International Journal of Clinical Medicine
Vol.05 No.21(2014), Article ID:52734,7 pages
10.4236/ijcm.2014.521176
Analysis of Autonomic, Respiratory and Motor Function of Infants in Pre- and Post-Liver Transplantation
Denise de Souza Rolim1, Évelim Leal de Freitas Dantas Gomes2, Andrea de Oliveira Franco Queiroga1, Juliana Angi Roche1, Audrey Borghi-Silva3, Luciana Maria Malosá Sampaio2
1Hospital Sírio-Libanês, São Paulo, Brazil
2Post-Graduation Program Science Rehabilitation, Nove de Julho University, São Paulo, Brazil
3Cardiopulmonary Physiotherapy Laboratory, Federal University of São Carlos, São Carlos, Brazil
Email: evelimgomes@uninove.br
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 20 October 2014; revised 19 November 2014; accepted 1 December 2014
ABSTRACT
Purpose: Children with liver impairment are likely to develop changes in autonomic nervous function and delay in motor development. The assessment and identification of these dysfunctions may allow an appropriate physiotherapeutic care. Method: Cross sectional study of 18 infants, 11 controls and 7 infants (post-liver transplantation) with an average age of 10 ± 4.5 months, was evaluated in pre- and post-liver transplant. All infants underwent to assessments of motor skills, body composition, chest and abdominal motion, and cardiac autonomic modulation was measured by heart rate variability. Results: Motor delay and malnutrition were found in all infants. The gravity index (PELD)―pediatric end-stage liver disease―showed a negative correlation with the Alberta Infant Motor Scale (r = 0.83, p = 0.01). In addition, reduced parasympathetic modulation was demonstrated by the rMSSD, SD1 and ApEn, pre- and post-transplant. Conclusion: Infants with liver disease, even after transplantation, have delay in motor development, as well as changes in their nutritional and autonomic dysfunction.
Keywords:
Autonomic Nervous System, Liver Transplant, AIMS, Respiratory System

1. Introduction
The liver has an important role in the maintenance of blood glucose, through gluconeogenesis and glycogen storage, converting amino acids into glucose. It acts in fat metabolism through fast oxidation of fatty acids for energy and formation of most lipoproteins. The liver also plays a vital role in the formation of plasma proteins and urea, and deamination of amino acid [1] . When liver disease is present as a result of changing these functions, there is a development of protein-calorie malnutrition. Consequentially, there is loss of muscle mass and subcutaneous tissue [2] -[4] .
Malnutrition in children also contributes to delays in growth and in motor development [5] . These factors interfere with these children’s interactions with the environment (hospitalizations, invasive medical procedures, use of enteral probe or other devices), preventing the exploitation of the environment and hindering the acquisition of motors mark [5] [6] . The beginning of life-threatening complications secondary to liver failure or chronic terminal liver disease corresponds to primary indication of liver transplantation. In addition, autonomic dysfunction has been observed in chronic liver diseases [1] [3] .
For this reason, strategies that could identify early secondary complications of liver disease, mainly during the period of convalescence hospital post-transplantation in these infants may positively impact in the motor abilities and postural changes as well as in the respiratory complications.
The PELD score (Pediatric End-Stage Liver Disease) can be used as a way to estimate mortality and morbidity in children from 0 to 12 years with liver disease awaiting transplantation [7] . In addition, negative clinical status post-transplantation can indicate complications and is the important cause of morbidity and mortality [8] . However, measurements of motor skills have been studied in infant’s pre-transplantation and may constitute important tool to identify reduced motor abilities caused by liver disease and prolonged hospitalization in these population.
Additionally, heart rate variability (HRV)―a physiological measure of fluctuation of the cardiac cycle in time that reflects the complex control mediated by the autonomic nervous system (ANS)―is a reliable and non- invasive tool to provide quantitative information on the cardiac system and its sympathetic and parasympathetic components.
Neuropathy is known as a common manifestation in chronic liver disease. Some studies have evaluated the role of ANS after liver transplantation in adults. However, to our knowledge, HRV was not analyzed in infants after liver transplantation. From the standpoint of respiratory, autonomic and motor development, and needs of identification of these amendments by the physiotherapist are of great importance for the choice of the best treatment of these infants [9] [10] .
2. Materials and Methods
The study is a prospective observational type held from June 2011 to October 2012 in philanthropy at the pediatric ambulatory units.
Patients (children between zero and 18 months) were recruited in pre-operative liver transplantation, able to perform measurements proposals. Excluded patients were: children with congenital (corrected or not), and/or musculoskeletal changes, as well as presence of lung diseases, tracheotomy, and children who had a first transplant. The protocol was approved by the Hospital’s Ethics Committee and Research, (protocol number HSL 2010/33) and the participant’s legal guardians signed informed consent.
The selected children underwent to four evaluations: anthropometric, autonomic, respiratory, and motor skills.
2.1. Anthropometric Evaluation
The first evaluation (anthropometric) was performed in pre-operative phase and the other two in the third and thirtieth post-operative of liver transplantation. The anthropometric assessment was calculated the circumference of the arm (AC) obtained by calculation: arm circumference (AC)―3.14× triceps skin fold (TSF).
The AC was held with tape measure, using as reference the middle third of the left arm. For the TSF, it was considered the value of the 50 percentile for age, obtained in the table proposed by Frisancho [11] . The weight of the participant children was measured during all three evaluations.
2.2. Respiratory Evaluation
The respiratory evaluation consisted in the verification of the presence of signs of respiratory distress (use of accessory muscles, retractions of sub-diaphragmatic, intercostals, furcula and nose wing-beating), time of invasive mechanical ventilation (IMV), time of oxygen supplementation, need for non-invasive mechanical ventilation (NIMV) and the thoracic and abdominal chest measurements (by use of measuring tape). The circumference measure was obtained at the end of inspiration and at the end of expiration [12] . The chest circumference was measured at axillaries line, and the abdominal circumference at the umbilical scar. From this measurements, the amplitude index (AI) [13] [14] thoracic and abdominal, calculated as:
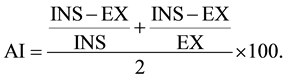
2.3. Motor Skills Assessment
The motor skills assessment was carried out through the Alberta Infant Motor Scale (AIMS), which evaluates the child’s sequential motor development until independent march to progressive independent development and integration of antigravity muscle control in four postures (prone, supine, sitting and standing). The score obtained is transferred to a chart, which determines the child’s percentile evaluated motor performance compared with normal values [15] .
2.4. HRV
Heart rate variability was analyzed on two occasions (pre- and post-operative) and comparison to this group was made to a control group, due to lack of reference values for this variable. R-R intervals were collected using a portable monitor of heart rate (Polar™ S810i-Finland). The data was collected 10 minutes in the supine position at rest and 10 minutes sitting before and after the transplant. The collection of the control group also followed the same sequence (10 minutes supine and 10 minutes sitting).
The indexes of HRV in the time domain used in this study were: average of iRR, SDNN (standard deviation of all iRR), STD HR and rMSSD (square root of the average of the square of the differences between the adjacent normal iRR). In addition, HRV indices in the frequency domain were: LF (0.04 - 0.15 Hz), HF (0.15 - 0.4 Hz), LF/HF. Non-linear analysis of Poincare Plot was (SD1, SD2), DFA α1, DFA α2 and ApEn were used (Figure 1).
3. Statistical Analysis
For this statistical analysis, it was used a comparison between pre-operative, third and 30 PO the ANOVA parametric test for normal distribution data. For the variables AI, AC and AIMS were used the Kruskal Wallis. The relationship between two variables was tested using Spearman test. For analysis of adherence to the Gauss curve, the Kolmogorov Smirnov test (KS) was used. A comparison of heart rate variability variables pre- and post-liver transplant, a paired nonparametric Wilcoxon test was used; and for pre- or post-liver transplant variables with the control group was used the unpaired and non-parametric Mann Whitney test. The significance level was considered p < 0.05. The software used was Minitab 14.
4. Results
Seven children with an average age of 10 ± 4.5 months were selected. The pre-operative diagnostics were bile atresia (86%) and Alagille Syndrome (14%), with average PELD 15.42 ± 8.28. The average time of surgery was 7.4 ± 1.7 hours. One child developed post-operative rejection (Table 1).
During times of evaluation, arm circumference (AC) did not significant alter (p = 0.46). As well as to the weight, (p = 0.90). Only one child showed signs of respiratory distress in the pre-operative evaluation. This was already in use of NIMV for presenting important ascites. All others were in room air in the pre-operative period. The IMV post-operative average was 32.6 ± 22.1 hours and oxygen was 8.2 ± 12.3 days. The same child who used pre-operative NIMV stood at IMV for 30 hours and was the only one that made use of post-operative NIMV.
Regarding the measurement, there was also no statistically significant change in the chest and abdominal motion thought the time (p = 0.82) in chest measurement with inspiratory and p = 0.809 in expiratory chest measurement and p = 0.95 in abdominal measurement with inspiratory and p = 0.975 in expiratory abdominal measurement (p = 0.97).
However, the amplitude index (AI) pre-operative thoracic versus abdominal motion were significant different
Figure 1. Flow chart.
Table 1. Sample data.
(p = 0.04) showing a predominance of thoracic breathing in pre-operative period.
For the AIMS, the medians obtained after the three measures were 20 (14 - 34), 20 (11 - 34) and 32 (10 - 46), respectively, with p = 0.53. As for the percentiles, 86% of the children were below the 5 percentile in pre- operative and 100% of them below the 5 percentile on the third post-operative period. On the 30th post-opera- tive, 86% of the participants were below the 5 percentile and the child who was in the 5 percentile pre-operative returned to this. We observed a negative correlation between AIMS and PELD (Figure 2).
In relation to HRV indices, we observed reduced rMSSD, HF and SD1 after liver transplantation, which did not reach the baseline. The values of ApEn were higher in pre- and post-liver transplantation group when compared to the control group (Table 2).
Figure 2. Correlation PELD × AIMS.
Table 2. HRV data.
Legend: *p < 0.05 pre-transplant × control. #p < 0.05 post-transplant × control. **p < 0.05 pre- × post-transplant.
5. Discussion
One month after liver transplant there was no change of nutritional status of these children, observed through anthropometric measures [16] , in a follow-up five years after liver transplantation in children, observed important improvement of AC after the first year of the transplant. The linear growth does not normalize so quickly. The study of Evans [17] showed that the liver transplantation helps to restore growth to a limited degree, not finding restoration of normal height in almost a quarter of children accompanied in seven years. Both papers noted that the growth is faster in children at the time of transplant. If we had a larger tracking time in our sample, we could observe any significant change in anthropometric measurements collected.
In relation to respiratory aspect, unlike found in literature [18] -[21] , not observed post-operative respiratory complications of liver transplantation, possibly because of the surgeries are elective. The only child who needed post-operative NIMV was already using it pre-operative due to the mechanics of breathing being limited by an important ascites. As for the breathing pattern, it was observed a pre-operative thoracic predominance, with a trend towards a mixed pattern, indicating progressive increase of post-operative diaphragm activation, perhaps by reducing the intra-abdominal pressure after the surgery.
Regarding the motor evaluation, there was a moderate negative correlation between PELD and AIMS, that is, the higher PELD, the lower t AIMS, and therefore, children with worse liver function have greater delays in motor development. Significant improvement in motor development was not observed on the evaluated children. Wayman et al. [22] evaluated the motor development of children with atresia of bile ducts, finding persistent delay after a year of transplant, rising as risk factors prolonged hospitalizations and malnutrition. Thevenin et al. [23] also observed this persistence of delays in motor development seven months after transplant and suggested immediate physical therapy intervention after transplantation.
Studies have shown that the intervention of physiotherapy on neurologically normal children with risk of present delay in motor development (preterm infants) is beneficial to the performance motor [24] -[27] . It is possible that the intervention of post-operative therapy of liver transplant can promote a better motor development on children. For both, we suggest a future randomized clinical trial comparing a group of children submitted to the liver transplant receiving physical therapy sessions with a control group.
As to the HRV variables a depression, in particular, of the parasympathetic tone has been described and found in the evaluated sample, in chronic [26] -[28] liver diseases. Neuropathy is known as a common manifestation. The underlying mechanism of systemic inflammation decreased HRV is unknown. However, it seems that the inflammatory process can correlate significantly with rates of depression of HRV in many clinical conditions. The HRV is also reduced in patients with chronic liver disease. The inflammatory process in these patients is increased even in the absence of active infection and this process can explain the decrease of this variable observed in these patients.
The autonomic disorder represents a severe complication and represents an increased risk of mortality in patients with chronic liver disease. In a longitudinal study of patients waiting for liver transplant, mortality was significantly higher in patients with autonomic disorder (27%) compared to those without disorder (0%), suggesting that one should take into consideration this issue for early liver transplantation in patients with advanced liver disease [29] .
In a longitudinal study, the presence of autonomic neuropathy and the severity of liver disease were independent risk factors for mortality [9] .
A nonlinear analysis on approximate entropy (ApEn), quantifies the regularity of patterns in data sets assigning a non-negative number for a time series, with higher values representing greater randomness of apparent process. The ApEn has been applied and has shown alterations in many states of disease and aging.
Lee et al. [10] found lower values normalized ApEn in patients with autonomic dysfunction and higher mortality in this group during the monitoring period, consistent with previous observations that the advanced disease is associated with less complexity.
Larger ApEn values were found in the evaluated group than in the control group; that can be explained by the fact that liver disease in infants produce an autonomic change different than degenerative liver diseases in adults and perhaps is liable to any cardiac homeostasis attempt at compensation. No studies to evaluate this variable in infants with liver disease were found.
The measurement of heart rate variability offers simple, non-invasive assessment of cardiovascular and autonomic effects in liver disease, particularly to those awaiting liver transplantation. Our study presents as limitation this sample’s follow up time, in which we could find significant change in some of the evaluated outcomes.
6. Conclusion
Children with liver disease have delayed motor development, which persists in the first month after the liver transplant, as well as changes on their nutritional and autonomic state. Future studies are needed to determine whether or not the intervention of physiotherapy in children after the transplant brings benefits to these variables.
References
- Guyton, A.C. and Hall, J.E. (1997) Tratado de Fisiologia Médica. 9th Edition, Guanabara Koogan.
- Campos, A.C.L., Matias, J.E.F. and Coelho, J.C.U. (2002) Nutritional Aspects of Liver Transplantation. Current Opinion in Clinical Nutrition and Metabolic Care, 5, 297-307. http://dx.doi.org/10.1097/00075197-200205000-00010
- Maio, R., Dichi, J.B. and Burini, R.C. (2000) Conseqüências Nutricionais das Alterações Metabólicas dos Macro- nutrientes na Doença Hepática Crônica. Arquivos de Gastroenterologia, 37, 52-57. http://dx.doi.org/10.1590/S0004-28032000000100011
- Zhao, V.M. and Ziegler, T.R. (2010) Nutrition Support in End-Stage Liver Disease. Critical Care Nursing Clinics of North America, 22, 369-380. http://dx.doi.org/10.1016/j.ccell.2010.02.003
- Alonso, E.M. (2008) Growth and Developmental Considerations in Pediatric Liver Transplantation. Liver Transplantation, 14, 585-591. http://dx.doi.org/10.1002/lt.21488
- Bagner, D.M., Rodrigue, J.R., Hemme, J., Adkins, J. and Gonzalez-Peralta, R. (2005) Developmental Considerations in the Context of Pediatric Transplantation. Annals of Transplantation, 10, 13-16.
- Kamath, B.M. and Olthoff, K.M. (2010) Liver Transplantation in Children: Update 2010. Pediatric Clinics of North America, 57, 401-414. http://dx.doi.org/10.1016/j.pcl.2010.01.012
- Mueller, A.R., Platz, K.P. and Kremer, B. (2004) Early Postoperative Complications Following Liver Transplantation. Best Practice & Research Clinical Gastroenterology, 18, 881-900. http://dx.doi.org/10.1016/j.bpg.2004.07.004
- Carey, E.J., Gautam, M., Ingall, T. and Douglas, D.D. (2008) The Effect of Liver Transplantation on Autonomic Dysfunction in Patients with End-Stage Liver Disease. Liver Transplantation, 14, 235-239. http://dx.doi.org/10.1002/lt.21350
- Fleisher, L.A., Fleckenstein, J.F., Frank, S.M. and Thuluvath, P.J. (2000) Heart Rate Variability as a Predictor of Autonomic Dysfunction in Patients Awaiting Liver Transplantation. Digestive Diseases and Sciences, 45, 340-344. http://dx.doi.org/10.1023/A:1005468711494
- Frisancho, A.R. (1974) Triceps Skin Fold and Upper Arm Muscle Size Norms for Assessment of Nutritional Status. The American Journal of Clinical Nutrition, 27, 1052-1058.
- Caldeira, V.S., Starling, C.C.D., Britto, R.R., Martins, J.A., Sampaio, R.F. and Parreira, V.F. (2007) Precisão e acurácia da cirtometria em adultos saudáveis. Jornal Brasileiro de Pneumologia, 33, 519-526. http://dx.doi.org/10.1590/S1806-37132007000500006
- Jamami, M., Pires, V.A., Oishi, J. and Costa, D. (1999) Efeitos da intervenção fisioterápica na reabilitação pulmonar de pacientes com doença pulmonar obstrutiva crônica (DPOC). Revista de Fisioterapia da Universidade de São Paulo, 6, 140-153.
- Costa, I.P., Gomes, E.L.F.D., Grecco, L.A.C., Dias, F.D., Pupin, D., Negrini, F., Oliveira, C.S. and Sampaio, L.M.M. (2011) Efeitos do exercício diafragmático e da espirometria de incentivo na função respiratória de crianças portadoras de miopatia nemalínica: Uma série de casos. Terapia Manual, 9, 608-612.
- Piper, M.C. and Darrah, J. (1994) Motor Assessment of the Developing Infant. Saunders, Philadelphia.
- Holt, R.I., Broide, E., Buchanan, C.R., Miell, J.P., Baker, A.J., Mowat, A.P., et al. (1997) Orthotopic Liver Transplantation Reverses the Adverse Nutritional Changes of End-Stage Liver Disease in Children. The American Journal of Clinical Nutrition, 65, 534-542.
- Evans, I.V.R., Belle, S.H., Wei, Y., Penovich, C., Ruppert, K., Detre, K.M., et al. (2005) Post-Transplantation Growth among Pediatric Recipients of Liver Transplantation. Pediatric Transplantation, 9, 480-485. http://dx.doi.org/10.1111/j.1399-3046.2005.00326.x
- Mack, C.L., Millis, J.M., Whitington, P.F. and Alonso, E.M. (2000) Pulmonary Complications Following Liver Transplantation in Pediatric Patients. Pediatric Transplantation, 4, 39-44. http://dx.doi.org/10.1034/j.1399-3046.2000.00080.x
- Pirat, A., Özgur, S., Torgay, A., Candan, S., Zeyneloglu, P. and Arslan, G. (2003) Risk Factors for Postoperative Respiratory Complications in Adult Liver Transplant Recipients. Transplantation Proceedings, 36, 218-220. http://dx.doi.org/10.1016/j.transproceed.2003.11.026
- Manczur, T.I., Greenough, A., Rafferty, G.F., Dimitrou, G., Baker, A.J., Mieli-Vergani, G., et al. (2002) Diaphargmatic Dysfunction after Pediatric Orthotopic Liver Transplantation. Transplantation, 73, 228-232. http://dx.doi.org/10.1097/00007890-200201270-00013
- Smyrniotis, V., Andreani, P., Muiesan, P., Mieli-Vergani, G., Rela, M. and Heaton, N.D. (1998) Diaphragmatic Nerve Palsy in Young Children Following Liver Transplantation: Successful Treatment by Plication of the Diaphragm. Transplant International, 11, 281-283.
- Wayman, K.I., Cox, K.L. and Esquivel, C.O. (1997) Neurodevelopmental Outcome of Young Children with Extrahepatic Biliary Atresia 1 Year after Liver Transplantation. The Journal of Pediatrics, 131, 894-898. http://dx.doi.org/10.1016/S0022-3476(97)70039-8
- Thevenin, D.M., Baker, A., Kato, T., Tzakis, A., Fernandez, M. and Dowling, M. (2006) Neurodevelopmental Outcomes for Children Transplanted under the Age of 3 Years. Transplantation Proceedings, 38, 1692-1693.
- Lekskulchai, R. and Cole, J. (2001) Effect of a Developmental Program on Motor Performance in Infants Born Preterm. Australian Journal of Physiotherapy, 47, 169-176. http://dx.doi.org/10.1016/S0004-9514(14)60264-6
- Formiga, C.K.M.R., Pedrazzani, E.S. and Tudella, E. (2004) Desenvolvimento motor de lactentes pré-termo partici- pantes de um programa de intervenção fisioterapêutica precoce. Revista Brasileira De Fisioterapia, 8, 239-245.
- Laghi, F., Adiguzel, N. and Tobin, M.J. (2010) Decreased Heart Rate Variability in Patients with Cirrhosis Relates to the Presence and Degree of Hepatic Encephalopathy. European Respiratory Journal, 35, 934.
- Keresztes, K., Istenes, I., Folhoffer, A., Lakatos, P.L., Horvath, A., Csak, T., Varga, P., Kempler, P. and Szalay F. (2004) Autonomic and Sensory Nerve Dysfunction in Primary Biliary. World Journal of Gastroenterology, 10, 3039- 3043.
- Hendrickse, M.T., Thuluvath, P.J. and Triger, D.R. (1992) The Natural History of Autonomic Neuropathy in Chronic Liver Disease. The Lancet, 339, 1462-1464. http://dx.doi.org/10.1016/0140-6736(92)92042-E
- Fleckenstein, J.F., Frank, S. and Thuluvath, P.J. (1996) Presence of Autonomic Neuropathy Is a Poor Prognostic Indicator in Patients with Advanced Liver Disease. Hepatology, 23, 471-475. http://dx.doi.org/10.1002/hep.510230311




