International Journal of Clinical Medicine
Vol.5 No.15(2014), Article
ID:48653,5
pages
DOI:10.4236/ijcm.2014.515123
Malignant Peripheral Nerve Sheath Tumors of the Scalp: Case Report and Review of Literature
Touria Bouhafa1, Abderahmann Elmazghi1, Hayat Baissel1, Hind El Fatmi2, Afaf Amarti2, Khalid Hassouni1
1Department of Radiotherapy, Université Sidi Mohammed Benabdellah, CHU HASSAN II, Fez, Morocco
2Department of Pathology, Université Sidi Mohammed Benabdellah, CHU HASSAN II, Fez, Morocco
Email: cancer05ino@yahoo.fr
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 10 June 2014; revised 19 July 2014; accepted 8 August 2014
ABSTRACT
Malignant peripheral nerve sheath tumors of the scalp are rare lesions of the nervous system. Only 16 cases have been reported to date. In this report, we present a case of a malignant peripheral nerve sheath tumor (MPNST) of the scalp and retrospectively analyze the clinical features, imaging findings, pathological features, and prognoses of these tumors.
Keywords:Malignant Peripheral Nerve Sheath Tumors, The Scalp, Treatment

1. Background
Malignant peripheral nerve sheath tumors of the scalp are rare neoplasms of the nervous system. A malignant peripheral nerve sheath tumor (also known as “Malignant schwannoma”, “Neurofibrosarcoma” and “Neurosarcoma) is a form of cancer of the connective tissue surrounding nerves. As defined by the World Health Organization, however, the universal or standard terminology should be a malignant peripheral nerve sheath tumor [1] -[5] . Given its origin and behavior it is classified as a sarcoma [1] -[4] . Although the incidence of a malignant peripheral nerve sheath tumor of the scalp is very low, it is highly malignant and is associated with poor prognosis [1] -[3] .
Approximately 25% to 50% of observed MPNSTs occur in patients with NF-1. Like other soft tissue sarcomas, MPNSTs have a tendency to recur locally and spread hematogenously. Despite aggressive surgery and adjuvant therapy, the prognosis for patients with MPNST remains poor; however, to date, prognostic factors have not been identified consistently in the literature [6] -[10] .
In this article we describe a new case of MPNSTs of the scalp.
2. Case Presentation
A 15-year-old male patient presented with a swelling in the occipital region that had been gradually increasing for past two years.
It had shown rapid growth during the year prior to being our hospital.
Initially, the swelling was the size of a peanut. The patient had no history of trauma, fever, or infection at this site.
On examination, we found a globular (goose egg size), non-tender, non-compressible, firm to fluctuant swelling measuring approximately 6 × 6 cm in the occipital region.
A cranial computed topography (CT) revealed a lesion that exhibited higher density to the brain parenchyma, with an adjacent bony defect measuring 46 × 37 mm in the occipital area.
Magnetic resonance imaging (MRI) revealed a swelling with bony and dural involvement measuring 47 × 38 × 52 mm in the occipital region, which showed nearly equal T1 and T2 signals. Mixed signals were also observed in various portions of the lesions, with partial enhancement in the lesion overall.
The tumor was misdiagnosed as a hemongioma in the occipital area before surgery. After excising the large mass, the wound healed well, and no surgical complications arose.
The diagnosis of malignant peripheral nerve sheath tumor was made through postoperative pathological examination. Light microscopy revealed that the tumor cells were spindle-shaped, with variable mitotic activity and nuclear pleomorphism (Figure 1). Focal hemorrhage and necrosis were also observed.
Immunohistochemistry revealed positive immunoreactivity with Sangtec 100 (S-100) (Figure 2).
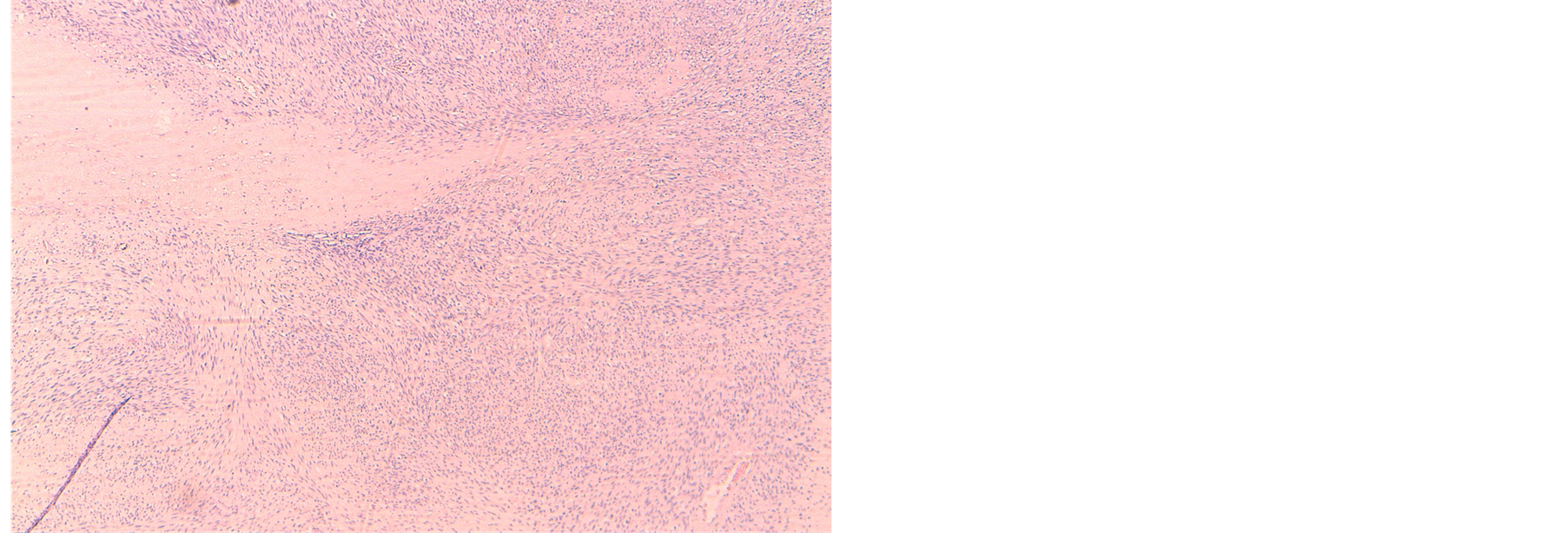
Figure 1. HES × 4: tumor cells were spindle-shaped, with high cell density, variable mitotic activity, nuclear pleomorphism and foci of necrosis.
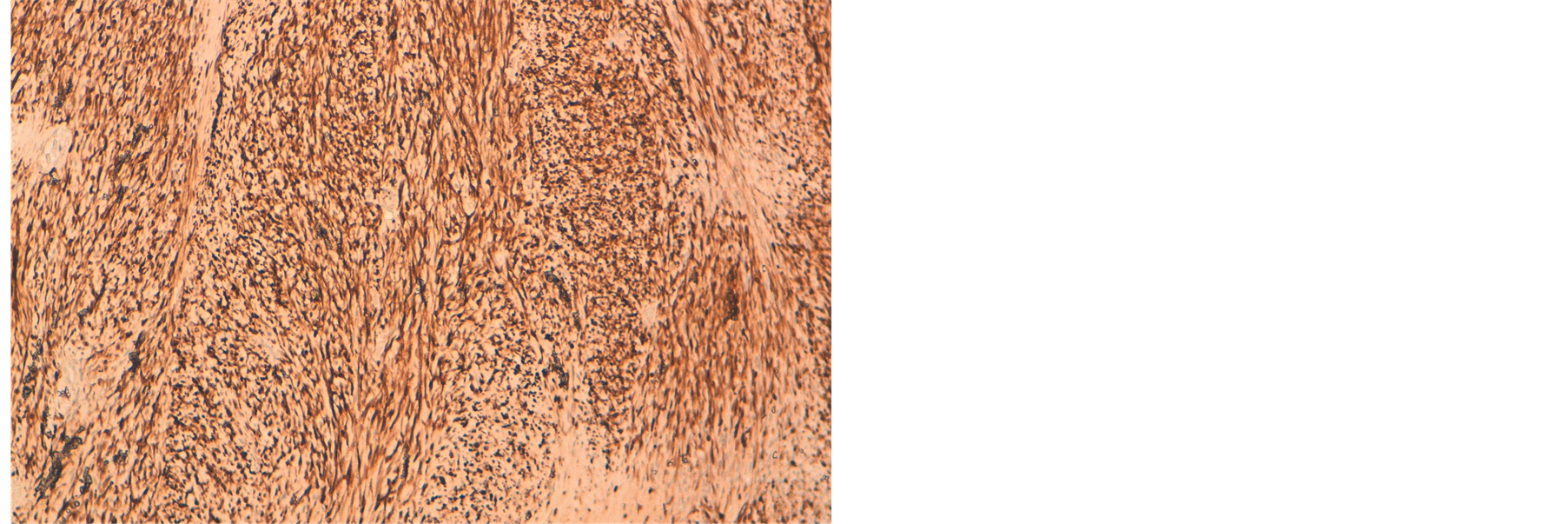
Figure 2. ×10: marquage positif des cellules tumorales à l’anti-corps anti PS100.
3. Discussion
Malignant peripheral nerve sheath tumors (MPNSTs) are rare, with an expected incidence of 0.1/100,000 per year [11] , they account for approximately 5% to 10% of all soft tissue tumors with slight male predominance [1] -[4] . Most studies show that the peak incidence of MPNSTs is in the seventh decade of life in the general population, but in the third or fourth decade in people with NF1 [12] ; however, these tumors can occur at a much younger age in either population [12] [13] .
MPNSTs are defined as any malignant tumor arising from or differentiating toward cells of the peripheral nerve sheath.
The etiology is unknown but they have a known association with neurofibromatosis type 1 (NF-1), an autosomal-dominant disorder that involves the NF1 tumor suppressor gene, which is located on chromosome 17, Up to 50% of MPNSTs occur in patients with NF1 [14] , and there is a higher incidence in patients with a history of radiation exposure [1] [14] .
MPNST is located mainly in the trunk and extremities, such as the buttocks, thighs, brachial plexus, sciatic nerve, and paraspinal region. Primary scalp MPNST is extremely rare, with only 16 cases reported to date in English literature [1] .
Theoretically, MPNST of the scalp can originate from any site on the scalp. Of the 16 reported cases in the literatutre, the occipital region seemed to be the most common site, accounting for more than 50%.
The initial clinical features of MPNST of the scalp are generally atypical. Most patients present with a gradually increasing swelling in the scalp (cutaneous or subcutaneous) [1] [15] -[21] . Symptoms of headache, vomiting, seizures, vertigo, visual impairment, or focal neurological deficits may affect patients with giant MPNST of the scalp based on the intracranial extension of the tumor [1] .
On local examination, the swelling is commonly globular, firm, non-tender, non-compressible and non-pulsatile [1] .
Although MPNSTs of the scalp generally lack specificity on imaging features at the early stage, CT or MRI scans are useful for demonstrating the relationship with surrounding structures.
On MRI scans, MPNSTs of the scalp are often hypointense or isointense in T1-weighted series and hyperintense in T2-weighted and Flair sections [1] [22] . MRI scans often reveal features of MPNSTs that differ from those of other scalp tumors, such as lipoma and malignant melamoma [1] . Tumors with cystic degeneration, hemorrhage, or calcification, however, may show mixed densities on CT or MRI scans, which contributes to the high chance of misdiagnosis. Of our case MRI scans demonstrated mixed densities in T1- and T2-weighted series.
The pathological diagnosis is the gold standard for diagnosing MPNST of the scalp. Although MPNST is a heterogeneous group of neoplasms that shows diverse differentiation potential, routine hematoxylin-eosin (H&E) and immunohistochemical staining techniques can help diagnose the majority of MPNSTs of the scalp [1] .
Malignant peripheral nerve sheath tumors are unencapsulated infiltrating tumors composed of spindle cells arranged in a whorling pattern with irregular nuclei, cyst formation, and nuclear palisading [23] .
Mitotic figures are readily visible, with more than one per hpf, and in 50% to 90% of cases the cells are immunoreactive to S-100 protein staining [24] . Necrosis, pseudocystic change, or hemorrhage may also be found. The pathological criteria for malignancy include invasion of surrounding tissues by tumor cells, vascular invasion, marked nuclear pleomorphism, necrosis, and the presence of mitoses [24] .
Immunohistochemical staining of the tumor tissue with a variety of antibodies can diagnose the disease and differentiate it from other diseases. The S-100, EMA, Vimentin (VIM), and CD34 antibodies are highly specific to MPNST of the scalp [4] [6] [25] .
Of the 16 cases described in the literature, 12 were immunoreactive for the S-100 protein (12 of 14 cases (86%); not available in two cases), suggesting a high specificity [1] .
Other markers, such as glial fibrillary acidic protein (GFAP), cell keratin (CK) and HMB, are usually not expressed in MPNST of the scalp and are therefore used only as references [1] . In general, a combination of antigens is used to help exclude other scalp tumors and to confirm the diagnosis of MPNST especially if S-100- negative.
The International Consensus Group has recommended that the current management of MPNST should be identical to that of any other soft tissue tumors [26] . Surgery, therefore, is the mainstay of treatment for MPNST of the scalp. The goal of surgery is to achieve complete excision of the tumor with wide (negative) margins (≥2 cm) [1] [21] [26] [27] . Therefore, patients who had positive surgical margins had a greater risk of dying, mainly from locoregional disease [10] .
As reported, surgical excision followed by adjuvant radiotherapy can improve local control [27] . Thus, some authors have recommended that adjuvant radiotherapy should been considered for all intermediateand highgrade MPNST of the scalp, as well as low-grade tumors with positive margins [27] .
Of the 16 cases, surgical excision followed by adjuvant radiotherapy had been done in eight cases. It seems that the current treatment of these highly malignant tumors, however, is not that pessimistic. As some authors have pointed out, the survival rates of patients with MPNSTs were significantly better for superficial tumors, such as MPNST of the scalp [5] . Therefore, further studies with greater numbers of patients are required to confirm the prognosis for MPNST of the scalp [1] .
4. Conclusion
The diagnosis of MPNST of the scalp is based on integrating clinical, imaging, histopathological and immunohistochemical findings. Surgical excision with wide margins (≥2 cm) and adjuvant radiation should be considered standard treatment.
References
- Wang, J., Ou, S.W. Guo, Z.Z., Wang, Y.J. and Xing, D.G. (2013) Microsurgical Management of Giant Malignant Peripheral Nerve Sheath Tumor of the Scalp: Two Case Reports and a Literature Review. World Journal of Surgical Oncology, 11, 269. http://dx.doi.org/10.1186/1477-7819-11-269
- Hajdu, S.I. (1993) Peripheral Nerve Sheath Tumors. Histogenesis, Classification, and Prognosis. Cancer, 72, 3549-3552. http://dx.doi.org/10.1002/1097-0142(19931215)72:12<3549::AID-CNCR2820721202>3.0.CO;2-Y
- Baehring, J.M., Betensky, R.A. and Batchelor, T.T. (2003) Malignant Peripheral Nerve Sheath Tumor: The Clinical Spectrum and Outcome of Treatment. Neurology, 61, 696-698. http://dx.doi.org/10.1212/01.WNL.0000078813.05925.2C
- Wanebo, J.E., Malik, J.M., Vandenberg, S.R., Wanebo, H.J., Driesen, N. and Persing, J.A. (1993) Malignant Peripheral Nerve Sheath Tumors: A Clinicopathological Study of 28 Cases. Cancer, 71, 1247-1253. http://dx.doi.org/10.1002/1097-0142(19930215)71:4<1247::AID-CNCR2820710413>3.0.CO;2-S
- Allison, K.H., Patel, R.M., Goldblum, J.R. and Rubin, B.P. (2005) Superficial Malignant Peripheral Nerve Sheath Tumor: A Rare and Challenging Diagnosis. American Journal of Clinical Pathology, 124, 685-692. http://dx.doi.org/10.1309/V8XMK5R78Q96V090
- Ducatman, B.S., Scheithauer, B.W., Piepgras, D.G., et al. (1986) Malignant Peripheral Nerve Sheath Tumours: A Clinicopathological Study of 120 Cases. Cancer, 57, 2006-2021. http://dx.doi.org/10.1002/1097-0142(19860515)57:10<2006::AID-CNCR2820571022>3.0.CO;2-6
- Doorn, P.F., Molenaar, W.M., Buter, J., et al. (1995) Malignant Peripheral Nerve Sheath Tumours in Patients with and without Neurofibromatosis. European Journal of Surgical Oncology, 21, 78-82. http://dx.doi.org/10.1016/S0748-7983(05)80073-3
- Wong, W.W., Hirose, T., Scheithauer, B.W., et al. (1998) Malignant Peripheral Nerve Sheath Tumour: Analysis of Treatment Outcome. International Journal of Radiation Oncology • Biology • Physics, 42, 351-360. http://dx.doi.org/10.1016/S0360-3016(98)00223-5
- Cashen, D.V., Parisien, R.C., Raskin, K., et al. (2004) Survival Data for Patients with Malignant Schwannoma. Clinical Orthopaedics and Related Research, 426, 69-73. http://dx.doi.org/10.1097/01.blo.0000131256.82455.c5
- Anghileri, M., Miceli, R., Fiore, M., Mariani, L., Ferrari, A., Mussi, C., Lozza, L., Collini, P., Olmi, P., Casali, P.G., Pilotti, S. and Gronchi, A. (2006) Malignant Peripheral Nerve Sheath Tumors. Prognostic Factors and Survival in a Series of Patients Treated at a Single Institution. Cancer, 5, 1065-1074
- Enzinger, F.M. and Weiss, S.W. (2001) Malignant Tumours of Peripheral Nerves. In: Enzinger, F.M. and Weiss, S.W., Eds., Soft Tissue Tumors. Vol. 31, CV Mosby Company, St. Louis, 1209-1263.
- Gupta, G. and Maniker, A. (2007) Malignant Peripheral Nerve Sheath Tumors. Neurosurgical Focus, 22, E12.
- Angelov, L., Davis, A., O’Sullivan, B., Bell, R. and Guha, A. (1998) Neurogenic Sarcomas: Experience at the University of Toronto. Neurosurgery, 43, 56-65. http://dx.doi.org/10.1097/00006123-199807000-00035
- Woodruf, J.M., Selig, A.M., Crowley, K. and Allen, P.W. (1994) Schwannoma (Neurilemmoma) with Malignant Transformation: A Rare Distinctive Peripheral Nerve Tumor. American Journal of Surgical Pathology, 18, 882-895. http://dx.doi.org/10.1097/00000478-199409000-00003
- George, E., Swanson, P.E. and Wick, M.R. (1989) Malignant Peripheral Nerve Sheath Tumors of the Skin. The American Journal of Dermatopathology, 11, 213-221. http://dx.doi.org/10.1097/00000372-198906000-00004
- Dabski, C., Reiman Jr., H.M. and Muller, S.A. (1990) Neurofibrosarcoma of Skin and Subcutaneous Tissues. Mayo Clinic Proceedings, 65, 164-172. http://dx.doi.org/10.1016/S0025-6196(12)65011-3
- Kikuchi, A., Akiyama, M., Han-Yaku, H., Shimizu, H., Naka, W. and Nishikawa, T. (1993) Solitary Cutaneous Malignant Schwannoma. Immunohistochemical and Ultrastructural Studies. The American Journal of Dermatopathology, 15, 15-19. http://dx.doi.org/10.1097/00000372-199302000-00003
- Demir, Y. and Tokyol, C. (2003) Superficial Malignant Schwannoma of the Scalp. Dermatologic Surgery, 29, 879-881.
- Garg, A., Gupta, V., Gaikwad, S.B., Mishra, N.K., Ojha, B.K., Chugh, M. and Sharma, M.C. (2004) Scalp Malignant Peripheral Nerve Sheath Tumor (MPNST) with Bony Involvement and New Bone Formation: Case Report. Clinical Neurology and Neurosurgery, 106, 340-344. http://dx.doi.org/10.1016/j.clineuro.2004.01.003
- Fukushima, S., Kageshita, T., Wakasugi, S., Matsushita, S., Kaguchi, A., Ishihara, T. and Ono, T. (2006) Giant Malignant Peripheral Nerve Sheath Tumor of the Scalp. The Journal of Dermatology, 33, 865-868. http://dx.doi.org/10.1111/j.1346-8138.2006.00197.x
- Kumar, P., Jaiswal, S., Agrawal, T., Verma, A., Verma, A. and Datta, N.R. (2007) Malignant Peripheral Nerve Sheath Tumor of the Occipital Region: Case Report. Neurosurgery, 61, E1334-E1335. http://dx.doi.org/10.1227/01.neu.0000306115.85668.ec
- Yamada, K., Harada, M., Kunitoku, N., Goto, S., Kochi, M. and Ushio, Y. (2004) MR Imaging Features of a Scalp Plexiform Schwannoma. American Journal of Neuroradiology, 25, 291-294.
- Cotran, R.S., Robbins, S.L. and Kumar, V. (1994) Robbins Pathologic Basis of Disease. WB Saunders, Philadelphia.
- Aguiar Vitacca, S., Sarrazin, D., Henry-Amar, M., Spielmann, M., Genin, J., Bernheim, A., et al. (1992) Neurosarcoma Associated with Von Recklinghausen Disease: Apropos of 25 Cases Observed at the Gustave Roussy Institute from 1967 to 1990. Bull Cancer, 79, 101-112
- Shintaku, M., Wada, K., Wakasa, T. and Ueda, M. (2011) Malignant Peripheral Nerve Sheath Tumor with Fibroblastic Differentiation in a Patient with Neurofibromatosis Type 1: Imprint Cytological Findings. Acta Cytologica, 55, 467-472. http://dx.doi.org/10.1159/000330676
- Jhawar, S.S., Mahore, A., Goel, N. and Goel, A. (2012) Malignant Peripheral Nerve Sheath Tumour of Scalp with Extradural Extension: Case Report. Turkish Neurosurgery, 22, 254-256.
- Voth, H., Nakai, N., Wardelmann, E., Wenzel, J., Bieber, T., Wendtner, C.M., Reinhard, G. and Schmid-Wendtner, M.H. (2011) Malignant Peripheral Nerve Sheath Tumor of the Scalp: Case Report and Review of the Literature. Dermatologic Surgery, 37, 1684-1688.

