International Journal of Clinical Medicine
Vol.5 No.14(2014), Article
ID:48133,16
pages
DOI:10.4236/ijcm.2014.514115
PPPM (Predictive, Preventive and Personalized Medicine) as a New Model of the National and International Healthcare Services and Thus a Promising Strategy to Prevent a Disease: From Basics to Practice
I. A. Sadkovsky1, O. Golubnitschaja2,3, M. A. Mandrik1, M. A. Studneva1, H. Abe4, H. Schroeder5, E. N. Antonova1, F. Betsou6, T. A. Bodrova7, K. Payne8, S. V. Suchkov1,9,10
1I.M. Sechenov First Moscow State Medical University, Moscow, Russia
2European Association for Predictive, Preventive and Personalized Medicine (EPMA), Brussels, EU
3Rheinische Friedrich-Wilhelms-University of Bonn, Bonn, Germany
4International Society for Personalized Medicine (ISPM), Tokyo, Japan
5Department of Medicine, Genetics and Comparative Pathology, University of Alabama at Birmingham (UAB), Birmingham, USA
6Université du Luxembourg and IBBL (Integrated BioBank of Luxembourg), Luxembourg, EU
7National Research University “Higher School of Economics”, Moscow, Russia
8Institute of Population Health and Health Economics, University of Manchester, Manchester, UK
9A.I. Evdokimov Moscow State Medical & Dental University, Moscow, Russia
10United Cultural Convention (UCC), Cambridge, UK
Email: ivan.sadkovsky94@mail.ru
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 27 May 2014; revised 23 June 2014; accepted 15 July 2014
ABSTRACT
Nowadays the system of public health is constructed in such a manner so that its main objective is the recovery of an already sick person, while prediction and prevention receive little attention. Meanwhile the development of these aspects of medicine can lead to the ability to control morbidity among the population, to identify chronic and genetic diseases in the early stages of development, and thus to prevent their further progression. This will reduce traditionally high costs of sick people treatment and the number of disabled population, and improve the quality and duration of life. The elaboration of new fields of science that are working on the study and interpretation of data obtained during laboratory and clinical research, creation of new methods for diagnosis, prognosis and treatment, provides an opportunity now to implement a new strategy, called PPPM, and gets promising results, which should lead to further development of an existing medicine.
Keywords:Predictive, Preventive, and Personalized Medicine, Biomarkers, Subclinical, Metabolomics, Genomics, Proteomics, Bioinformatics, Public Health, Predisposition

1. Introduction
Over the course of its history, medical science was much more focused on the treatment of clinical illnesses that have already arisen, than on detecting and preventing their occurrence. Practical experience over many years has shown that this approach is often ineffective against a number of diseases, and also requires a great deal of expenses [1] -[3] .
Medicine and thus healthcare services are now undergoing a major revolution that will transform the nature of healthcare from reactive and palliative to preventive and guaranteed [4] . The changes will be catalyzed by a new system approach to disease that will trigger the emergence of PPPM (Predictive, Preventive and Personalized Medicine)—a medicine that focuses on the integrated diagnosis, treatment and prevention of disease in individual patients (Figure 1).
Predicting the future is not a new calling neither even a new challenge. But unlike the predictions of the oracles of antiquity or new-age fortunetellers, PPPM is based on the science achievements that help to prevent the development of disease before symptoms appear. Most of the chronic disorders develop gradually over a period of time. It may take years to reach a point to be diagnosed definitively [3] -[5] .
To achieve the practical implementation of PPPM concept, it is necessary to create a fundamentally new strategy based upon the pre-early (subclinical) recognition of biomarkers of hidden imbalances long before the illness clinically manifests itself. This strategy would give a real opportunity to secure preventive measures whose personalization could have a significantly positive influence on demographics!
NIH (National Institutes of Health) has added PPPM to a list of the five most prioritized branches of the development in 21st century.
PPPM is also being actively supported and promoted by the European Commission, by FDA and by CDC.
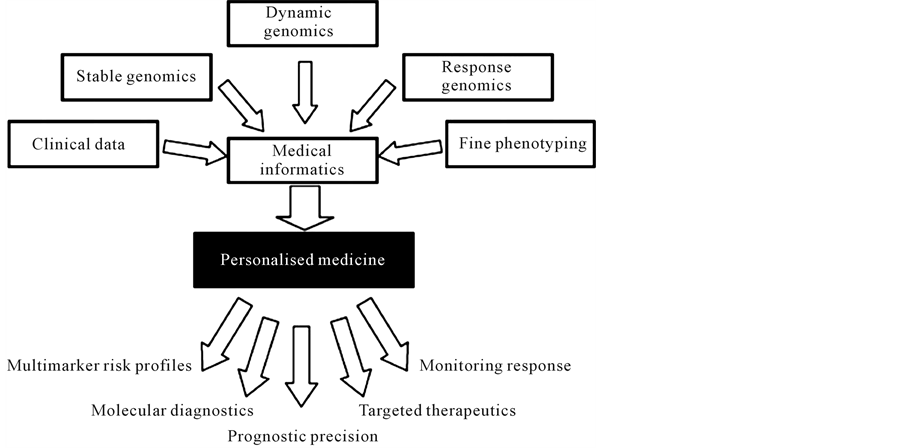
Figure 1. The scheme depicts the underpinnings of personalized medicine and principle approaches to be included into the PPPM protocols.
PPPM actively employs the latest scientific achievements, which help prevent disease, and thereby avoid a failure of performance, quality of life and life expectancy.
Within this framework, a key role is given to the prediction and prevention diseases. Therefore, the search for new methods of diagnosis which would allow identifying the disease at an early stage, before the formation of the pathological process and the development of effective means of drug-based prevention have become the main objectives of the medical science of the 21st century (Figure 2).
Thus, the main task PPPM includes:
• Determination of signs of the disease at the stage of preclinical pathology in conjunction with the identification of targets for adequate prevention;
• Targeted correction of the violations with the goal of pharmacological prevention, which belongs to the category of preventive measures that contribute to the suppression of the pathological process in the preclinical stage [3] .
2. Fundamentals of PPPM
PPPM as the big change to forecast, to predict and to prevent is rooted from the achievements of genomics, proteomics, metabolomics and bioinformatics which are being implemented into the daily practice to secure visualizing of lesion foci that was previously unknown to clinicians.
The change mentioned is rooted in new science covering systems biology and biomedicine and utilizing a new generations of translational tools to implement the new concepts and technologies into clinical practice. And therefore it is necessary to create a new strategy that takes into account individual features of the organism. Complete diagnostics of the organism, the creation of individual genetic passports, development and correction of treatment strategies based on the individual features of genetics, metabolism, microbiota of particular organism—all these tasks are belongs to PPPM. However, holding such a colossal amount of expensive and complicated research would be impossible without translation of the latest scientific achievements into practical medicine [3] [6] -[8] (Figure 3).
2.1. Genomics
Genomics deals with the common principles of genome composition and function by analyzing DNA structure and function using a combination of sequencing and genetic polymorphism assays. The latter has revealed single nucleotide polymorphisms (SNPs) as families of genomic biomarkers that account for some of the genetic variability between individuals, and made possible the exploitation of genome-wide association studies (GWAS) to identify genetic variations and to thus define risks for common diseases (Figure 4).
In reality, genomics as a set of molecular tools to probe genome and to thus identify and to select genomic biomarkers has allowed for identifying newer genes and newer genetic variations that affect health to form subclinical risks to be screened and unveiled, then the subclinical pathology to be diagnosed, monitored and terminated to prevent illness [3] [9] .

Figure 2. The overarching idea of personalized (individualized) medicine with basic branches aimed at subclinical diagnosis and preventive measures.
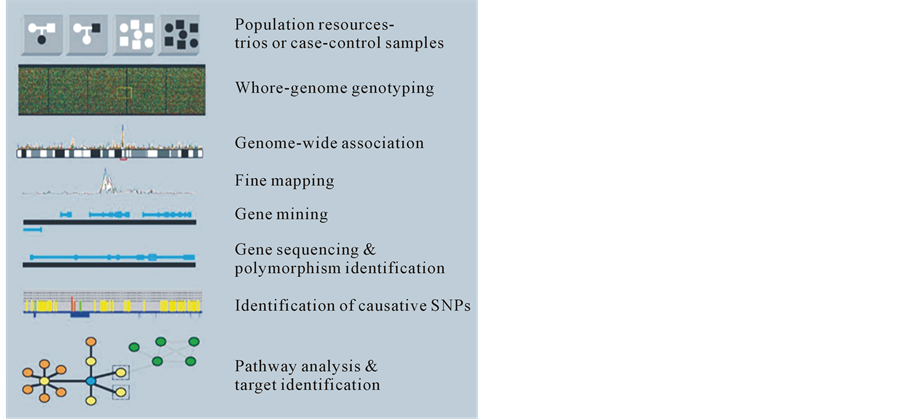
Figure 3. Ways to implementation of the main tasks of genomic diagnostics for new target identification and screening of causative mutations.
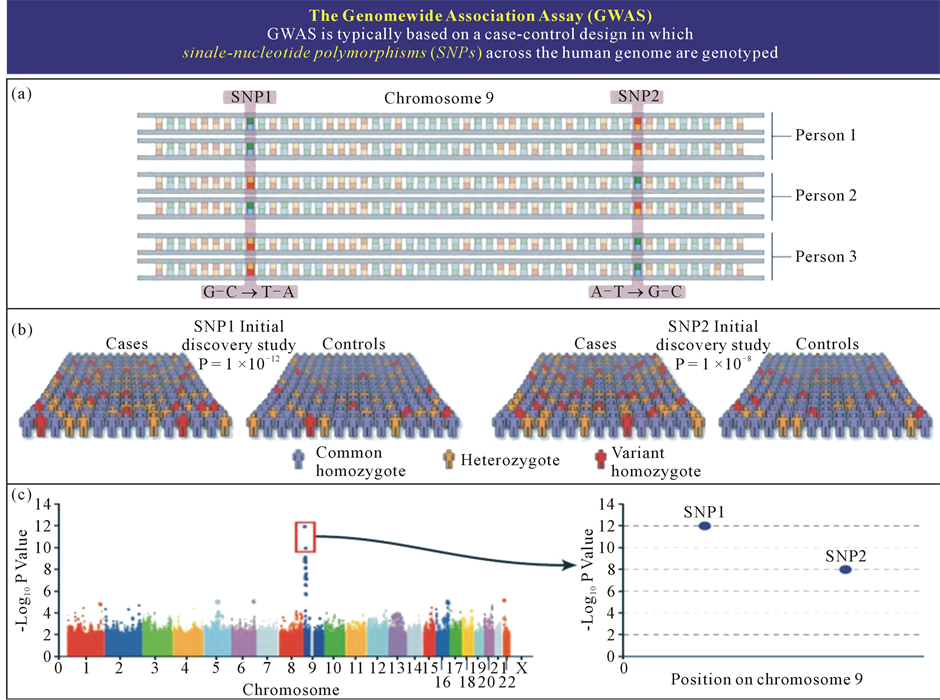
Figure 4. Bioinformatics as a valuable tool for processing of large data values in indicative research, genome-wide analysis and large-scale population studies (a). Simultaneous genotyping of more than 500,000 - 1,000,000 SNPs (b). Initial discovery study with large patient and control sample collection (c). Statistical analysis (probability plot for association with a certain disease) and independent replication of top results.
Among the best-validated genomic biomarkers are cancer-related and autoimmunity-related ones to be broadly known (Figure 5 and Figure 6).
As an allied portion of genomics and thus an area of study to examine the impact of genetic variations on the response to medications is pharmacogenomics.
The latter is aimed at tailoring drug therapy at a dosage that is most appropriate for an individual patient, with the potential benefits of increasing the clinical efficacy and safety. Pharmacogenomics will thus guide therapeutic decisions and monitor the response to therapy on one hand and speed the development of novel therapeutics, on the other one [10] .
Genomic information is now increasingly available out-side traditional medical settings to suit the model of PPPM. Patients can now instigate their own testing and often know more than their clinicians about particular genetic topics. Indeed, healthcare providers are increasingly bypassed altogether, as patients embrace direct-toconsumer (DTC) genetic tests and turn to social networks for help in interpreting their results.
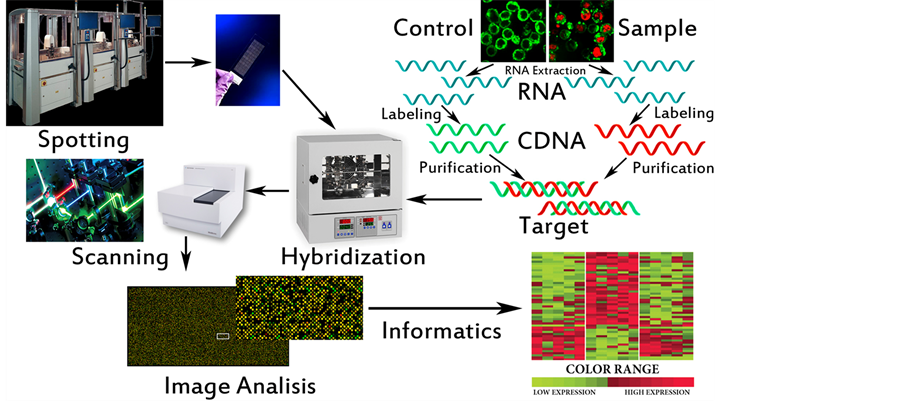
Development of genomics pathologies, including autoimmune, not only provides their molecular genetic diagnostics, but also allows determining the intensity of RNA and protein synthesis (collectively-transcriptome) associated with the occurrence and development of disease. The study of transcriptomics also referred to as expression profiling, examines the expression level of mRNAs in a given cell population, often using high throughput techniques based on DNA microarray technology. Also, recent advances in RNAi screening and next-generation sequencing technologies enable a synergistic application of all of these genomic technologies to the discovery of predictive biomarkers [3] [5] . Thus comparison of the transcriptome of normal and pathological samples allows to identify new biomarkers and biopredictors, control changes in their expression levels in time and, therefore, to make conclusions about the dynamics of disease and efficiency of treatment, predicting its outcome (Figure 7).
Genes can say a lot about an individual’s predisposition to a disease, but cannot reveal what is happening in
(a) Non-immune Genes (b) Immune Genes
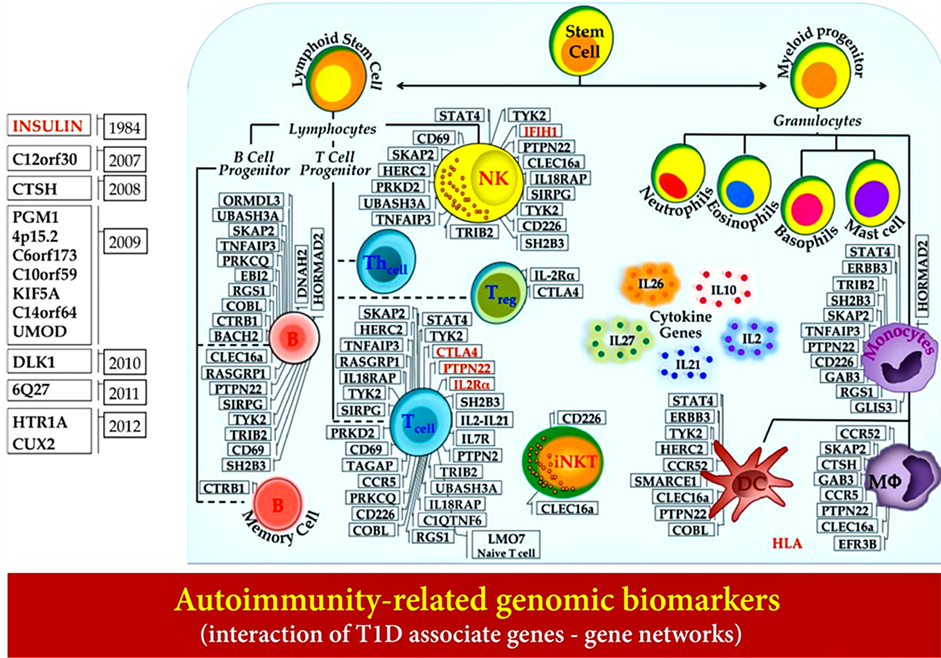
Figure 5. Autoimmunity-related genomic biomarkers (interaction of T1D associated genes-gene networks).
 (a) (b)
(a) (b)
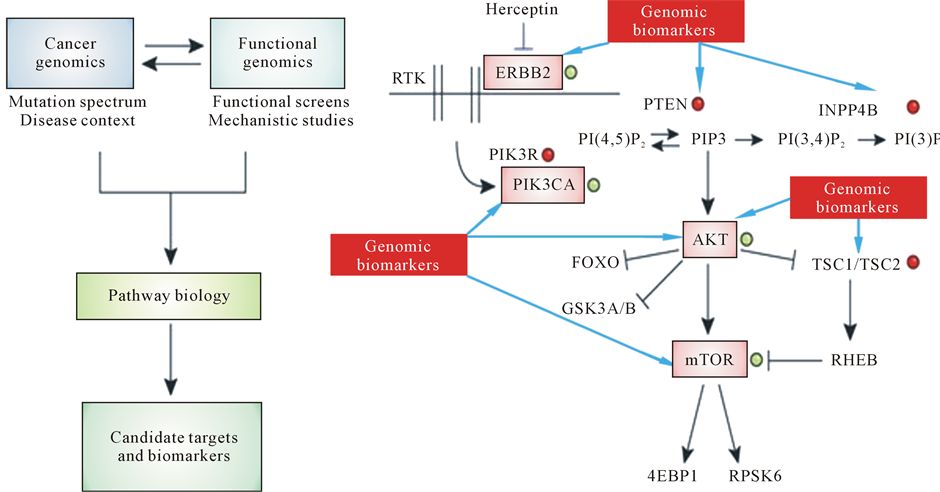
Figure 6. Genomic biomarkers and their impact into pathway-targeted cancer therapies. (a) Routine sequencing of cancer genomes will identify many new genes that are involved in cancer. (b) Detailed mechanistic studies will be required to determine how these genes contribute to tumorigenesis and how they influence therapeutic efficacy. Black arrows and lines illustrate the gene interactions in signaling pathways of cancerogenesis. Black arrows mean induction and lines mean repression.
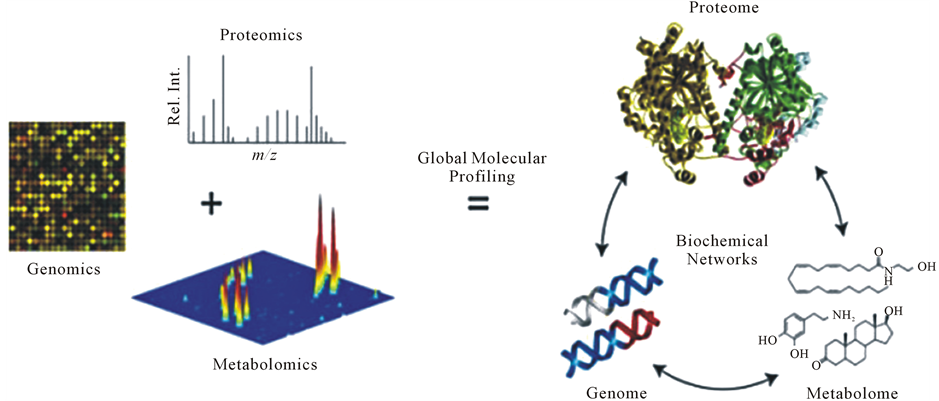
Figure 7. The interrelations between the basis of PPPM (genomics, proteomics, and metabolomics) and their application for global molecular profiling.
cells at the protein level. The latter would attribute to proteomics to identify within interactomes individual proteins and their epitopes to be valuable for predictive diagnosing and thus may eventually have a great impact on PPPM.
Proteomics
Proteomics provides opportunity to characterize the cell within the framework of current point of time by fixing all of its RNA transcripts and proteins in real time.
Proteomic analysis is focused on the simultaneous study of many individual proteins and consequently researches include immunochemical tests, micro sequencing of proteins, high performance liquid chromatography (HPLC) and mass-spectrometry as well as application protein microarrays with various types of detection. Such approach allows to detect subclinical defects and imbalances that are suitable for preventive intervention, including cancerand autoimmunity-related ones [3] .
A combination of genomic and proteomic biomarkers are becoming of great significance to predict risks of the chronization and thus of the disabling since chronic diseases are preceded by a long subclinical (symptomfree) phase or a period of latency (Figure 8).
At this point, most of the FDA approved targeted therapeutics are directed at proteomic, not genomic biomarkers. And protein-based assays were the first “companion diagnostic” assays to comprise both diagnostic and therapeutic resources and to be approved by FDA (Figure 9).
2.2. Metabolomics
Metabolomics shows the functional state of the cell in real time at the level of its metabolism, requiring use of the term “metabolome”, which reflects the totality of all the metabolic pathways in the cell at a given time, which makes metabolomics highly effective means of early detection of disease, monitoring of treatment outcomes and toxic impact on the body (Figure 10).
Metabolomics is based on combined application of the methods of physical chemistry (HPLC, gas chromatography, etc.) and computer analysis (pattern recognition). Progress in this field has led to the creation of methods such as metabolic fingerprinting (classification bioassay based on the characteristics of involved metabolites) and metabolic profiling (analysis of metabolites belonging to the same chemical class or a specific biochemical pathway), which allow to define thousands of metabolites in the bioassay. In fact, metabolomics today is development of global profile of metabolite concentrations in the sample in order to detect metabolic changes, that are inherent to the point of initiation of the disease and its dynamics as well as regularities metabolic response to therapy.
However, for correlation data with the overall picture of metabolism and prediction of the nature of the interlayer interaction based on the received regularities, we need to implement their data integration and visualisation. Bioinformatics is designed to solve this task using mathematical modeling.
Bioinformatics
Over the past 40 years, science has accumulated tremendous amount of experimental and theoretical material in
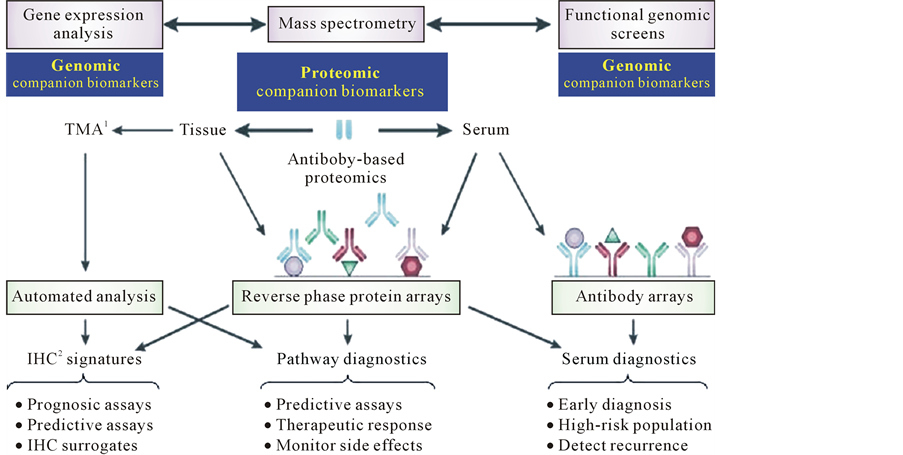
Figure 8. Discovering of proteomic companion biomarkers. IHC1: immunohistochemistry. TMAs2: tissue microarrays.
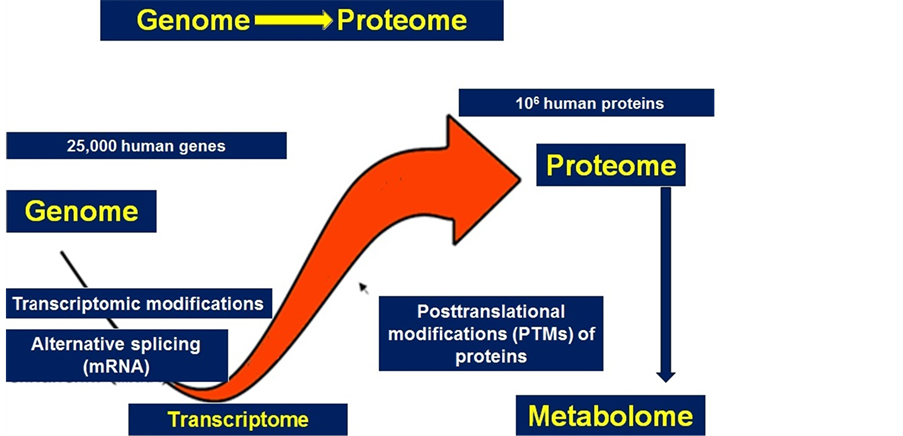
Figure 9. In reality, proteomics per se is the continuation of functional genomics and, at the same time, a prologue to metabolomics.
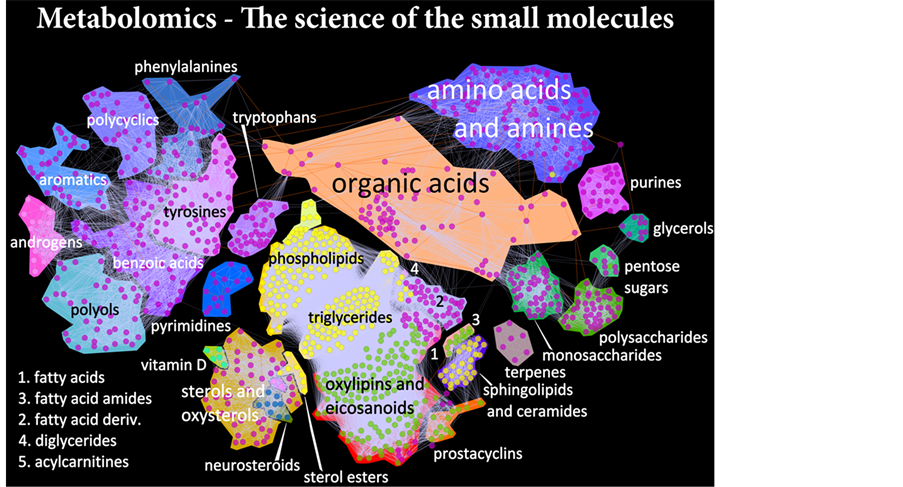
Figure 10. Metabolomics-the science of the small molecules.
the field of molecular biology. Huge amount of data on the structure and functioning of biological molecules (proteins and nucleic acids) require advanced computer methods for their analysis and development of databases on the basis of the received information. Therefore a new science has appeared—bioinformatics, combining methods and approaches of computational molecular biology (Figure 11). Depending on the task, bioinformatics can be divided into several areas:
2.3. Sequence Analysis
Development of effective methods of decoding the nucleotide sequences resulted in automation of research and the accumulation of large amounts of information, in which it is impossible to find or compare (align) any parts of nucleotide sequences without application of computer methods.
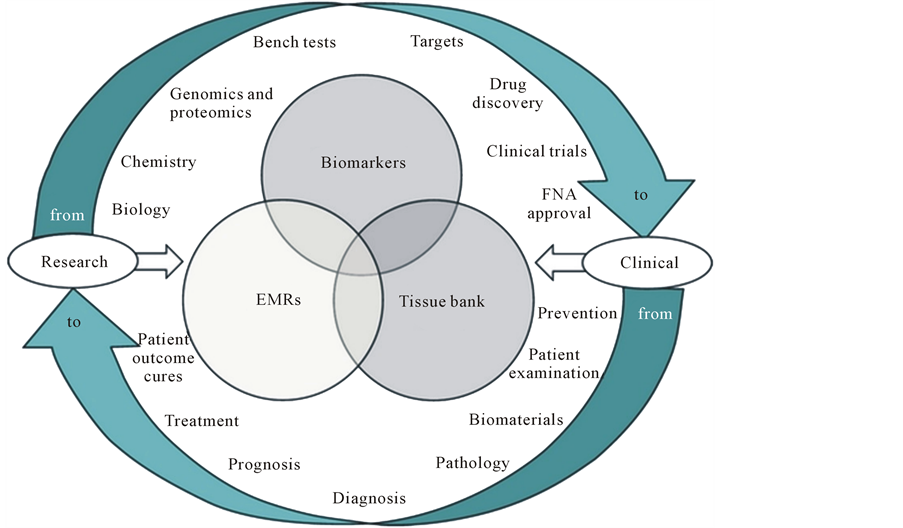
Figure 11. Knowledge received from scientific researches, as well as from clinical practice is exposed to careful processing and interpretation through bioinformatics.
2.4. Structural Bioinformatics
The unique spatial structure of a protein is one of its most important characteristics. Since the most drugs are chemicals whose molecules are able to form complexes with protein molecules, thereby influencing their action, the study of protein structure, finding binding sites to determine the structure of protein complexes with drug molecules are important objectives of bioinformatics.
2.5. Computer Genomics
Definition of full nucleotide sequence is only step to understanding the functioning of the cell. Application of computer analysis can significantly accelerate these studies that promotes to effectively search of potential drug targets, and studying of the metabolic pathways that can be used in biotechnology of future.
Biobanks
Biobanks would provide the proper information about a patient’s proteomic, genetic and metabolic profiles to be used to tailor medical care due to the individual’s needs and personalized scenarios. An under-standing of the factors underlying the burden of a disorder and later on of the clinical illness would provide policymakers, healthcare providers and medical educators with an opportunity to guide preventive initiatives at both individual and community levels.
Prevention cannot be imagined without the use of a personalized approach, which involves the creation of individual databases that should be fully structured and analyzed by bioinformatics tools (Figure 12).
3. PPPM as a Technological Armamentarium of a New Generation to Get It into the Practice Implemented
Currently, there is an integration of fundamentals of PPPM, which described above, in clinical practice. PPPM uses a new generation of diagnostic tests, based in particular on the knowledge of genomic, proteomic and metabolomicsbiomarkers that allow us to individually determine the health status of a person predisposed to the disease and to reveal the agents of the probable or the already existing pathology.
Predictive branch of PPPM is mainly designed to meet the interests of healthy individuals, its purpose being
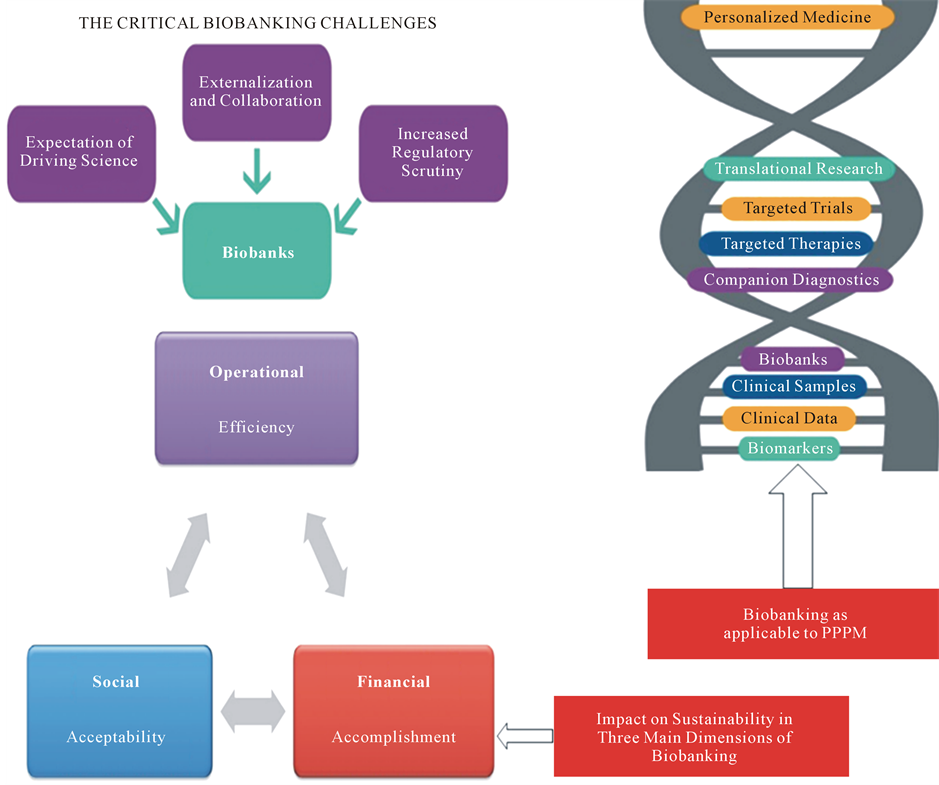
Figure 12. Biobankingas applicable to PPPM.
to determine whether susceptibility to a particular disease is increased or not.
Preventive branch is aimed at taking measures to avoid development of clinical manifestations rather than cure or treat it on manifestation.
Personalized medicine proposes the customization of healthcare tailored to the individual patient and/or to the person-at-risk by the mutual integration of family history, medical records and other information including genomic, proteomic and metabolomic biomarkers-based profiles to be integrated via bioinformatics [11] [12] .
In general, key benefits of PPPM to the patient, person-at-risk and the system would include new abilities to:
• Detect disease at an earlier stage, when it is easier and less expensive to treat effectively;
• Stratify patients into cohorts that enable the selection of optimal and preventive therapy;
• Improve the selection of new targets for drug discovery;
• Shift the emphasis from reaction to prevention and from disease to wellness.
Generally, there is a multilevel infrastructure to demonstrate and to operate three levels desirable for providing optimal subclinical and clinical medical care services:
It includes:
1) Determining genetic predisposition to a defined pathology to utilise updated protocols of genotyping.
This step requires the use of such technologies as genetic polymorphism testing and DNA sequencing, as well as the analysis of information available from the genealogical tree, anamnesis morbi, and anamnesis vitae (Figure 13(a) and Figure 13(b)).
Technologically, those goals can be accomplished by BioChip methodology (every disease has individual
 (a)
(a)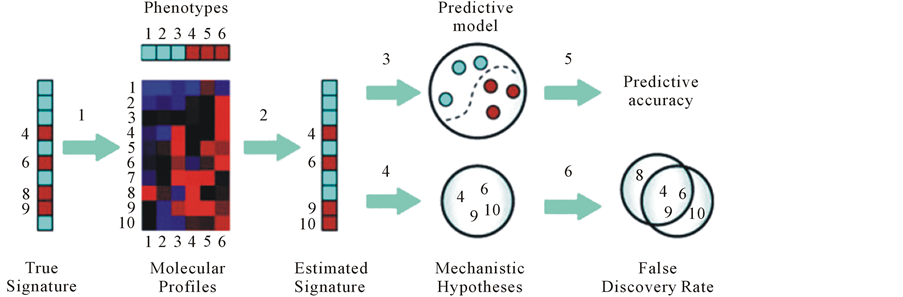 (b)
(b)
Figure 13. The fundamentals of BioChip methodology.
fingerprints and/or molecular signatures: changes in gene expression/transcription levels that are indicative of a nosology) [3] [13] .
2) Survey using the target group phenotypic biomarkers (phenotyping protocol) of patients selected at the first stage.
3) Application of preventive measures including monitoring of dynamic changes in the levels of biomarkers and biopredictors and precise control of physiological reactions in response to drug-based preventive measures.
We illustrate the ability to predict based on the diagnostic tests, regardless of basic mechanism of disease:
а) HLA-related biomarkers in combination with autoAbs to monitor chronic autoimmune disorders (T1D, MS, SLE).
b) genomic biomarkers in combination with cancer-associated antigens to monitor cancerogenesis.
For chronic autoimmune diseases there are two typical examples that best illustrate the specificity of the topic: T1D and MS.
The basis of autoimmune diseases is a universal degenerative and inflammatory process, which comprises a number of stages, including the stage of subclinical pathology.
Each stage is proved to be determined by a set of specific parameters, i.e.:
a) Appearance of anti-islet autoAbs and an upsurge in the autoAbs titers in T1D, the most important biopredictive factor of T1D at the subclinical stage.
b) Gene expression products of the key (e.g., functional transcripts) and anti-myelinautoAbs with proteolytic activity directed towards myelin antigens (Abs-proteases) [2] .
It was found that the main factor of the clinical manifestations of chronic underlying disease is postinfectious autoimmune syndrome (PIFAS), which was then proposed as a universal model of autoimmune disease (Figure 14).
From HLA-related biomarkers most informative genetic markers that increase the risk of developing T1D are DR4, DR3, DQ, DR2, DR6, and DR7, as their certain combinations promote the progression of PIFAS as a biopredictor of T1D-related clinical illness at a subclinical stage.
Concerning MS, the most important and informative gene combinations that have to date been associated with MS include 509 TGFB1, C DRB*18(3), CTLA4*G and 238TNF*B1, 308TNF*A2, and CTLA4*G. Such combinations support the formation of PIFAS-related signs at the subclinical stage, which are highly informative biopredictors to monitor a process of demyelination signs at the subclinical stage, which are highly informative biopredictors to monitor a process of demyelination [3] [14] -[16] .
Applicable to predictions of autoimmune diseases, proteomics is not less important than genomics (Figure 15).
It is known that T1D patients begin expressing auto Abs as early as 5 - 10 years before the clinical onset of the disease. Most of the data available indicate that this phenomenon also occurs in SLE, RA, Addison’s disease, celiac disease, and MS [3] [7] .
A combination of these data with the geno-and phenotyping of persons having both categories (genomics-and proteomics-related ones simultaneously) allows to significantly increase the predictive index (up to 85% - 90%), thereby improving the selection of persons for further drug-based therapeutic prevention.
With regard to cancer diseases, it is known that tumor initiation is provided by oncogenic mutations and inactivation of tumor-suppressor genes and depends on the stepwise acquisition of specific functions by cancer stem cells (CSC) and circulating tumor cells (CTCs) which may be defined by genomic tailoring approach on one hand and proteomic and immunonomic approaches, on the other hand (see Figure 6).
A strategy of preventive treatment should contain, at least, two critical steps.
For chronic autoimmune and/or infectious diseases:
1) Quenching of autoaggression or blocking the infectious process;
2) Restoration of the tissue affected.
For cancerogenesis:
1) Killing the malignancy and prevention of metastatic formation;
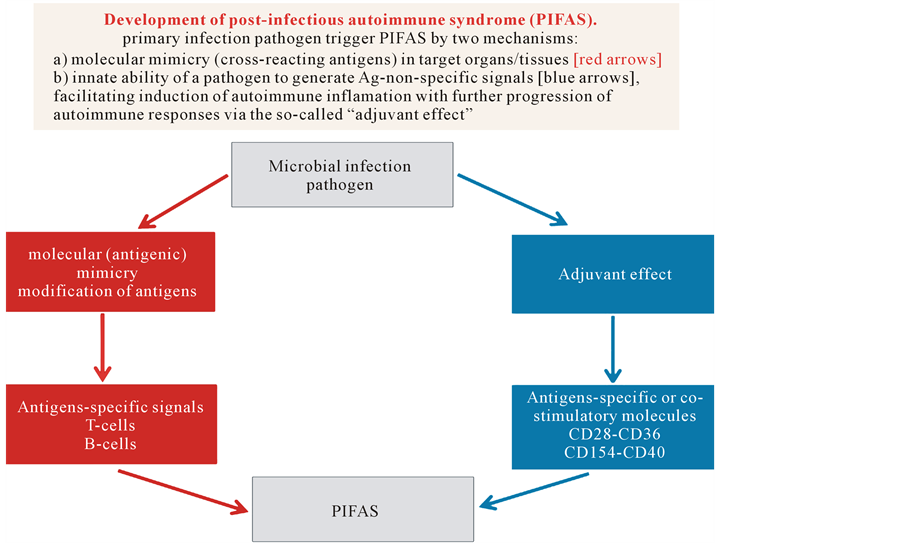
Figure 14. Two major mechanisms of PIFAS: molecular mimicry and adjuvant effect that are usually the common causes of most autoimmune diseases.
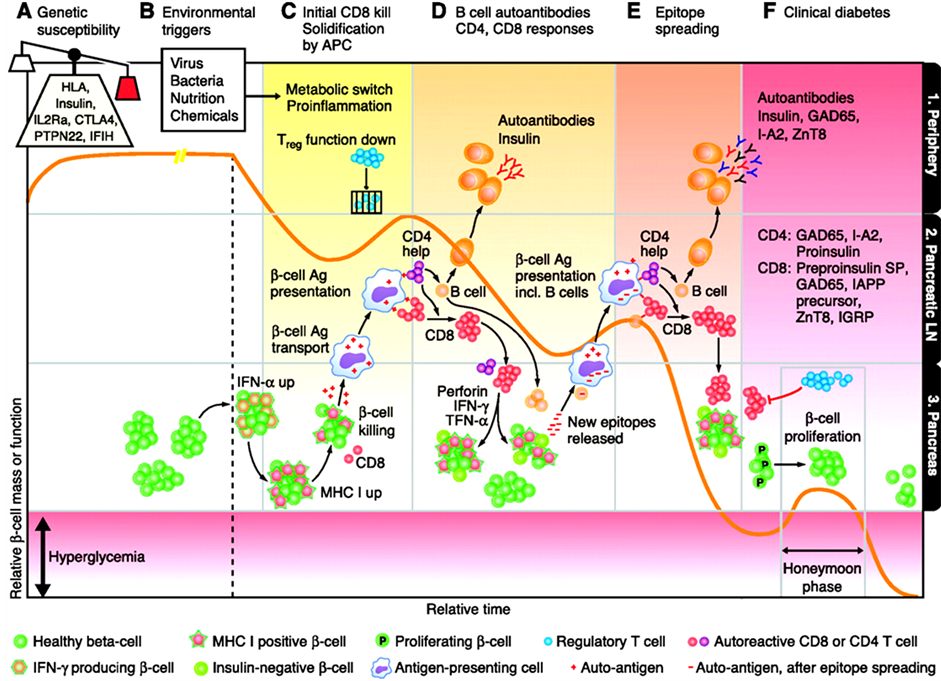
Figure 15. Autoantibodies and diabetes risk.
2) Restoration of the primary tissue affected.
The latter can successfully be achieved through the practical realisation of a number of strategies, particularly celland/or gene-based therapy, allogenic or xenogeneic transplantation, and stem cell technologies. In addition, a principally new technology that holds promise in modern medicine is the application of Abs-proteases as novel tools for drug-based therapeutic prevention [3] [17] .
4. Conclusions
21 century marked the beginning of a global transformation in medicine, which characterized by a changing in the fundamental principles of treatment and prevention of diseases. Translation of the advanced discoveries of fundamental sciences directly into clinical practice not only provides creating of new methods and the campaigns which are designed for “protection of individual health”, but also allows focusing on diagnostics of diseases, that have not yet arisen, and consequently gives an opportunity to prevent them.
PPPM is a new healthcare model that notifies people of the health conditions they’re disposed to and it reveals the biomarkers and thus the agents to improve and to thus secure the health and individual biosafety. Meanwhile, implementation of PPPM would require the adjusted technology for proper interpretation of diagnostic and predictive data before the current model “physician-patient” could be gradually displaced by a “medical advisor-healthy persons-at-risk” model.
This approach should be based on postulates which will change the incarnate culture and social mentality [3] .
First of all, it is the impact of human responsibility for the own health and for the health of their children, and active involvement into the preventive measures for strengthening of the public health and country’s biosafety.
Secondly, a creation of legal basis to satisfy all society needs for the protection of individual health—regulations of the state insurance in the PPPM.
And, for sure, it’s necessary to radically change the system of medical training, and designing novel approaches to build the academic schools of new generations.
Due to our viewpoint, all healthcare professionals of the future should be educated to deliver patient-centric care as members of inter-disciplinary teams, emphasizing evidence-based practice, quality improvement approaches and bioinformatics. That concerns the need for novel training programs since the society is in bad need of large-scale dissemination of novel systemic thinking and minding. And upon construction of the new educational platforms in the rational proportions, there would be not a primitive physician created but a medical artist to be able to enrich flow-through medical standards with creative elements to gift for a patient a genuine hope to survive but, in turn, for a person-at-risk—a trust for being no diseased. So, the existing medical education would strongly need to be restructured to involve along with traditional graduate and post-graduate training, pre-graduate preliminaries to disclose for schoolchildren the mysteries of the evidence-based medicine and PPPM as the entity.
Currently, many of countries are stressing their national goal to establish a continuous and intensive system of education throughout life consciously. In addition to basic educational programs, it is important to include in the training program of additional education, which provides an individualization of educational process and assists the children to build their own pathway to learn and to know, whilst disclosing their own talents.
The process of vocational training of highly skilled specialists in the new generation involves 10 - 12 years of the general and 4 - 5 years of vocational training. Thus, a multistep procedure of forming a competent specialist should not be restricted by the post-university internship training, but be broadened at a pre-university (secondary school) stage, in other words, simultaneously with the profiling seminars and workshops to secure the positive mental motion. In this regard, we suggest creating classes, which will specialize on medicine and biology at schools where students are aimed at the participation in health care modernization. The system of selection of talented youth and its involvement in scientific activity is important at a pre-university level.
The training of specialists who are able to build the interdisciplinary structure of health protection will be based on the new generation principles, taking into consideration the following:
• Peculiarities of introduction of school-university partnership onto international educational ground;
• Role of extra-classes education as the basis for such ground;
• Project principles in the framework of extra-classes education and design of research and engineering games;
• Tough necessity of multi-level testing and dialogues in the partnership student-teacher, their personal features considered, while forming vocationally self-sufficient, promising and highly-skilled specialist with personality for the future;
• Importance of innovation-related risks of educational process and possibility to manage those risks in crises.
The integration of science, education and production has an important role in the quality improvement of science staff training at the undergraduate and postgraduate levels. Such cooperation gives the unique opportunity to combine training on the basis of the fundamental knowledge received at university, with practical experience of production work. At the same time association of the large integrated production structures with universities will allow solving a problem of shortage of experts at the enterprises and in the scientific organizations of a high-tech complex. So, one of the most effective forms of science and education integration is the creation of basic professional chairs at the enterprises and the research laboratory in higher education institutions.
Based on current trends and own experience, we have tried a non-canonical approach towards reshuffling the traditional educational tandem “school-university” to create a team of talented and gifted teenagers to be engaged into PPPM-related areas. The team has been given a roof under the aegis of European Association of Predictive, Preventive and Personalized Medicine (EPMA), Brussels, EU, and started up to move ahead now.
Our global challenge is that the new guidelines should create the robust juristic and economic platforms for advanced medical services utilizing the cost-effective models of risk assessments followed by tailored preventive treatments focused on the precursor stages of chronic diseases.
5. Comments
Individuals to be under regular monitoring that helps to detect pathological shifts at subclinical stages have a higher life expectancy and are able-bodied up to 8 - 15 years more than those under traditional treatment. This means that the society would save more than US $20,000 - 40,000 per person annually [18] .
At the community level, the annual savings from each individual may vary from several thousands to several tens of thousands US dollars. In the area of oncology, for instance, the latter means that as little as a 10 percent reduction in cancer would translate into a savings of 4.4 trillion US dollars to society. As you might feel, besides the scientific and clinical challenges, there are economic hurdles.
So, PPPM promises to sharply reverse the ever-escalating costs of healthcare-introducing diagnosis to stratify patients and disease, decrease high cost of approaches to drug discovery, preventive medicine and wellness. Already now, the implementation of aspects of PPPM improves the results of treatment, as well as expands the powers of the physician and the patient’s ability. To ensure the widespread introduction of PPPM it is necessary to eliminate the key technical and societal (ethical, societal, policy, legal, economic, etc.) barriers, that requires a total and comprehensive transformation of Health Care System. On the speed they will be implemented with depends the quality and duration of life not only of the future but also of the present generations.
The opportunity arises for unusual and, even extraordinary, strategic partnerships between governments, academic and business sectors. The healthcare industry, public policy sector, and consumer industries will be required to develop new and creative business models and products.
And, no doubt, next generations will speak about the XXI century as a time, when medicine became preventive and personified, and its outcomes—predictive and guarantied.
References
- Ray, R. (2012) The Future of Medicine. American Journal of Medicine, 125, 236-239. http://dx.doi.org/10.1016/j.amjmed.2011.06.035
- Gabibov, A.G., Paltsev, M.A. and Suchkov, S.V. (2011) Antibody-Associated Proteolysis in Surveillance of Autoimmune Demyelination: Clinical and Preclinical Issues. Future Neurology, 6, 303-305.http://dx.doi.org/10.2217/fnl.11.17
- Bodrova, T.A., Kostyushev, D.S., Antonova, E.N., Slavin, S., Gnatenko, D.A., Bocharova, M.O., Legg, M., Pozzilli, P., Paltsev, M.A. and Suchkov, S.V. (2012) Introduction into PPPM as a New Paradigm of Public Health Service: An Integrative View. EPMA Journal, 3, 16. http://www.epmajournal.com/content/3/1/16
- Golubnitschaja, O. and Costigliola, V. (2010) Common Origin but Individual Outcomes: Time for New Guidelines in Personalised Healthcare. Personalized Medicine, 7, 561-568. http://dx.doi.org/10.2217/pme.10.42
- Nair, V.S., Maeda, L.S. and Ioannidis, J.P. (2012) Clinical Outcome Prediction by microRNAs in Human Cancer: A Systematic Review. Journal of the National Cancer Institute, 104, 528-540. http://dx.doi.org/10.1093/jnci/djs027
- Jones, T. and Price, P. (2012) Development and Experimental Medicine Applications of PET in Oncology: A Historical Perspective. Lancet Oncology, 13, e116-e125. http://dx.doi.org/10.1016/S1470-2045(11)70183-8
- Patel, N.R., McPhail, M.J., Shariff, M.I., Keun, H.C. and Taylor-Robinson, S.D. (2012) Biofluid Metabonomics Using (1)H NMR Spectroscopy: The Road to Biomarker Discovery in Gastroenterology and Hepatology. Expert Review of Gastroenterology and Hepatology, 6, 239-251. http://dx.doi.org/10.1586/egh.12.1
- Hood, L., Balling, R. and Auffray, C. (2012) Revolutionizing Medicine in the 21st Century through Systems Approaches. Biotechnology Journal, 7, 992-1001. http://dx.doi.org/10.1002/biot.201100306
- Tran, B., Dancey, J.E., Kamel-Reid, S., McPherson, J.D., Bedard, P.L., Brown, A.M., Zhang, T., Shaw, P., Onetto, N., Stein, L., Hudson, T.J., Neel, B.G. and Siu, L.L. (2012) Cancer Genomics: Technology, Discovery, and Translation. Journal of Clinical Oncology, 30, 647-660. http://dx.doi.org/10.1200/JCO.2011.39.2316
- Scott, S.A. (2011) Personalizing Medicine with Clinical Pharmacogenetics. Genetics in Medicine, 13, 987-995.http://dx.doi.org/10.1097/GIM.0b013e318238b38c
- Saubermann, A.J. and Lagasse, R.S. (2012) Prediction of Rate and Severity of Adverse Perioperative Outcomes: “Normal Accidents” Revisited. Mount Sinai Journal of Medicine, 79, 46-55. http://dx.doi.org/10.1002/msj.21295
- Lemke, H.U. and Berliner, L. (2011) Patient Specific Modeling and Model Guided Therapy. EPMA Journal, 2, 181-182. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3405420/
- Farra, N., Manickaraj, A.K., Ellis, J. and Mital, S. (2012) Personalized Medicine in the Genomics Era: Highlights from an International Symposium on Childhood Heart Disease. Future Cardiology, 8, 157-160.http://dx.doi.org/10.2217/fca.12.3
- Kostyushev, D., Tsarev, I., Gnatenko, D., Paltsev, M. and Suchkov, S. (2011) Myelin-Associated Serological Targets as Applicable to Diagnostic Tools to Be Used at the Preclinical and Transient Stages of Multiple Sclerosis Progression. Open Journal of Immunology, 1, 80-86.http://www.scirp.org/journal/PaperInformation.aspx?paperID=16662#.U3_Xavnud8E
- Jain, K.K. (2012) Role of Nanodiagnostics in Personalized Cancer Therapy. Clinics in Laboratory Medicine, 12, 15-31.http://dx.doi.org/10.1016/j.cll.2011.10.001
- Khitrov, A.N., Shogenov, Z.S., Tretyak, E.B., Ischenko, A.I., Matsuura, E., Neuhaus, O., Paltsev, M.A. and Suchkov, S.V. (2007) Postinfectious Immunodeficiency and Autoimmunity: Pathogenic and Clinical Values and Implications. Expert Review of Clinical Immunology, 3, 323-331. http://dx.doi.org/10.1586/1744666X.3.3.323
- Robinton, D.A. and Daley, G.Q. (2012) The Promise of Induced Pluripotent Stem Cells in Research and Therapy. Nature, 481, 295-305. http://dx.doi.org/10.1038/nature10761
- Sheiman, I. and Shishkin, S. (2010) New Challenges and New Objectives Problems of Economic Transition. Russian Health Care, 52, 4-49.http://connection.ebscohost.com/c/articles/49240513/russian-health-care-new-challenges-new-objectives

