American Journal of Analytical Chemistry
Vol.4 No.4(2013), Article ID:30460,5 pages DOI:10.4236/ajac.2013.44026
Determination of 17α-Methyltestosterone in Freshwater Samples of Tilapia Farming by High Performance Liquid Chromatography
1Centre for Pharmaceutical Studies, Faculty of Pharmacy, University of Coimbra, Coimbra, Portugal
2Faculty of Pharmacy, University of Coimbra, Coimbra, Portugal
3Department of Biology & CESAM, University of Aveiro, Aveiro, Portugal
Email: ibarbosa@ff.uc.pt
Copyright © 2013 Isabel R. Barbosa et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 1, 2013; revised March 29, 2013; accepted April 19, 2013
Keywords: 17α-Methyltestosterone; High Performance Liquid Chromatography; Environment; Tilapia Farming
ABSTRACT
17α-methyltestosterone is used to induce the sex reversal of Tilapia sp. to obtain cultures mono-sex to an economically viable. This practice may lead to environmental contamination and problems in human health. Therefore methods need to be developed to detect residues of 17α-methyltestosterone in aqueous matrices. A simple high-performance liquid chromatographic method using ultraviolet detection (245 nm) and testosterone as internal standard has been developed for the monitoring 17α-methyltestosterone in freshwater samples of tilapia aquaculture. The method described involves limited sample preparation as it includes a filtration followed by a single solid-phase extraction step using C18 cartridge. Validation data indicated that the HPLC-UV method for 17α-methyltestosterone determination in the concentration range of 50 - 2000 μg/L provided good linearity, sensitivity, accuracy and precision. Method performance was efficiently applied to monitoring the freshwater samples of fish ponds and the surrounding aquatic channels.
1. Introduction
One of the most cultivated fishes of freshwater in many developing countries is the Nile tilapia (Oreochromis niloticus). Oreochromis species are characterised by a precocious sexual maturity and a high reproductive efficacy, resulting in over population in ponds. To overcome these negative aspects, tilapia culture has traditionally involved all-male populations. The most common method for producing monosex populations (all-male) is the sex reversal of larvae with the use of feeds containing synthetic sex hormones for 21 days. 17α-methyltestosterone (MT), 17β- hydroxy-17α-methylandrost-4-ene-3-one (Figure 1), is a synthetic anabolic androgenic steroid, commonly used in newly hatched tilapia fry for sex reversal at a concentration of 60 mg per one kilogram of feed [1-3].
Although it was shown that the use of hormone does not result in the accumulation of residues in tissues of fish treated [4,5] there are still concerns about their release into the environment and the reaction of consumers.
Since it is very common to overfeed the fry, residual MT

Figure 1. Molecular structure of 17α-methyltestosterone (MT, I) and internal standard (testosterone, T, II).
in the MT-impregnated food from the masculinizing process may accumulate in the ponds and be released into the receiving water body when the pond water is released or when the ponds are cleaned. The residual MT from uneaten and unmetabolized MT-impregnated food remains in the ponds and, if released, it can contaminate the environment around the discharge points. MT is an endocrine disrupter at part per billion levels [6,7] and can interfere with normal functions of the reproductive systems of humans and animals.
Several high performance liquid chromatography (HPLC) and liquid chromatography-mass spectrometry (LC-MS) methods have been reported for the analysis of MT in various biological matrices and pharmaceutical preparations [8-17]. To our knowledge, the analysis of MT in aqueous matrices has not been performed. The purpose of this work was to develop a simple, effective and sensitive method for determination of MT residues in aqueous matrices. The work presented here used HPLC with UV detector for the determination of MT following a solid-phase extraction (SPE). This method was validated and used to monitor MT residues in water samples of tilapias farming.
2. Experimental
2.1. Chemicals and Materials
All reagents used during the extraction and analysis were analytical reagent grade. 17α-methyltestosterone (17β- Hydroxy-17α-methyl-4-androsten-3-one, CAS n: 58-18-4, purity 97.7%) and internal standard, testosterone (17α- hydroxyandrost-4-en-3-one, CAS no: 58-22-0, purity 99%), were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, acetonitrile and ethanol were all HPLCgrade and purchased from Merck (Darmstadt, Germany). Ultrapure water was obtained in the laboratory by MilliQ® system (Millipore Corporation, Milford, MA, USA). The cartridges used for, SPE were Discovery® DSC-18 μm, 6 mL, 500 mg, (Supelco, USA).
2.2. Apparatus and Chromatographic Conditions
The method was developed on a Shimadzu HPLC system LC-2010C HT with quaternary pumps, autosampler, column oven, UV detector, degasser, and controlled by Shimadzu LC Solutions® software. The chromatographic separation was achieved with a C18 reverse-phase (RP-C18) column (ACE, 5 μm particle size, 250 × 4.6 mm) at 25˚C. All injections were performed automatically using 20 μl loop on autosampler. Detection of analyte was carried out using UV detector at 245 nm. The analyte were separated by running a mobile phase consisted of ace-tonitrile and ultrapure water (45:55, v/v) at a flow rate of 1 mL·min−1.
2.3. Method and Sample Preparation
2.3.1 Sample Collection and Preparation
A total of twenty-six surface water samples were collected in 200 mL PET bottles at different fish ponds and the surrounding aquatic channels. The water samples were frozen at −20˚C. The freshwater samples of Tilapia farming were previously defrosted, spiked appropriate amounts of with internal standard (testosterone, IS), filtered through 0.2 μm membrane filter (Schleicher & Schuell) and then extracted by solid phase extraction (SPE).
2.3.2. Standard Solutions
Individual stock standard solutions (100 mg/L) were prepared in methanol. The working standard solutions of 17α- methyltestosterone were prepared through appropriated dilutions to obtain concentrations between 50 - 2000 μg/L. The working standard solutions of testosterone were prepared to have the concentration of the 500 μg/L. The stock standard solutions were stored at −20˚C and the working standard solutions were stored at 4˚C for 6 months.
2.3.3. Extraction and Clean-Up
The cartridge was preconditioned sequentially with 5 mL of acetonitrile followed by same volume of Milli-Q water. The water samples were loaded on the preconditioned cartridge under vacuum. The washing step was performed with Milli-Q water. Then the cartridge was eluted with 5 mL of ethanol at a flow rate of 2 mL/min. The eluate was evaporated to dryness at 37˚C - 40˚C and the residue was redissolved in 200 μL of acetonitrile. The volume of eluate injected was 20 μL.
3. Results and Discussion
3.1. Optimization of HPLC Conditions
Prior to the analytical determination of 17α-methyltestosterone with reversed phase HPLC/UV-Vis method the UV region was scanned to obtain a shared absorption wavelength for 17α-methyltestosterone and testosterone as IS. As a result, 245 nm was selected as the optimum wavelength for simultaneous determination of these compounds. Feasibility of different solvent systems such as acetonitrile—water and methanol—water mixtures in different compositions, pumped at different flow rates (in the range of 0.5 - 1.5 ml/min) at different column oven temperatures (in the range of 25˚C - 35˚C) were evaluated. Best results were obtained using acetonitrile-water in the ratio of 45:55, v/v at a flow rate of 1 mL·min-1 with a column oven temperature at 25˚C on the analysis of both steroids. The chromatogram in the Figure 2(a) shows the separation of analytes obtained by the proposed method.
3.2. Optimization of Extraction and Clean-Up
The clean-up efficiencies for Discovery® DSC-18 μm, 6 mL/500 mg cartridges were studies by adjusting the following parameters: the solvents used in the washing steps, the eluent solvents, and the volumes for eluting 17α- methyltestosterone from the cartridge. The polymeric
 (a)
(a)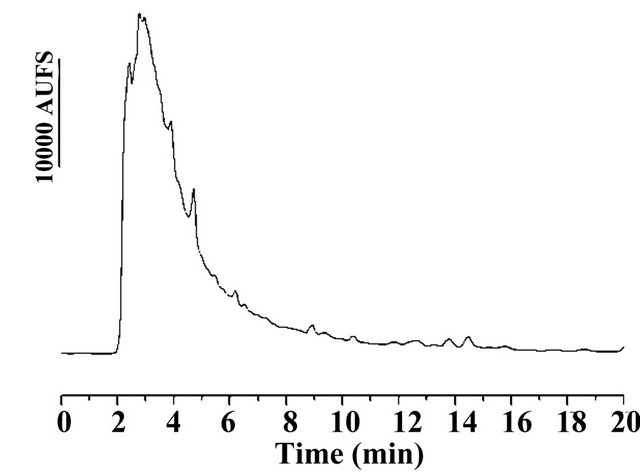 (b)
(b) (c)
(c) (d)
(d)
Figure 2. (a) (b) (c) (d) Chromatogram of (a) Standard solution with MT and T (IS) with 600 µg/L respectively; (b) Water blank sample; (c) Spiked water blank with 100 µg/L of MT and PI; (d) Freshwater sample of tilapia aquaculture containing 617.4 µg/L of MT.
reversed-phase sorbent showed no effect in the analyte. The cartridges were washed with 5 ml of Milli-Q water to obtain clean blank chromatograms without interferences. The elution of 17α-methyltestosterone was study with different solvents and volumes. Thus our results show that 5 mL of ethanol is necessary and sufficient to elute all 17α-methyltestosterone from cartridge. This method was therefore applied to monitoring 17α-methyltestosterone in freshwater samples of fish ponds and the surrounding aquatic channels.
3.3. Method Validation
In the validation procedure of the analytical method, the following criteria were considered: sensitivity; linearity; recovery; precision and evaluation of the matrix effect.
3.3.1. Linearity
The calibration curves were prepared using linear regression analysis and gave good fits over the range 50 - 2000 μg/L. The mean regression coefficients (R2) were 0.999 for 17α-methyltestosterone.
3.3.2. Stability Studies
The stability of standard solutions and sample extracts was evaluated. The stock standard solutions were stored at −20˚C and analysed during one-month period, and the working standard solutions stored at 4˚C were analysed during a one-week period. We did not observe any degradation of 17α-methyltestosterone and testosterone in this study period. The stability of 17α-methyltestosterone during sample storage at −20˚C was tested during one week, and no degradation was observed.
3.3.2. Limit of Quantification and Accuracy
The limit the quantification, calculated according to the lower concentration that provides repeatabilities lower than 20%, was 58.3 μg/L for 17α-methyltestosterone. The limit of detection it was 19 μg/L at a signal-to-noise of 3:1.
In order to verify the absence of potential interfering substances around the retention time of 17α-methyltestosterone and testosterone, water blank samples (n = 5) were analysed in order to assess the specificity of the method. No interferences were observed in the region of interest where the 17α-methyltestosterone and testosterone were eluted (Figure 2(b)).
The results demonstrate that real sample matrices had no effect on the performance of the proposed method, which is therefore suitable for analysis of trace levels of 17α-methyltestosterone in surface water of fish ponds and the surrounding aquatic channels.
The accuracy of the method was studied by spiking water samples at three fortification levels (100, 600 and 2000 μg/L). Figure 2(c) represents the chromatogram obtained for fortification assay.
Within-day accuracy and precision data were determined by analysing, on the same day, three replicates of spiked samples at three levels, and one blank (to check the interferences). The between-day accuracy and precision were also determined by extracting batches of three fortification levels and analysing them on the five consecutive days. Accuracy and intra-day and inter-day precision are shown in Table 1. Recoveries for the lower fortification level were generally greater than 100% for 17α-methyltestosterone. For the three fortification levels, the relative standard deviation for all fortification levels on each day for 17α-methyltestosterone was less than 12%, demonstrating good method precision.
Table 1. Recovery, intra-day and inter-day precision and accuracy (n = 5).

3.4. Application of the Proposed Method
A total of twenty-six samples of water were analysed under the conditions described, and 17α-methyltestosterone were detected in six samples at level higher than the LOQ in the concentration of 59.9 > 2000 μg/L. Figure 2(d) represents the chromatogram obtained with one of water sample fish ponds analysed.
4. Conclusion
The monitoring of 17α-methyltestosterone residues is an important issue in order to avoid possible residues in water systems release into the environment from Tilapia farming. In the present study, we developed a simple HPLC method to identify and quantify trace levels of release into the environment in real environmental surface water samples from one of Tilapia farming of Thailand. The use of a RP-column with UV detection allows the rapid and sensitive analysis required for this type of analysis. The proposed HPLC method was proved to be convenient and effective for monitoring the waters in the fish ponds (MT unmetabolized and surplus food with MT) and the waters at different points of discharge in the farming.
REFERENCES
- W. L. Gale, M. S. Fitzpatrick, M. Lucero, W. M. Contreras-Sanchez and C. B. Schreck, “Masculinization of Nile Tilapia (Oreochromis niloticus) by Immersion in Androgens Original,” Aquaculture, Vol. 178, No. 3-4, 1999, pp. 349-357. doi:10.1016/S0044-8486(99)00136-2
- J. A. Beardmore, G. C. Mair and R. I. Lewis, “Monosex Male Production in Finfish as Exemplified by Tilapia: Applications, Problems, and Prospects,” Aquaculture, Vol. 197, No. 1-4, 2001, pp. 283-301. doi:10.1016/S0044-8486(01)00590-7
- T. J. Pandian and S. G. Sheela, “Hormonal Induction of Sex Reversal in Fish,” Aquaculture, Vol. 138, No. 1-4, 1995, pp. 1-22. doi:10.1016/0044-8486(95)01075-0
- J. S. Abucay and G. C. Mair, “Hormonal Sex Reversal of Tilapias: Implications of Hormone Treatment Application in Closed Water Systems,” Aquaculture Research, Vol. 28, No. 11, 1997, pp. 841-845. doi:10.1111/j.1365-2109.1997.tb01008.x
- L. R. Curtis, F. T. Diren, M. D. Hurley, W. K. Seim and R. A. Tubb, “Disposition and Elimination of 17α-Methyltestosterone in Nile Tilapia (Oreochromis niloticus),” Aquaculture, Vol. 99, No. 1-2, 1991, pp. 193-201. doi:10.1016/0044-8486(91)90298-L
- L. Andersen, R. Goto-Kazeto, J. M. Trant, J. P. Nash, B. Korsgaard and P. Bjerregaard, “Short-Term Exposure to Low Concentrations of the Synthetic Androgen Methyltestosterone Affects Vitellogenin and Steroid Levels in Adult Male Zebrafish (Danio rerio),” Aquatic Toxicology, Vol. 76, No. 3-4, 2006, pp. 343-352. doi:10.1016/j.aquatox.2005.10.008
- S. Wason, G. Pohlmeyer-Esch, C. Pallen, X. Palazzi, G. Espuña and R. Bars, “17α-Methyltestosterone: 28-Day Oral Toxicity Study in the Rat Based in the ‘Enhanced OECD Test Guideline 407’ to Detect Endocrine Effects,” Toxicology, Vol. 192, No. 2-3, 2003, pp. 119-137. doi:10.1016/S0300-483X(03)00265-8
- D. Teichert-Coddington, B. Manning, J. C. Eya and D. Brock, “Concentration of 17µ-Mehtyltestosterone in Hormone-Treated Feed: Effects of Analytical Technique, Fabrication, and Storage Temperature,” Journal of the World Aquaculture Society, Vol. 31, No. 1, 2000, pp. 42-50. doi:10.1111/j.1749-7345.2000.tb00696.x
- W. Van Thuyne and F. T. Delbeke, “Validation of a GCMS Screening Method for Anabolizing Agents in Aqueous Nutritional Supplements,” Journal of Chromatography Science, Vol. 43, No. 1, 2005, pp. 2-6.
- C. A. Goudie, “Extraction of a Synthetic Androgen from Fish Muscle and Quantitation by High Performance Liquid Chromatography,” Steroid, Vol. 44, No. 3, 1984, pp. 241-252. doi:10.1016/0039-128X(84)90005-9
- E. A. I. Daeseleire, A. De Guesquière and C. H. Van Peteghem, “Multiresidue Analysis of Anabolic Agents in Muscle Tissues and Urines of Cattle by GC-MS,” Journal of Chromatography Science, Vol. 30, No. 10, 1992, pp. 409- 414.
- B. L. Lampert and J. T. Stewart, “Determination of Anabolic Steroids and Zeranol in Human Serum by Isocratic Reverse Phase HPLC on Silica,” Journal of Liquid Chromatography, Vol. 12, No. 16, 1989, pp. 3231-3249.
- M. H. Blokland, H. J. van Rossum, H. A. Herbold, S. S. Sterk, R. W. Stephany and L. A. Van Ginkel, “Metabolism of Methyltestosterone, Norethandrolone and Methylboldenone in a Heifer,” Analytica Chimica Acta, Vol. 529, No. 1-2, 2005, pp. 317-323. doi:10.1016/j.aca.2004.10.064
- P. Regal, C. Nebot, B. I. Vásquez, A. Cepeda and C. A. Fente, “Determination of the Hormonal Growth Promoter 17α-Methyltestosterone in Food-Producing Animals: Bovine Hair Analysis by HPLC-MS/MS,” Meat Science, Vol. 84, No. 1, 2010, pp. 196-201. doi:10.1016/j.meatsci.2009.08.047
- R. Chiba and Y. Ishii, “Simultaneous Determination of Yohimbine Hydrochloride, Strychnine Nitrate and Methyltestosterone by Ion-Pair High-Performance Liquid Chromatography,” Journal of Chromatography A, Vol. 588, No. 1-2, 1991, pp. 344-347. doi:10.1016/0021-9673(91)85044-G
- A. Cappiello, G. Famiglini, F. Mangani, P. Palma and A. Siviero, “Nano-High-Performance Liquid Chromatography-Electron Ionization Mass Spectrometry Approach for Environmental Analysis,” Analytica Chimica Acta, Vol. 493, No. 2, 2003, pp. 125-136. doi:10.1016/S0003-2670(03)00868-7
- A. Marwah, P. Marwah and H. Lardy, “Development and Validation of a High Performance Liquid Chromatography Assay for 17α-Methyltestosterone in Fish Feed,” Journal of Chromatography B, Vol. 824, No. 1-2, 2005, pp. 107-115. doi:10.1016/j.jchromb.2005.07.005

