added to 50 ml of water to cover the concentration range from 1.0 × 106 to 1.0 × 102 M. For higher concentrations, separate solutions were used. In this solution, the sensor and the reference electrodes were immersed and the emf was recorded after 10 seconds, at 25˚C, after each addition, and plotted versus—log [Pm.Cl].
2.5.2. Effect of pH on the Electrode Potential
The effect of pH of the test solution on the potential values of the electrode system concentrations of 1.0 × 102 and 1 × 103 M of Pm.Cl was studied. Aliquots of the drug solution [50 ml] were transferred to 100 ml cell and the tested electrode in conjunction with an Ag/AgCl/3M KCl reference electrode, and a combined glass electrode were immersed in this solution. The potential and pH readings were simultaneously recorded. The pH of the solution was varied over the range of 1.0 - 12.0 by addition of small volumes of 0.1 - 1.0 M HCl and/or NaOH solutions. The mV-readings were plotted against the pH of the above mentioned concentrations.
2.5.3. Potentiometric Determination of Promethazine Hydrochloride
The standard addition method was applied [33], in which small known increments of standard Pm.Cl solution 0.1 M was added to 50 ml aliquot samples containing various amounts of pure Pm.Cl or the pharmaceutical preparations. The change in potential reading was recorded for each increment and used to calculate the concentration of Pm.Cl in the sample solution using the following equation:
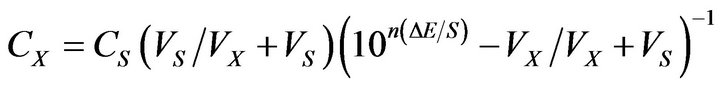
where: CX and VX are the concentration and the volume of the unknown, respectively, Cs and VS are the concentration and the volume of the standard, respectively, S is the slope of the calibration graph, and ∆E is the change in milli volt due to addition of the standard.
For analysis of pharmaceutical preparations, appropriate volumes from syrup, containing 5.00, 10.00, 15.00, 20.00, 25.00 and 35.00 mg of promethazine were taken as samples and completed to 50 ml by doubly distilled water and the standard addition technique was applied as described above.
In potentiometric titration, aliquots containing 16.0 - 96.0 mg of Pm.Cl were transferred into 100 ml titration cell and diluted to 50 ml with distillated water. The resulting solutions were titrated against 10−2 mol/L NaTPB solution. The end points were determined from the conventional S-shaped curves and the first derivative method. The same procedure was applied for the pharmaceutical preparations.
3. Results and Discussion
3.1. Composition and Characteristics of the Electrode
In preliminary experiments carbon paste electrodes with and without ion-exchanger were prepared. The paste with no exchanger displayed no measurable response toward promethazine cations, whereas, in the presence of the proposed ion-exchanger Pm-TPB, the optimized sensor demonstrated appreciable response and remarkable selectivity for Pm+ cations over several common organic and inorganic cations. We examined also the influence of the plasticizer type and concentration on the characteristics of the electrode using four plasticizers with different polarities including DBP, DINP, ONPOE and DOP (Figure 1). An electrode modified by 0.6 % Pm-TPB and 6.2 % DOP as a plasticizer has a slope of 58 mV per concentration decade (Table 1) and a linearity range of 1 × 10−5 to 6 × 10−2 M of Pm.Cl. This composition was chosen for carrying out the subsequent studies and its electrochemical performance characteristics were systematically evaluated according to IUPAC recommendations [34].
3.2. Electrode Response
Response characteristics of the electrode under investigation are summarized in (Table 2). Detection limit was evaluated according to IUPAC recommendation [34].
3.3. Effect of pH of the Test Solution
Representative curves for the electrode are shown in
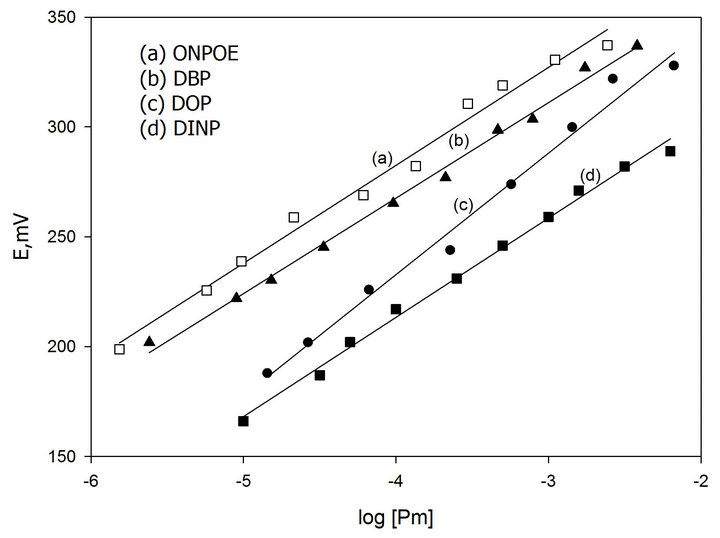
Figure 1. Effect of type and concentration of plasticizer on potential response of Pm-TPB MCPE.
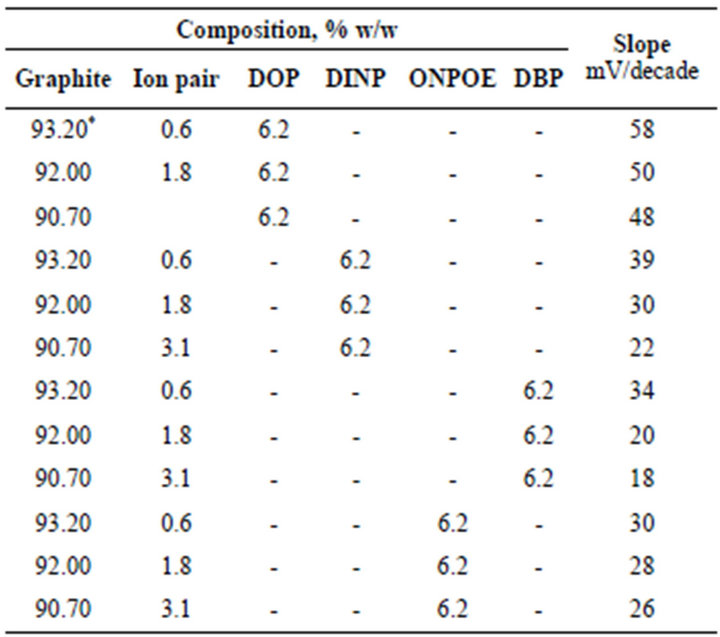
Table 1. Composition of the Pm modified carbon paste electrode and the corresponding slopes of the calibration graphs at 25˚C ± 0.1˚C.
*Optimum composition.
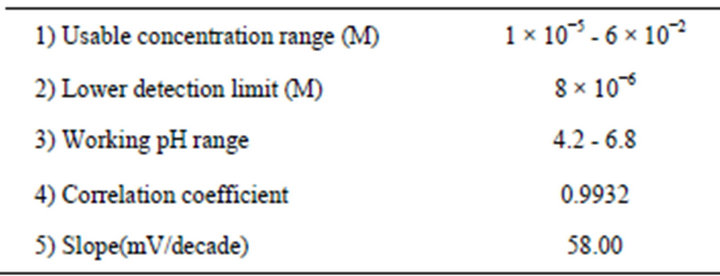
Table 2. Response characteristics of the promethazine modified carbon paste electrode.
(Figure 2). The results indicate that at pH range of 4.2 - 6.8, the potential of the electrode is independent of the value of pH. At pH values lower than 4.2, the potential slightly increased, which can be related to penetration of hydronium ion, while the decreased potential that took place at pH values higher than 6.8, is most probably attributed to the formation of the free promethazine base in the test solution.
3.4. Selectivity of the Electrode
The selectivity coefficients 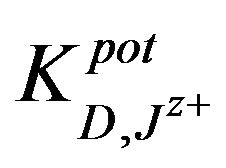 were evaluated by separate solution method SSM [35] for ions with charge numbers, applying the following equation:
were evaluated by separate solution method SSM [35] for ions with charge numbers, applying the following equation:
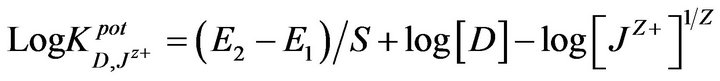
where E1 and E2 are the electrode potentials in 10−2 M Pm.Cl and in 10−2 M interferent Jz+ solutions, respectively and S is the slope of the calibration graph.
The, 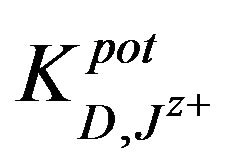 was also determined by the matched potential method MPM [36] for all types of ions and mole-
was also determined by the matched potential method MPM [36] for all types of ions and mole-
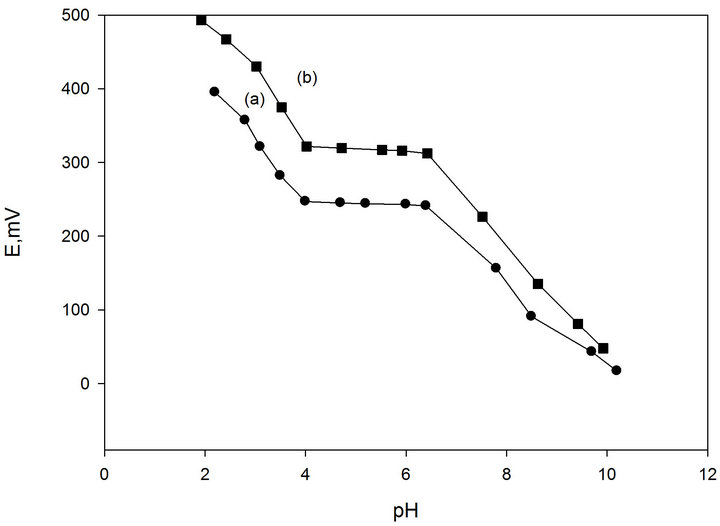
Figure 2. Effect of pH of test solution, 10−3 M (a) and 10−2 M (b) Pm.Cl on the potential response of Pm-TPB CMCPE.
cules. In this method, the potentiometric selectivity coefficient was defined as the activity ratio of primary and interfering ions that gives the same potential change under identical conditions.
At first, a known concentration ( ) of the primary ion is added to a reference solution that contains a fixed concentration (CD) of primary ions, and the corresponding potential difference (∆E) was recorded and a solution of an interfering ion was added to the reference solution until the same potential change (∆E) is recorded. The change in potential produced at the constant background of the primary ion must be the same in both:
) of the primary ion is added to a reference solution that contains a fixed concentration (CD) of primary ions, and the corresponding potential difference (∆E) was recorded and a solution of an interfering ion was added to the reference solution until the same potential change (∆E) is recorded. The change in potential produced at the constant background of the primary ion must be the same in both:
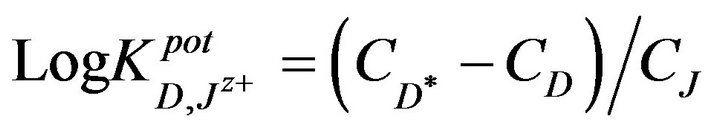
where, CJ is the concentration of the interfering ion. The determined selectivity coefficients (Table 3) reflect a very high selectivity of the investigated electrode toward promethazine cations, with respect to many common inorganic cations, sugars and amino acids which are frequently present in biological fluids and pharmaceutical preparations. The inorganic cations did not interfere due to the differences in their motilities and permeabilities as compared to Pm+ cations.
4. Analytical Applications
Promethazine was determined, in pure solutions and in pharmaceutical preparations, potentiometrically using the investigated electrode by both standard addition and potentiomertic titration methods. Representative titration curves are shown in (Figure 3). The mean recovery and the relative standard deviation values, for pure solution and pharmaceutical preparations, were calculated and summarized in (Table 4).
In pharmaceutical analysis, it is important to test the selectivity toward the excipients and fillers added to the pharmaceutical preparations. It is clear from the results
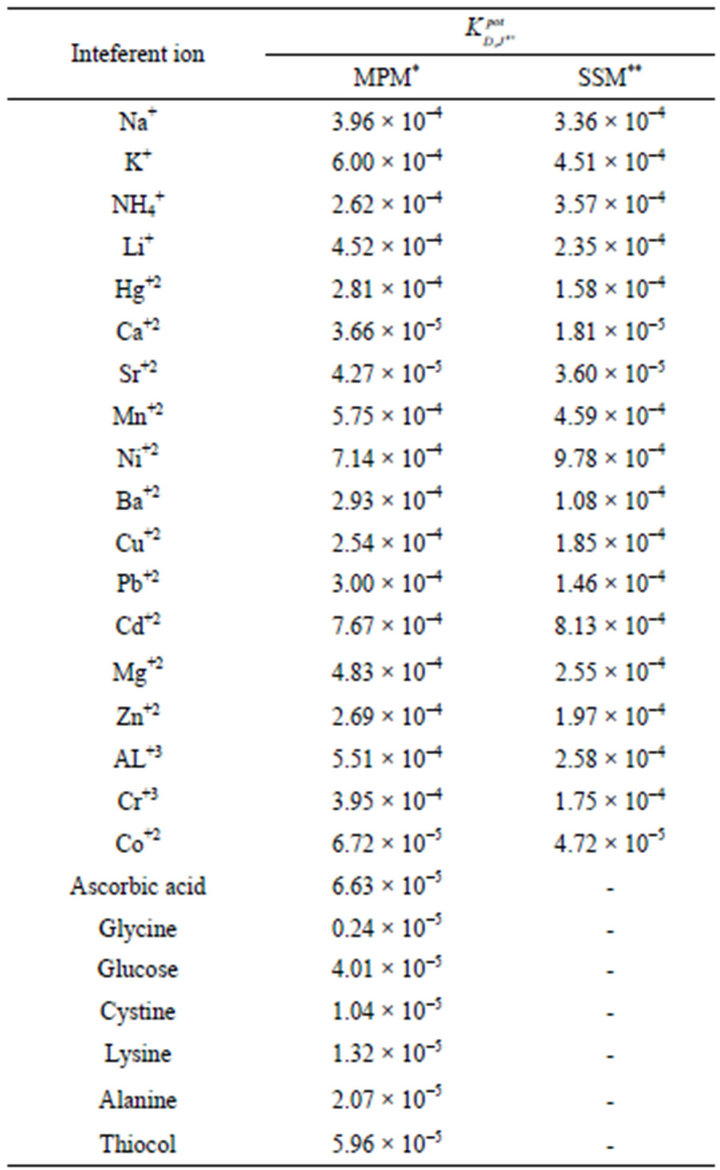
Table 3. Selectivity coefficients of Pm-TPB CMCP electrode.
*SSM: Separate Solution Method; **MPM: Matched Potential Method.
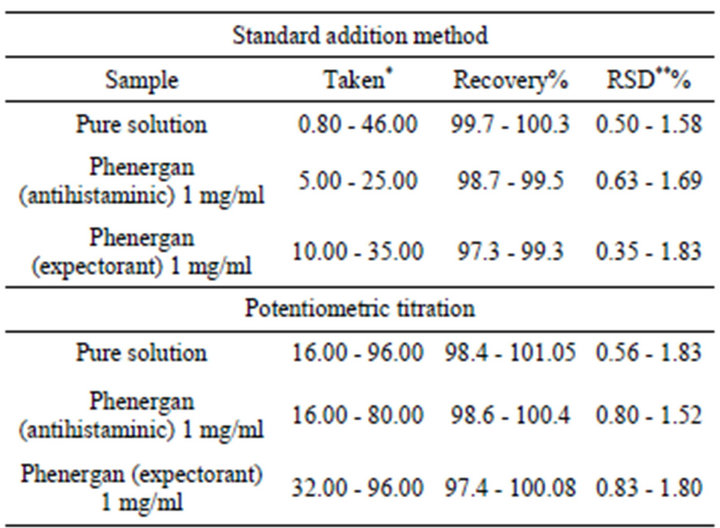
Table 4. Determination of promethazine in pure form and in pharmaceutical preparations by applying standard addition and potentio-metric titration methods.
*Taken (mg per 50 ml); **RSD, relative standard deviation (four determinations).
obtained for pharmaceutical preparations (Table 4) that these components and excipients do not interfere as indicated by high recovery and low standard deviation values. The results of the proposed electrode indicate high accuracy and precision of the present work (Table 5). These results were compared with those obtained from the official method (based on non aqueous titration of the drug with 0.1 N perchloric acid in the presence of anhydrous acetic acid and acetic anhydride and the end point was determined potentiometrically [37]) by applying of F-and t-tests [37]. The calculated F-values were found to be in the range 2.68 - 2.82 which were lower than the tabulated value [9.12 at 95% confidence limit and 4 degrees of freedom] While, the t-values were found in the range 4.19 - 5.17 which were lower than the tabulated value [5.84 at 99.9% confidence limit and 4 degrees of freedom]. This means that the present methods were of comparable precision to that of the official method and there was no significant difference between the mean values obtained by both methods. In order to establish whether the proposed methods exhibit any fixed or proportional bias, a simple linear regression of the taken milligrams against found was calculated [38] and the results of statistical treatments of the data were shown in (Table 5).
5. Conclusion
The proposed electrode based on Pm-TPB ion exchanger as the paste modifier offer a valuable technique for the determination of promethazine in pure solution and in pharmaceutical preparations. The inherent advantages of the proposed electrode were its rapid response, simple operation, and precise results, low cost, applicability over reasonable pH range with minimal sample pretreatment and direct application to the determination of promethazine in complex matrix without prior separation. The proposed methods of determination with the prescribed electrode were simple, sensitive, highly specific and had advantages over the previously described procedures for Pm.Cl determination since the interferences of the excipients and impurities were nullified.
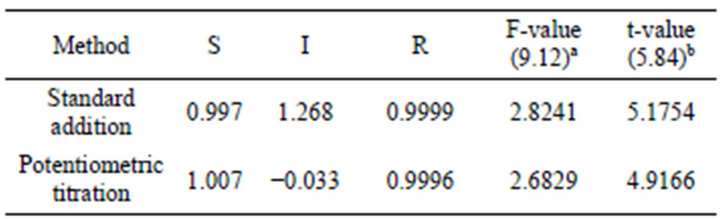
Table 5. Statistical treatment of the data obtained for Promethazine using modified carbon paste electrode in comparison with the official method.
S: Slope of the regression line of mg taken versus mg found. I: intercept of the regression line. R: Correlation coefficient of the regression line. aFtabulated at 95% confidence limit. bt-tabulated at 99.9% confidence limit.
REFERENCES
- P. Liu, S. Liang, B. J. Wang and R. C. Guo, “Development and Validation of a Sensitive LC-MS Method for the Determination of Promethazine Hydrochloride in Human Plasma and Urine,” European Journal of Drug Metabolism and Pharmacokinetics, Vol. 34, No. 3-4, 2009, pp. 177-184.
- S. L. Yang, L. O. Wilkin and C. R. Clark, “Ahigh-Performanceliquid-Chromatographic Method for the Simultaneous Assay of Aspirin, Caffeine, Di-Hydro Codeine Tartarate and Promethazine Hydrochloride in a Capsule Formulation,” Journal of Medical Economics, Vol. 11, No. 4, 1985, pp. 799-814.
- D. D. Borkar, V. P. Godse, Y. S. Bafana and A. V. Bhosale, “Simultaneous Estimation of Paracetamol and Promethazine Hydrochloride in Pharmaceutical Formulationsby a RP-HPLC Method,ˮ International Journal of ChemTech Research, Vol. 1, No. 3, 2009, pp. 667-670.
- B. J. Meakin, D. J. Davies, N. Cox and J. Stevens, “Chromatographic Determination of Promethazine Hydrochloride in Aqueous Solution,ˮ Analyst, Vol. 101, No. 1206, 2009, p. 720. doi:10.1039/an9760100720
- S. X. R. Spring, Y. H. L. Hong, S. X. R. Spring and Y. H. L. H. Ling, “High Performance Liquid Chromatography for Determination of Promethazine Hydrochloride Injection,ˮ Pharmaceutical Analysis Journal, Vol. 25, No. 9, 2005, pp. 139-141.
- S. Stavchanskya, J. Wallaceb, M. Chuea and J. Newburgera, “High Pressure Liquid Chromatographic Determination of Promethazine Hydrochloride in the Presence of Its Thermal and Photolytic Degradation Products,ˮ Journal of Liquid Chromatography, Vol. 6, No. 7, 1983, pp. 1333-1344. doi:10.1080/01483918308080002
- J. E. Wallace, J. E. Shimek, S. C. Harris and S. Stavchansky, “Determination of Promethazine in Serum by Liquid Chromatogphy,ˮ Clinical Chemistry Journal, Vol. 27, No. 2, 1981, pp. 253-255.
- S. Stavchanskya, J. E. Wallaceb, M. Chuea and P. Wua “Gas Liquid Chromatographic Determination of Promethazine Hydrochloride in Cocoa Butter-White Wax Suppositories,ˮ Indian Pharmaceutical Journal, Vol. 9, No. 6, 1983, pp. 989-998. doi:10.3109/03639048309042837
- B. J. Meakin, D. J. G. Davies, N. Cox and J. Stevens, “Chromatographic Determination of Promethazine Hydrochloride in Aqueous Solution,ˮ Analyst, Vol. 101, No. 1206, 1976, pp. 720-727. doi:10.1039/an9760100720
- S. Stavchanskya, J. E. Wallaceb, M. Chuea and P. Wua, “Gas Liquid Chromatographic Determination of Promethazine Hydrochloride in Polyethylene Glycol Suppositories Using the Hall’s Electrolytic Conductivity Detector,ˮ Analytical Letters Journal, Vol. 15, No. 16, 1982, pp. 1361-1372.
- J. M. Calatayud, S. N. Sarrion, A. S. Sampedro and C. G. Benito, “Determination of Promethazine Hydrochloride with Bromophenol Blue by a Turbidmetric Method and Flow Injection Analysis,ˮ Micro chemical Journal, Vol. 45, No. 2, 1992, pp. 129-136. doi:10.1016/0026-265X(92)90004-M
- J. L. Francisco, A. M. García, C. F. Alés, Barrero and J. M. B. Sendra. “Determination of Thiazinamium, Promazine and Promethazine in Pharmaceutical Formulations Using a CZE Method,ˮ Analytica Chimica Acta Journal, Vol. 535, No. 1-2, 2005, pp. 101-108.
- P. Kubacák, P. Mikus, I. Valásková and E. Havránek, “Determination of Promethazine Hydrochloride in Pharmaceuticals by Capillary Isotachophoresis,” Methods and Findings in Experimental and Clinical Pharmacology, Vol. 27, No. 8, 2005, p. 529.
- N. N. Yong and B. Q. Zheng, “Differential Kinetic Spectrophotometric Determination of Chlorpromazine Hydrochloride and Promethazine Hydrochloride by Chemometric Method,ˮ Guang Pu Fenxi Chinese Journal, Vol. 22, No. 7, 2002, pp. 1364-1367.
- Q. Zhang, X. Zhan, C. Li, T. Lin, L. Li, X. Yin, N. He and Y. Shi, “Determination of Promethazine Hydrochloride and Its Preparations by Highly Accurate Nephelometric Titration,ˮ International Journal of Pharmaceutics, Vol. 302, No. 1-2, 2005, pp. 10-17.
- A. M. Idris, F. N. Assubaie and S. M. Sultan, “Ion-Pair Formation in Pharmaceutical Analysis Conduct Metric Determination of Promazine, Chlorpromazine, Promethazine, Imipramine and Ciprofloxacin Hydrochlorides in Pure Form, Drug Formulations and Urine,ˮ Micro Chimica Acta Journal, Vol. 134, No. 1-2, 2000, pp. 9-14.
- F. W. P. Ribeiro, A. S. Cardoso, R. R. Portela, J. E. S. Lima, S. A. S. Machado, P. L. Neto, D. D. Souza and A. N. Correia, “Electro analytical Determination of Promethazine Hydrochloride in Pharmaceutical Formulations on Highly Boron-Doped Diamond Electrodes,ˮ Electro Analysis Journal, Vol. 20, No. 18, 2008, pp. 2031-2039.
- X. Y. Hu and Z. Z. Leng, “Spectrophotometric Determination of Promethazine Hydrochloride,ˮ Yaoxue Xuebao, Vol. 27, No. 4, 1992, pp. 283-286.
- Y. Ni, L. Wanga and S. Kokot, “Voltammetric Determination of Chlorpromazine Hydrochloride and Promethazine Hydrochloride with the Use of Multivariate Calibration,ˮ Analytica Chimica Acta Journal, Vol. 439, No. 1, 2001, pp. 159-168.
- Z. S. Yang, J. Zhao, D. P. Zhang and Y. C. Liu, “Electrochemical Determination of Trace Promethazine Hydrochloride by a Pretreated Glassy Carbon Electrode Modified with DNA,ˮ Analytical Science Journal, Vol. 23, No. 5, 2007, pp. 569-572.
- X. Xi, L. Ming and J. Liu, “Voltammetric Determination of Promethazine Hydrochloride at a Multi-Wall Carbon Nanotube Modified Glassy Carbon Electrode,ˮ Drug Testing and Analysis Journal, Vol. 3, No. 3, 2011, pp. 182-186.
- P. Xiao, W. Wu, J. Yu and F. Zhao, “Voltammetric Sensing of Promethazine on Multi-Walled Carbon Nanotubes Coated Gold Electrode,ˮ International Journal of Electro Chemical Science, Vol. 2, No. 2, 2007, pp. 149-157.
- A. K. Hassan, B. Saad, S. A. Ghani, R. Adnan, A. A. Rahim, N. Ahmad, M. Mokhtar, S. T. Ameen and S. M. A. Araji, “Ionophore-Based Potentiometric Sensors for the Flow-Injection Determination of Promethazine Hydrochloride in Pharmaceutical Formulations and Human Urine,ˮ Academic Journal, Vol. 11, No. 1, 2011, pp. 1028-1042.
- N. S. Nassory, S. A. Maki and B. A. Phalahy, “Preparation and Potentiometric Study of Promethazine Hydrochloride Selective Electrodes and Their Use in Determining Some Drugs,ˮ Journal of Chemistry, Vol. 32, No. 2, 2008, pp. 539-548.
- H. S. Gowda and K. A. Padmaji, “Spectrophotometric Studies on Platinum Promethazine Hydrochloride Complex,ˮ Micro Chemical Journal, Vol. 25, No. 3, 1980, pp. 396-402. doi:10.1016/0026-265X(80)90281-7
- D. Daniel and I. G. R. Gutz, “Flow Injection Spectro Electro Analytical Method for the Determination of Promethazine Hydrochloride in Pharmaceutical Preparations,ˮ Analytical Chimica Acta, Vol. 494, No. 1-2, 2003, pp. 215-224.
- M. B. Devani, B. N. Suhagia and S. A. Shah, “Spectrophotometric Determination of Promethazine Hydrochloride in Bulk Powder and In Its Dosage Forms,” Indian Journal of Pharmaceutical, Vol. 61, No. 2, 1999, pp. 110-112.
- M. J. Saif and J. Anwar, “New Spectrophotometer Method for the Determination of Promethazine-HCl from Pure and Pharmaceutical Preparations,ˮ Talanta Journal, Vol. 31, No. 5, 2005, pp. 869-872.
- K. C. Ramesh, B. G. Gowda and J. Keshavayya, “Spectrophotometric Determination of Promethazine Hydrochloride in Pharmaceutical Formulations,ˮ Indian journal Pharmaceutical Science, Vol. 65, No. 4, 2003, pp. 432- 435.
- M. Q. A. Abachi, T. S. A. Ghabsha and E. S. Salih, “Application of Promethazine Hydrochloride as a Chromogenic Reagent for the Spectrophotometric Determination of Aniline and Its Substituent’s,ˮ Micro Chemical Journal, Vol. 41, No. 1, 1990, pp. 64-71. doi:10.1016/0026-265X(90)90096-N
- S. M. Sultan, Y. A. M. Hassan and A. M. Abulkibash, “Chemiluminescence Assay of Promethazine Hydrochloride Using Acidic Permanganate Employing Flow Injection Mode Operated with Syringe and Peristaltic Pumps,ˮ Talanta Journal, Vol. 59, No. 6, 2003, pp. 1073-1080.
- H. Ibrahim, Y. M. Issa and H. M. A. Shawish, “Chemically Modified Carbon Paste Electrode for the Potentiometric Determination of Dicylomine Hydrochloride in Batch and in FIA Conditions,ˮ Analytical Sciences, Vol. 20, No. 6, 2004, pp. 911-916. doi:10.2116/analsci.20.911
- S. E. A. Karim, R. M. E. Nashar and A. H. Abadi, “Potentiometric Determination of Imatinib under Batch and Flow Injection Analysis Conditions,ˮ International Journal of Electro Chemistry Science, Vol. 7, No. 10, 2012, pp. 9668-9681.
- R. P. Buck and E. Lindner, “Recommendations for Nomenclature of Ion Selective Electrodes. IUPAC Recommendations,ˮ Pure and Applied Chemistry, Vol. 66, No. 12, 1994, pp. 227-236.
- G. G. Guilbault, R. A. Drust, M. S. Frant, H. Freiser, E. H. Hansehen, T. S. Light, E. Pungor, G. A. Rechnitz, N. M. Rice, T. J. Rohm, W. Simon and J. D. R. Thomas, “Picoliter Cell Volume Potentiometric Detector for Open-Tubular Column LC,” Pure and Applied Chemistry, Vol. 48, No. 29, 1976, pp. 127-132.
- Y. Umezawa, P. Buhlmann, K. Umezawa, K. Tohda and S. Amemiya, “Potentiometric Selectivity Coefficient of Ion Selective Electrodes, Part one, Inorganic Cations,ˮ Pure and Applied Chemistry, Vol. 72, No. 10, 2000, pp. 1851-2082.
- J. C. Miller and J. N. Miller, “Statistics in Analytical Chemistry,ˮ Ellis Horwood Chichester, England, 1988.
- British Pharmacopeia, “Medicinal and Pharmaceutical Substances,” Vol. 1, Stationary Office Ltd., Cambridge, 2002.
NOTES
*Corresponding author.

