American Journal of Analytical Chemistry
Vol.3 No.11(2012), Article ID:24928,6 pages DOI:10.4236/ajac.2012.311100
Flow-Injection Flame Atomic Absorption Determination of Hexavalent Chromium with On-Line Preconcentration on an Anion Imprinted Polymer
1Department of Analytical Chemistry, Nutrition and Bromatology, Faculty of Chemistry, University of Santiago de Compostela, Santiago de Compostela, Spain
2Department of Analytical Chemistry, Escuela Universitaria Politécnica, University of A Coruña, A Coruña, Spain
3Technological Institute for the Monitoring of the Maritime Environment of Galicia (INTECMAR), Vilagarcía de Arousa, Spain
Email: *mcarmen.yebra@usc.es
Received September 11, 2012; revised October 14, 2012; accepted October 25, 2012
Keywords: Hexavalent Chromium; Flame Atomic Absorption Spectrometry; Preconcentration; Flow Injection; Environmental Samples
ABSTRACT
A flow injection preconcentration system for the flame atomic absorption spectrometric determination of hexavalent chromium has been developed. The method employs on-line preconcentration of Cr(VI) on a minicolumn packed with Cr(VI)-imprinted poly(4-vinyl pyridine-co-2-hydroxyethyl methacrylate) placed into a flow injection system. Hexavalent chromium was eluted with a small volume of diluted hydrochloric acid into the nebulizer-burner system of a flame atomic absorption spectrometer. An enrichment factor of 550 and a 3σ detection limit of 0.04 µg×L−1 along a sampling frequency of 4 h−1 at a sample flow rate of 3.5 mL×min–1. The relative standard deviation is 2.9% for 1 µg×L−1 Cr(VI) (n = 11). The flow injection system proposed has the advantage of being simpler because the use of expensive and sophisticated instruments is avoided. Ease of use, continuous process and selectivity make this method suitable for Cr(VI) determination in different environmental samples such as sea and river waters, soils and sediments.
1. Introduction
Chromium exists in small quantities throughout the environment. Chromite ore (FeCr2O4) is the most important commercial ore and is usually associated with ultramafic and serpentine rocks. Chromium has two predominant oxidation states in the environment: Cr(III) is an essential nutrient in low doses and is a known essential element which potentiates the action of insulin in peripheral tissue, while Cr(VI) is highly toxic and carcinogenic. Hexavalent chromium compounds are widely used in the chemical industry for different applications, such as manufacture of pigments, in metal finishing and chrome plating, in stainless steel production, in hide tanning, as corrosion inhibitors, and wood preservation. As a result, Cr(VI) is released into the environment and can be available to human beings [1-3].
Flame atomic absorption spectrometry (FAAS) is a widely used technique that offers many advantages. However, there are analytical situations in which flame methodology is limited in sensitivity and selectivity [4]. Therefore, in order to increase its sensitivity, achieve the separation of both chromium species and isolate the analyte from interfering matrix constituents were proposed several methodologies including solid phase extraction methods involving anion exchange resins [5,6], adsorption of Cr(VI) complexes with dithiocarbamate, or diphenylcarbazide on solids sorbents as macroporous polystyrene-divinylbenzene, Amberlite XDA, C18 bonded silica or activated alumina and chelating resins to determine by difference between total Cr and Cr(III) the Cr(VI) concentration [7-14]. Nevertheless, these methods are tedious and subject to potential interferences because require preliminary complexation with a chelating reagent or involve reduction of Cr(VI) to Cr(III). By the other hand, other kind of methods separate Cr(III) and Cr(VI) species in elution, which require a controlled elution step to avoid interconversion of both oxidation states of chromium. More recently, molecular imprinted (MIPs) or ion-imprinted polymers (IIPs) have been recognized as suitable materials and they are increasingly used in trace analysis because they are suitable for applications where analyte selectivity is essential. Between IIPs, mainly were synthesized cationic materials to be selective to trace metals [15-17]. Nevertheless, only few anions imprinted polymers (AIPs) were proposed to the selective separation of anions. These are high selective for recognizing chromate [18,19] and phosphate anions [20,21]. However, these AIPs were not applied for analytical purposes.
Manual separation procedures are labor, time, and reagent-intensive, and require large volumes of sample. Flow injection (FI) on-line preconcentration systems coupled with FAAS have demonstrated to be effective in terms of enhanced sensitivity, efficient removal of the matrix, high sample throughput, minimal sample and reagents consumption, easy operation and high precision [22].
In the present work, a cheap, easy and sensitive on-line FI-FAAS method for the determination of trace amounts of hexavalent chromium is described. An AIP (Cr(VI)- imprinted poly(4-vinylpyrdine-co-2-hydroxy-ethyl methacrylate) (poly(4-VP-HEMA) synthesized as proposed Bayramoglu et al. [19] was used for the separation and preconcentration of Cr(VI) from different environmental matrices by complex formation between Cr(VI) anions and 4-pyridine groups of the polymer.
2. Experimental
2.1. Instrumentation
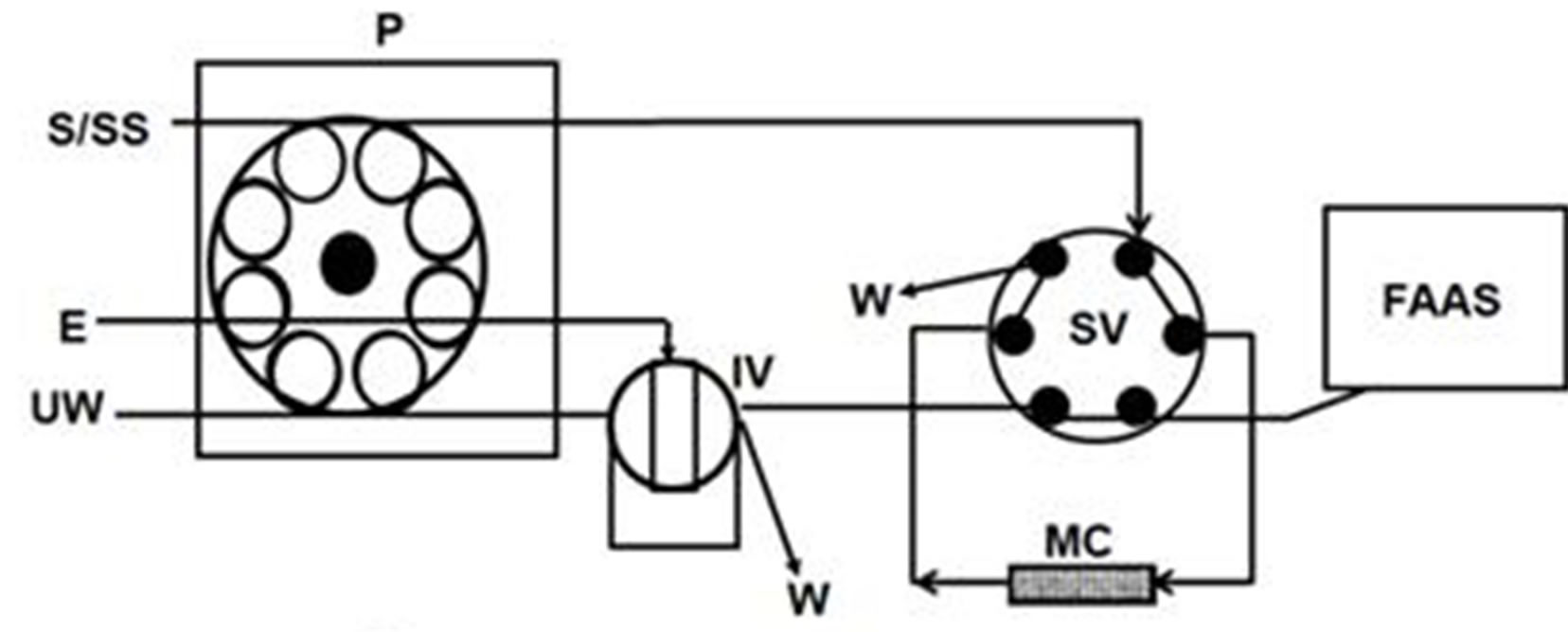
The measures of absorbance were performed with a Perkin-Elmer flame atomic absorption spectrometer Model 5000 (PerkinElmer Life and Analytical Sciences, USA). The hollow cathode lamp used as the radiation source was operated at a wavelength of 357.9 nm. The flow of acetylene and air was 4.5 and 15.0 L×min−1, respectively. The nebulizer flow rate was 3.0 mL×min−1. The on-line system used for preconcentration of Cr(VI) is represented by the Figure 1. It consists of a Gilson Minipuls-3 peristaltic pump (Gilson, France), two Rheodyne low-pressure injection valves model 5020 (Rheodyne, USA). A laboratory-made minicolumn (4.0 cm length and internal diameter of 1.8 mm) containing 30 mg of the AIP was inserted in the on-line system. A polyvinylchloride (PVC) tube was used in the construction of the minicolumn, which was sealed on each end with a small glass wool bed to prevent material losses. Numerical analyses of experimental designs were performed by means of the statistical package Statgraphics Plus 5.1 (Manugistic, Inc., Rockville, MD, USA).
2.2. Reagents
All reagents were of analytical grade, unless otherwise stated. Ultrapure water with a resistivity of 18.2 MΩ cm–1 was obtained with a Milli-Q™ purification device (Millipore Corporation, Bedford, MA, USA). Cr(VI) anion imprinted polymer was prepared as described
 (a)
(a)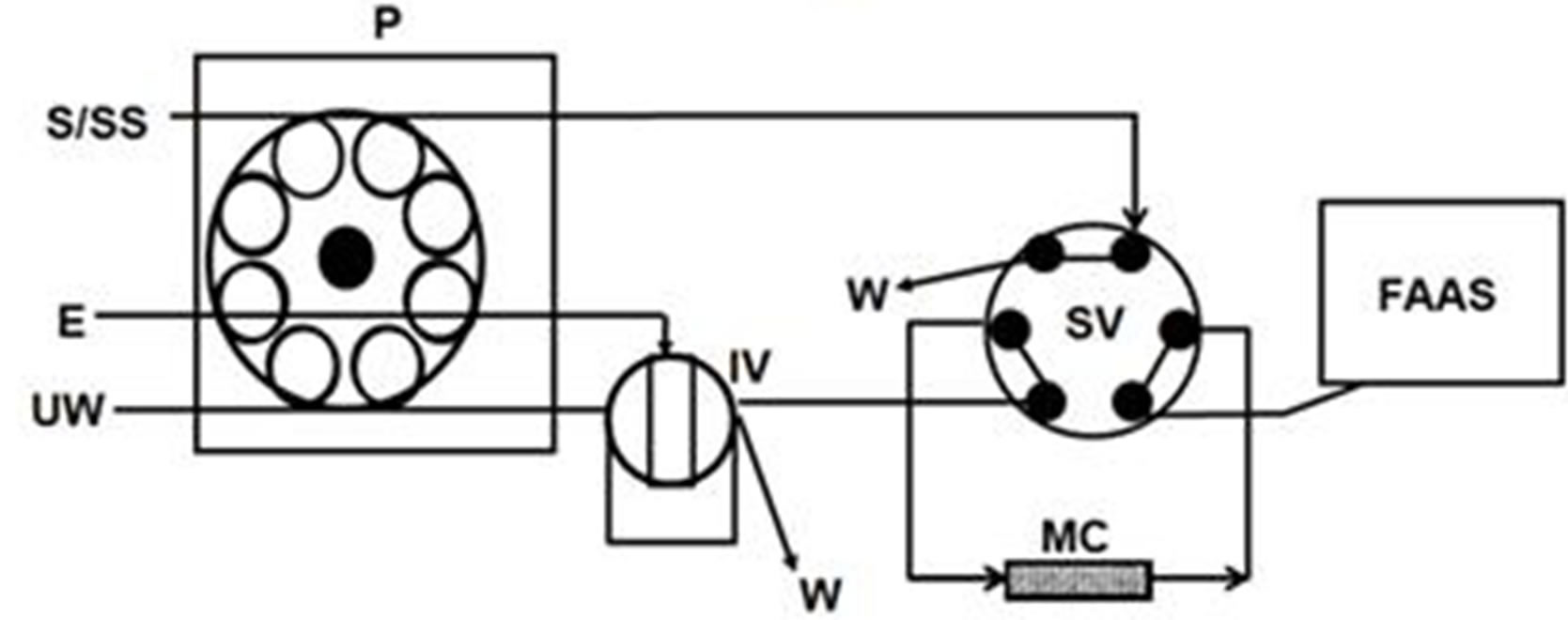 (b)
(b)
Figure 1. Schematic diagram of FI-manifold showing the two operational sequences for Cr(VI) determination by FAAS. (a) Preconcentration step; (b) Elution step. E: eluent; FAAS: flame atomic absorption spectrophotometer; IV: injection valve; MC: minicolumn; P: peristaltic pump; S/SS: sample/standard solution; SV: switching valve; UW: ultrapure water; W: waste.
elsewhere [19] using 4-vinyl pyridine as a complexing monomer and copolymerized with 2-hydroxyethyl methacrylate (co-monomer) and ethyleneglycol dimethacrylate (cross-linker) with a ratio 0.3:1.7:1.0 (v/v/v). Hydrochloric acid was of Suprapur quality (Merck, Darmstadt, Germany). Standard solutions of Cr(VI) were made by dilution of a 1000 mg×L–1 stock solution made by dissolving 2.8290 g of dried potassium dichromate (Merck, Darmstadt, Germany) in 1000 mL of ultrapure water.
2.3. Environmental Water Collection
All water samples were collected from beaches and coasts (Atlantic Ocean and Cantabrian Sea) and from rivers of Galicia (north-west Spain) in pre-cleaned high density polyethylene bottles. The samples were filtered through a Millipore cellulose membrane (0.45 mm) and were analyzed immediately after collection.
2.4. Soil and Sediment Samples Collection and Preparation
Soil and sediment samples were collected in pre-cleaned hermetic plastic containers. Surface marine sediment samples were from coasts (Atlantic Ocean and Cantabrian Sea) of Galicia (north-west Spain). Soil samples were collected from industrial, agricultural and forest areas located in Galicia (north-west Spain). The samples were dried in an oven at 60˚C and homogenized with a sieve and completely homogenized in order to avoid unrepresentative sampling. Sample preparation used was EPA Method 3060A alkaline extraction procedure [23]. Thus, samples (2.5 g) were digested using 50 mL of an extractant solution containing 0.28 mol×L–1 Na2CO3 and 0.5 mol×L–1 NaOH and heating at 90˚C - 95˚C for 60 minutes to dissolve the Cr(VI) and stabilize it against reduction to Cr(III).The cooled extract was then filtered through a 0.45 mm membrane filter. Samples were digested within 30 days of sample collection and the digestate was analyzed within seven days of preparation.
2.5. Procedure for the FI Determination of Cr(VI)
As shown in Figure 1, the preconcentration cycle runs through two steps. Step 1: Selective retention of Cr(VI). With the injection valve (IV) in the load position (Figure 1(a)), the sample (25 mL adjusted to pH 4 with diluted hydrochloric acid) was loaded into the minicolumn which was assembled to the FI manifold. The sample was passed through the minicolumn at a flow rate of 3.5 mL×min−1. Step 2: Elution and transportation of the eluate toward the detector. The selecting valve (SV) was switched to its other position and with the IV in the inject position (Figure 1b), 90 µL of eluent (2 mol×L–1 hydrochloric acid) were injected into an ultrapure water carrier stream which flowed at 3.0 mL×min–1 through the minicolum in opposite direction to the sample stream. Thus, the eluate was transported to the detector where hexavalent chromium was continuously monitored.
3. Results and Discussion
3.1. Optimization of Experimental Parameters
To optimize the FI system for Cr(VI) determination, the main efforts were focused on the conditions for sample loading and Cr(VI) elution from the minicolumn, as well as the analytical FI-FAAS system which was coupled on-line with the preconcentration/separation unit in order to obtain highly sensitive, accurate and reproducible results. For the optimization of the FI preconcentration procedure, six variables were considered; namely, sample flow-rate, sample pH, minicolumn diameter, eluent (hydrochloric acid solution) concentration, eluent volume and elution flow-rate. The response variable was the percentage of Cr(VI) recovery. Optimization experiments were performed on standard solutions of 25 mL containing known concentrations of Cr(VI) (5 µg×L–1). A Plackett-Burman design 2^6*3/6 type III resolution allowing six degrees of freedom and involving 12 randomized runs plus one centre points was built for a screening study of the behavior of the six variables potentially affecting the preconcentration process. The upper and lower values given to each factor were selected from the available data and experience gathered in the preliminary experiments. Both the tested and the optimum values obtained for each variable are shown in Table 1(a). Nu-
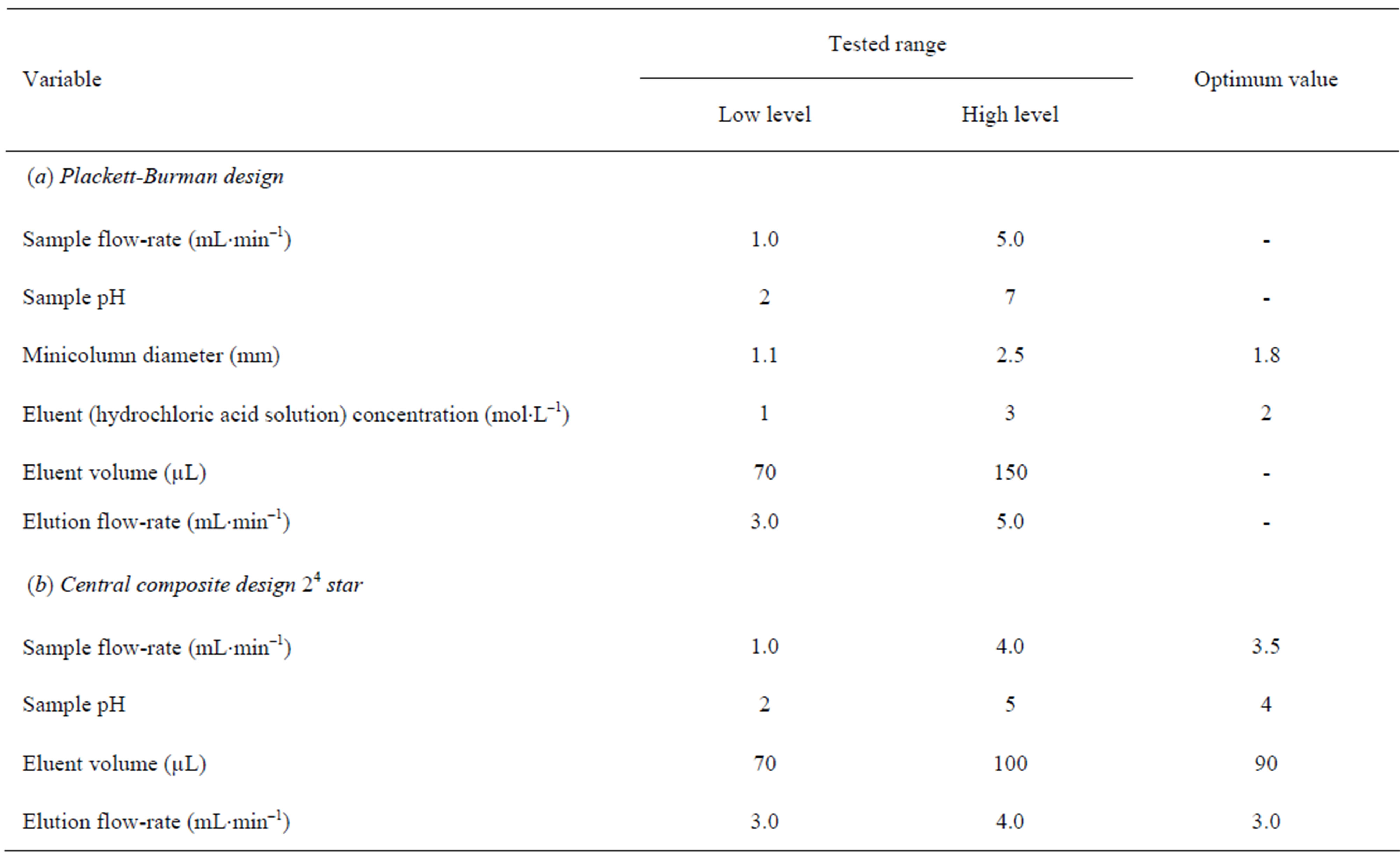
Table 1. Factor levels in the (a) Plackett-Burman design 26*3/6; (b) Central composite design 24 star.
merical analysis of the results produced the standardized main effects Pareto Chart (Figure 2). The conclusions of this screening study were that sample pH and eluent volume were statistically influential factors with negative and positive sign, respectively at 95% confidence level. Although sample and elution flow-rates are non significant variables within the ranges under study, they have a high negatitive estimated effect, and its optimum value was obtained by a surface response design. Minicolumn diameter and eluent concentration, were also not statistically influential factors at 95% confidence level within the ranges under study but they have estimated effects very low. Thus, intermediate values for these variables, 1.8 mm and 2 mol×L–1 for minicolumn diameter and eluent concentration, respectively, were selected for subsequent experiments.
Significant variables (sample pH and eluent volume) and sample and elution flow-rates were optimized by using an orthogonal central composite design 24 star with two center points, resulting in 26 randomized runs with eleven degrees of freedom. Axial distance (α) was selected having a value of 1.48258 in order to establish the orthogonality condition. Results obtained confirmed that sample pH, eluent volume and elution flow-rate were statistically influential factors at the 95% confidence level in the ranges studied (Table 1(b)). The sample flow-rate and the eluent volume are critical parameters in preconcentration procedures. The sample flow-rate largely determines the residence time of analytes in the minicolumn, but it is also a crucial factor affecting the sample throughput. The eluent volume is intended to be the minimum possible in order to obtain the highest preconcentration factor. As the elution flow-rate was a statistically influential factor with negative sign the lowest value tested (3 mL×min–1) was fixed as optimum. The sample pH has a positive sign, but with an estimated effect very low, so a pH 4 was fixed as optimum. Taking into account these considerations, in order to determine
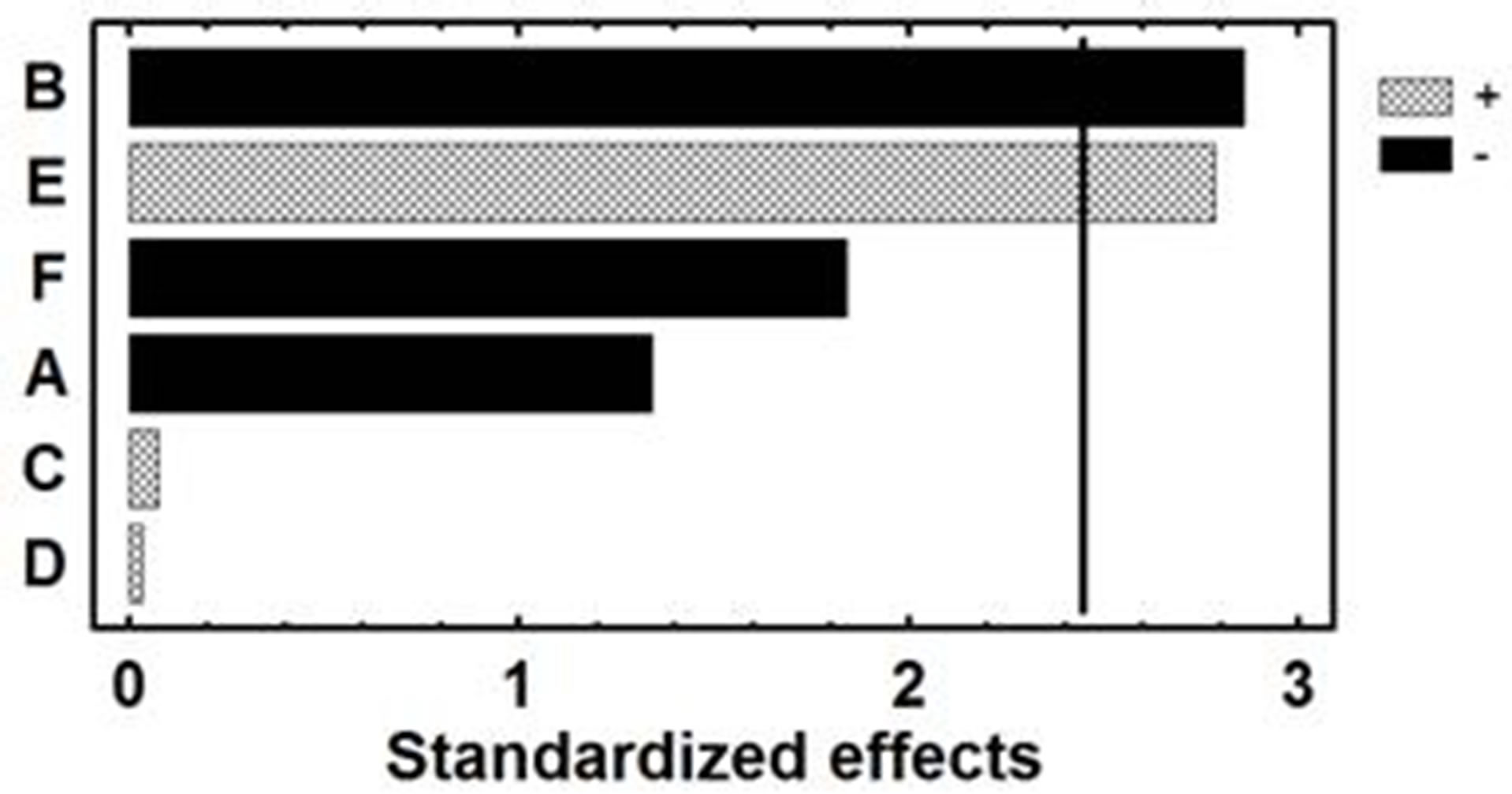
Figure 2. Pareto chart for the Plackett-Burman design applied to optimize the FI preconcentration of Cr(VI). A: sample flow-rate; B: sample pH; C: minicolumn diameter; D: eluent (hydrochloric acid solution) concentration; E: eluent volume; F: elution flow-rate.
the highest sample flow-rate and the lowest eluent volume that could be used obtaining a quantitative recovery, a response surface was obtained (Figure 3). In this figure, can be observed that a flow-rate of 3.5 mL×min–1 and an eluent volume of 90 µL are adequate for quantitative recovery of Cr(VI). Optimum values of all variables are shown in Table 1.
3.2. Performance of the Method
The characteristic performance data of the FI-FAAS system for hexavalent chromium determination are presented in Table 2. The detection and quantification limits were defined as the concentrations of analyte giving signals equivalent to 3 and 10 times, respectively, the standard deviation of the blank plus the net blank signal. The preconcentration factor was determined as the ratio of the slopes of the linear section of the calibration graphs before and after preconcentration, and the sample throughput was calculated as the number of samples analyzed per hour.
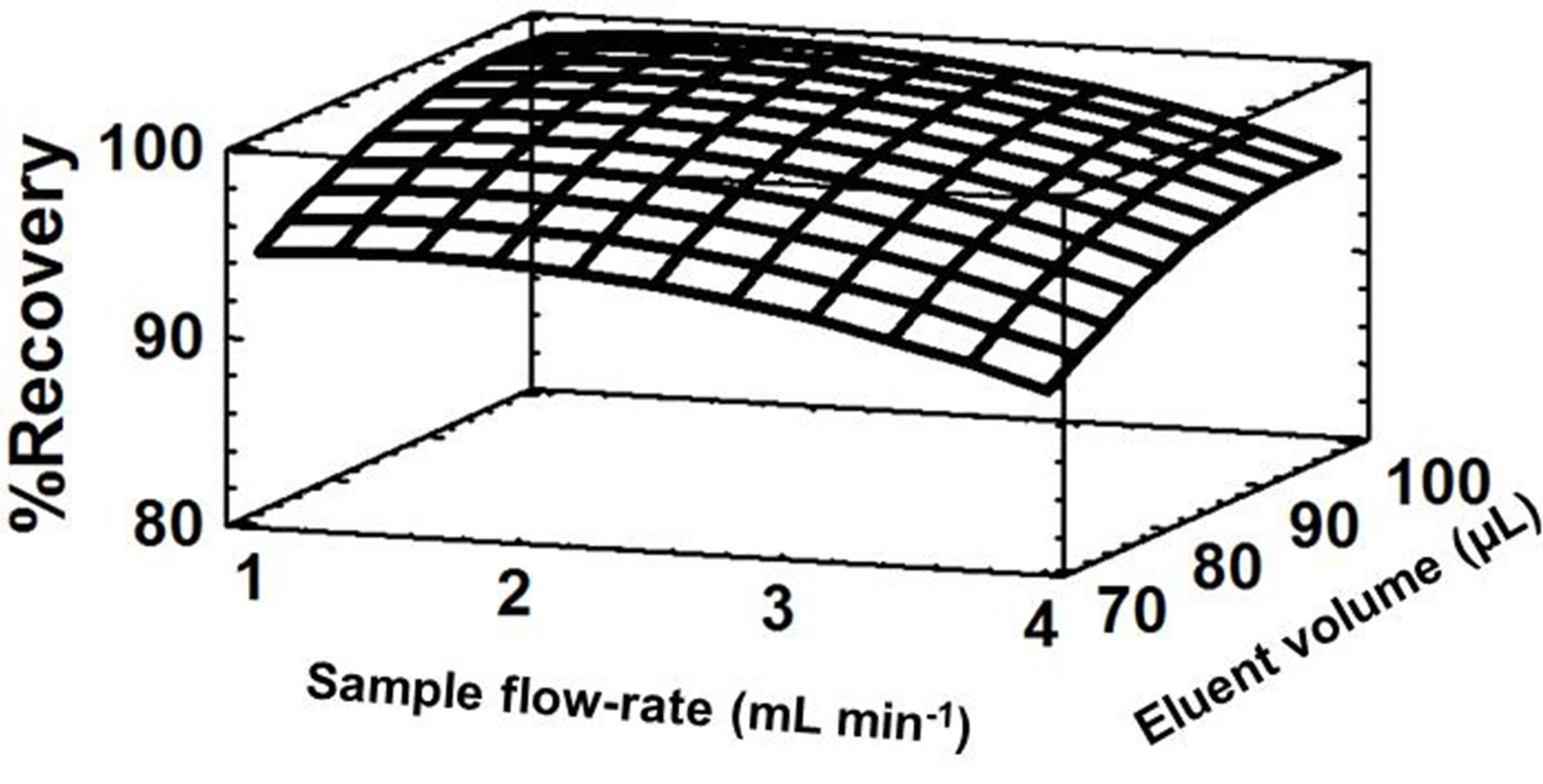
Figure 3. Response surface estimated for the central composite design applied to optimize the FI preconcentration of Cr(VI). Sample pH = 4 and elution flow-rate = 3.0 mL min–1.
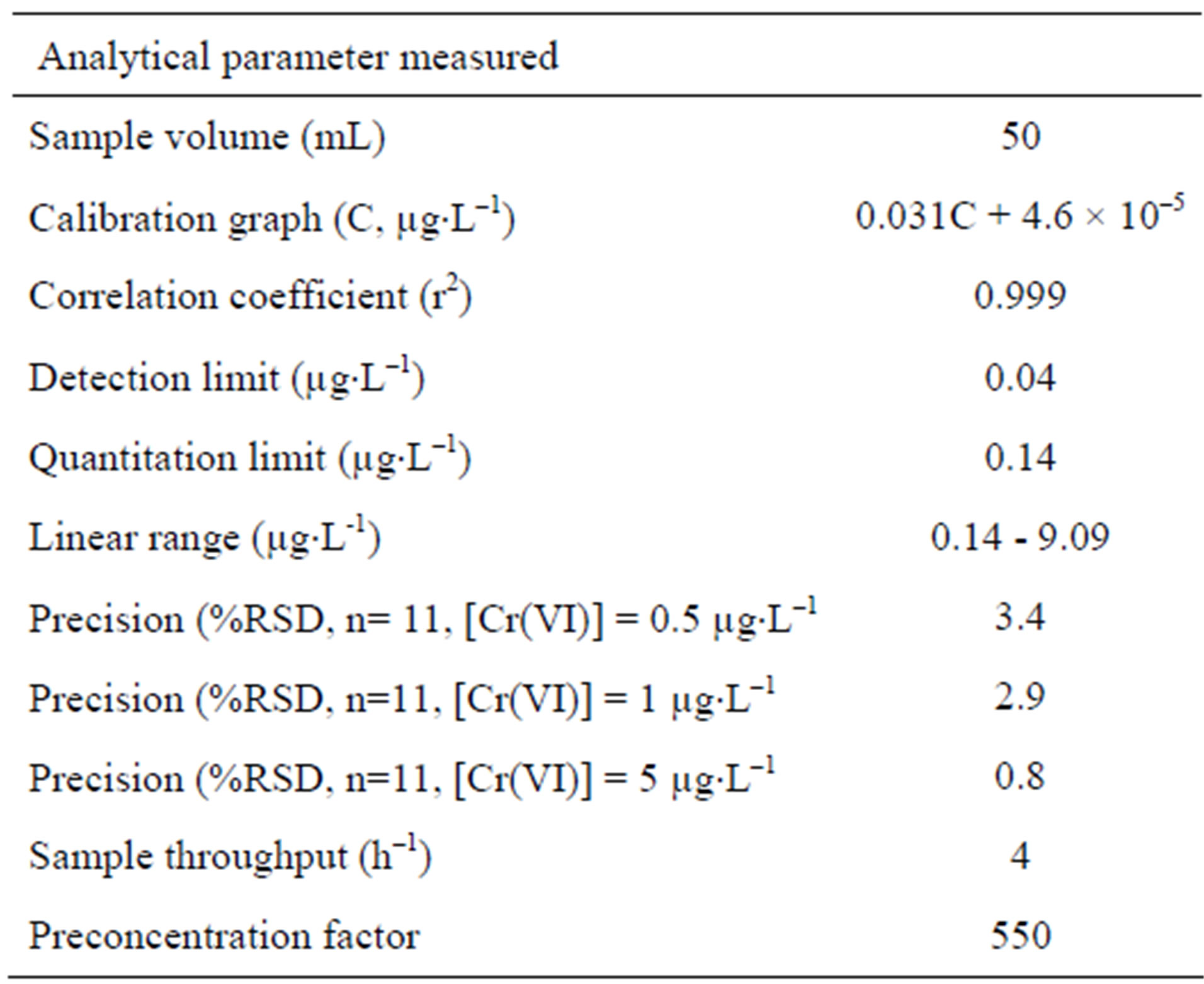
Table 2. Performance of the FI-FAAS system for hexavalent chromium determination under optimized conditions.
3.3. Determination of Cr(VI) in Presence of Cr(III)
In order to assess the selectivity of the proposed procedure for Cr(VI) determination, this was applied to various standard solutions using different concentration relations between the two chromium oxidation states. The results obtained demonstrated that Cr(VI) can be determined quantitatively in the presence of Cr(III).
3.4. Sample Analysis
The methodology for Cr(VI) determination described above was applied to the analysis of sea and river waters, soils and sediments from different origin in Galicia (NW, Spain). Results for liquid samples are shown in Table 3, and results for solid samples are shown in Table 4. It
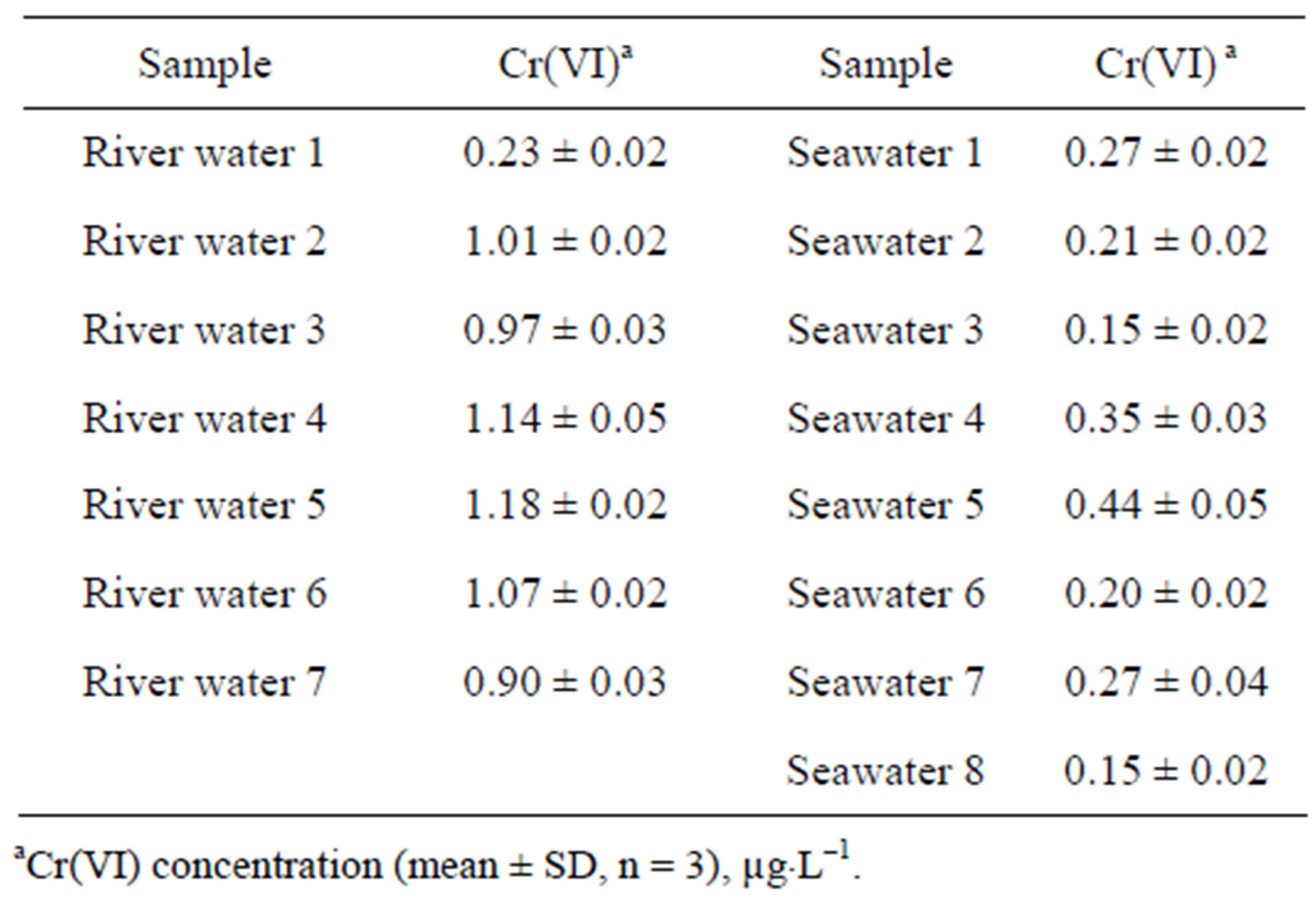
Table 3. Results for hexavalent chromium determination in environmental water samples.
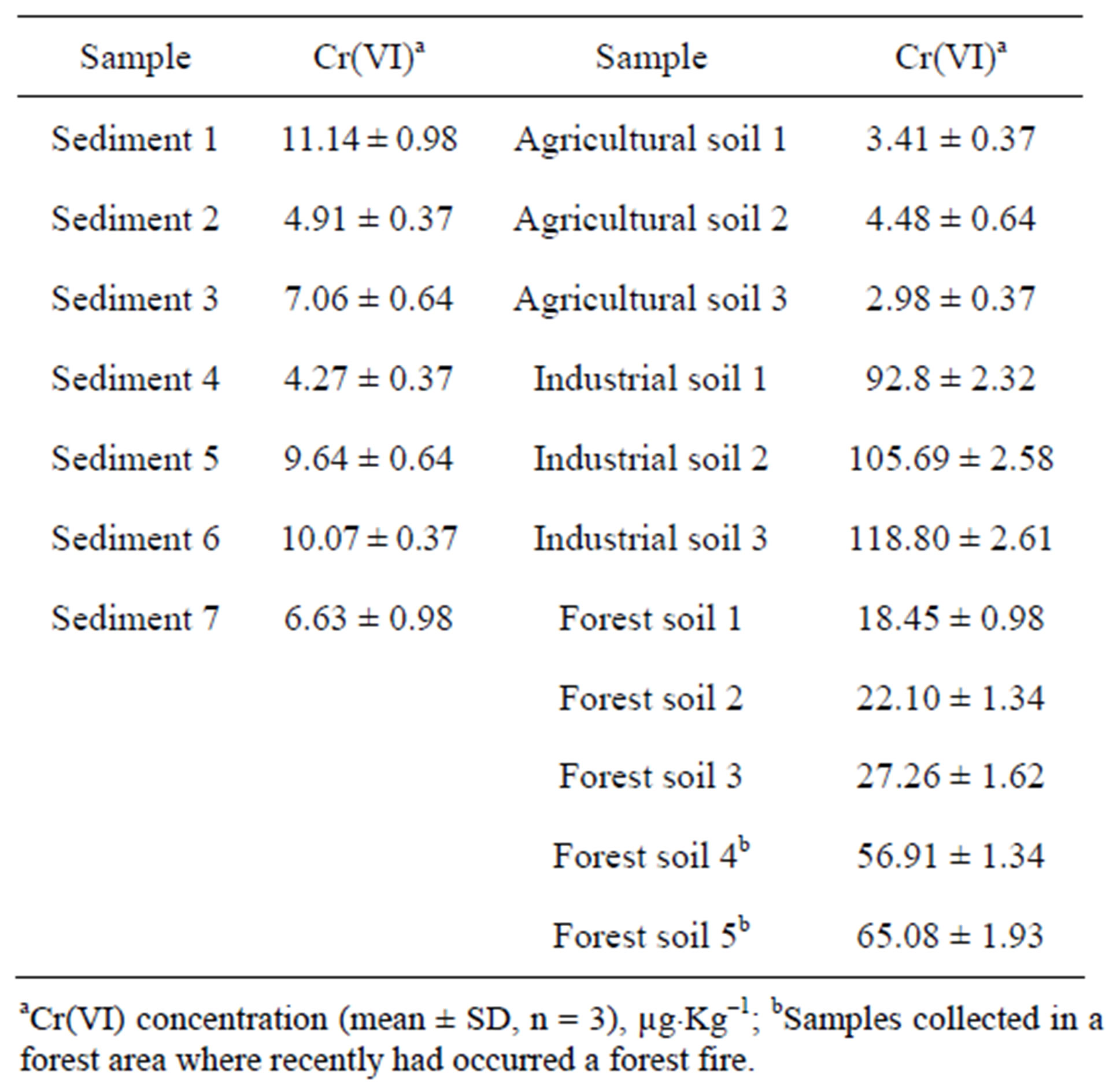
Table 4. Results for hexavalent chromium determination in soils and sediments samples.
was observed in this table that the highest hexavalent chromium concentration was located in industrial soil. However, is important to point out that forest soils samples number 5 and 6 have a concentration of Cr(VI) higher than the rest of forest samples analyzed. This can be explained by the fact that these samples were collected in a forest area where recently had occurred a forest fire and a partial oxidation of Cr(III) with atmospheric oxygen could have taken place. With regard to river and seawater, all samples analyzed were below a Cr(VI) concentration of 1.2 µg×L–1. The recovery of the method was checked for solid and liquid samples. Thus, for solid samples, were added 0.15 μg of Cr(VI) to the alkaline extractant solution and 0.25 µg directly to liquid samples, and these solutions were analyzed by the proposed methodology. The recovery obtained was in the range 97.5% - 100.1%. A comparison of the slopes of the calibration graph and the standard addition graph using a t-test (95% confidence level) was made and no difference was observed, which indicated that there is not a matrix effect.
4. Conclusion
Cr(VI)-imprinted poly(4-vinyl pyridine-co-2-hydroxyethyl methacrylate) (poly(4-VP-HEMA) proposed Bayramoglu et al [19] was applied to preconcentrate Cr(VI) from environmental samples such as river water, seawater, soils and sediments. FI preconcentration by using a minicolumn filled with poly(4-VP-HEMA particles results in a preconcentration factor of 550 (50 mL of sample and 90 µL of eluate), which offers a limit of detection of 0.04 µg×L–1 by using FAAS as detector. The FI system proposed in this paper has the advantage of being simpler because the use of expensive and sophisticated instruments is avoided. Ease of use, continuous process and selectivity make this method suitable for Cr(VI) determination in different environmental samples.
5. Acknowledgements
This work has been funded by the Spain’s Ministry of Science and Innovation, within the framework of Project CTQ2009-12282.
REFERENCES
- R. E. Hester and R. M. Harrison, “Electronic Waste Management,” Royal Society of Chemistry, Cambridge, 2009.
- A. Basu and T. M. Johnson, “Determination of Hexavalent Chromium Reduction Using Cr Stable Isotopes: Isotopic Fractionation Factors for Permeable Reactive Barrier Materials,” Environmental Science & Technology, Vol. 46, No. 10, 2012, pp. 5353-5360. doi:10.1021/es204086y
- B. Yaron, I. Dror and B. Berkowitz, “Soil-Subsurface Change: Chemical Pollutant Impacts,” Springer-Verlag, Berlin, 2012.
- M. Payehghadr, S. Esmaeilpour, M. K. Rofouei, and L. Adlnasab, “Determination of Trace Amount of Cadmium by Atomic Absorption Spectrometry in Table Salt after Solid Phase Preconcentration Using Octadecyl Silica Membrane Disk Modified by a New Derivative of Pyridine,” Journal of Chemistry, Vol. 2013, 2013. doi:10.1155/2013/417085
- E. Pehlivan and S. Cetin, “Sorption of Cr(VI) Ions on Two Lewatit-Anion Exchange Resins and Their Quantitative Determination Using UV-Visible Spectrophotometer,” Journal of Hazardous Materials, Vol. 163, No. 1, 2009, pp. 448-453. doi:10.1016/j.jhazmat.2008.06.115
- M. M. López Guerrero, E. Vereda Alonso, J. M. Cano Pavón, M. T. Siles Cordero and A. García de Torres, “OnLine Preconcentration Using Chelating and Ion-Exchange Minicolumns for the Speciation of Chromium(III) and Chromium(VI) and Their Quantitative Determination in Natural Waters by Inductively Coupled Plasma Mass Spectrometry,” Journal of Analytical Atomic Spectrometry, Vol. 27, No. 4, 2012, pp. 682-688. doi:10.1039/C2JA10290K
- M.J. Marqués, A. Morales-Rubio, A. Salvador and M. de la Guardia, “Chromium Speciation Using Activated Alumina Microcolumns and Sequential Injection Analysisflame Atomic Absorption Spectrometry,” Talanta, Vol. 53, No. 6, 2001, pp. 1229-1239. doi:10.1016/S0039-9140(00)00616-0
- A. TunÇeli and A. R. Türker, “Speciation of Cr(III) and Cr(VI) in Water After Preconcentration of Its 1,5-Diphenylcarbazone Complex on Amberlite XAD-16 Resin and Determination by FAAS,” Talanta, Vol. 57, No. 6, 2002, pp. 1199-1204. doi:10.1016/S0039-9140(02)00237-0
- A. C. Sahayam, “Speciation of Cr(III) and Cr(VI) in Potable Waters by Using Activated Neutral Alumina as Collector and ET-AAS for Determination,” Analytical and Bioanalytical Chemistry, Vol. 372, No. 7, 2002, pp. 840- 842. doi:10.1007/s00216-002-1261-7
- S. Kartal, S¸ Tokalloglu , and B . Özkan, “Speciation of Cr(III)/Cr(VI) in Tannery Waste Waters by Using IonExchange Resins,” Bulletin of the Korean Chemical Society, Vol. 27, No. 5, 2003, pp. 694-698. doi:10.5012/bkcs.2006.27.5.694
- I. Narin, A. Kars and M. Soylak, “A Novel Solid Phase Extraction Procedure on Amberlite XAD-1180 for Speciation of Cr(III), Cr(VI) and Total Chromium in Environmental and Pharmaceutical Samples,” Journal of Hazardous Materials, Vol. 150, No 2, 2008, pp. 453-458. doi:10.1016/j.jhazmat.2007.04.125
- M. E. Mahmoud, A. A. Yakout, S. B. Ahmed and M. M. Osman, “Speciation, Selective Extraction and Preconcentration of Chromium Ions via Alumina-FunctionalizedIsatin-Thiosemicarbazone,” Journal of Hazardous Materials, Vol. 158, No. 2-3, 2008, pp. 541-548. doi:10.1016/j.jhazmat.2008.01.114
- Z. Wang, D. M. Fang, Q. Li, L. X. Zhang, R. Qian, Y. Zhu, H. Y. Qu and Y. P. Du, “Modified Mesoporous Silica Materials for on-Line Separation and Preconcentration of Hexavalent Chromium Using a Microcolumn Coupled with Flame Atomic Absorption Spectrometry,” Analytica Chimica Acta, Vol. 725, No. 6, 2012, pp. 81-86. doi:10.1016/j.aca.2012.03.005
- H. M. Marwani, H. M. Albishri, E. M. Soliman and T. A. Jalal, “Selective Adsorption and Determination of Hexavalent Chromium in Water Samples by Chemically Modified Activated Carbon with Tris(hydroxymethyl) Aminomethane,” Journal of Dispersion Science and Technology, Vol. 33, No. 4, 2012, pp. 549-555. doi:10.1080/01932691.2011.574941
- S. Walas, A. Tobiasz, M. Gawin, B. Trzewik, M. Strojny, and H. Mrowiec, “Application of a Metal Ion-Imprinted Polymer Based on Salen-Cu Complex to Flow Injection Preconcentration and FAAS Determination of Copper,” Talanta, Vol. 76, No. 1, 2008, pp. 96-101. doi:10.1016/j.talanta.2008.02.008
- M. Saraji and H. Yousefi, “Selective Solid-Phase Extraction of Ni(II) by an Ion-Imprinted Polymer from Water Samples,” Journal of Hazardous Materials, Vol. 167, No. 1-3, 2009, pp. 1152-1157. doi:10.1016/j.jhazmat.2009.01.111
- Y. K. Tsoi, Y. M. Ho and K. S. Y. Leung, “Selective Recognition of Arsenic by Tailoring Ion-Imprinted Polymer for ICP-MS Quantification,” Talanta, Vol. 89, No. 30, 2012, pp.162-168. doi:10.1016/j.talanta.2011.12.007
- Y. Li, B. Gao and R. Du, “Studies on Preparation and Recognition Characteristic of Surface-Ion Imprinting Material IIP-PEI/SiO2 of Chromate Anion,” Separation Science and Technology, Vol. 46, No. 9, 2011, pp. 1472- 1481. doi:10.1080/01496395.2011.561821
- G. Bayramoglu and M. Y. Arica, “Synthesis of Cr(VI)-Imprinted Poly(4-vinyl Pyrdine-co-hydroxyethylmethacrylate) Particles: Its Adsorption Propensity to Cr(VI),” Journal of Hazardous Materials, Vol. 187, No. 1-3, 2011, pp. 213-221. doi:10.1016/j.jhazmat.2011.01.022
- I. Fujiwara, M. Maeda and M. Takagi, “Preparation of Ferrocyanide-Imprinted Pyridine-Carrying Microspheres by Surface Imprinting Polymerization,” Analytical Sciences, Vol. 19, No. 4, 2003, pp. 617-620. doi:10.2116/analsci.19.617
- E. B. Özkütük, A. Ersöz, A. Denizli and R. Say, “Preconcentration of Phosphate Ion onto Ion-imprinted Polymer,” Journal of Hazardous Materials, Vol. 157, No. 1, 2008, pp. 130-136. doi:10.1016/j.jhazmat.2007.12.118
- M. C. Yebra-Biurrun, “Flow Injection Analysis of Marine Samples,” Nova Science Publishers, New York, 2009.
- USEPA, “Method 3060A, Alkaline Digestion for Hexavalent Chromium. Tests Methods for Evaluating Solid Waste, Physical/Chemical Methods SW 846,” US Government Printing Office (GPO), Washington DC, 1996.
NOTES
*Corresponding author.

