Natural Resources
Vol.4 No.5(2013), Article ID:37169,6 pages DOI:10.4236/nr.2013.45050
Eco-Friendly Pest Control in Cucumber (Cucumis sativa L.) Field with Botanical Pesticides
![]()
1Institute of Environmental Science, University of Rajshahi, Rajshahi, Bangladesh; 2Department of Crop Science and Technology, University of Rajshahi, Rajshahi, Bangladesh.
Email: *akazad_ies@yahoo.com
Copyright © 2013 Abul Kalam Azad et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received July 11th, 2013; revised August 9th, 2013; accepted August 20th, 2013
Keywords: Cucumber; Chirata; Mahogany; Extract; Eco-Friendly
ABSTRACT
A field experiment on eco-friendly pest control in cucumber (Cucumis sativa L.) field was conducted at Rajshahi University during April, 2011 to June, 2011 with eight botanical pesticides prepared from the leaves and seeds of Bangladeshi plants. These botanicals are mahogany seeds, (Swietenia mahagoni), chirata leaves (Swertia chirata), jute seeds (Corchorus olitorius L.), garlic (Allium sativum L.), marigold leaves (Tagetes erecta) and carrot leaves (Daucus carota). One control treatment without botanicals was maintained during this experiment where only water was sprayed. Out of these botanicals, a less number of insect attacks on cucumber leaves (1.33 ± 0.19) were found in combined treatment of mahogany and chirata whereas a high number of insect attacks were observed in combined treatment of garlic and jute seed (5.89 ± 0.40) and control (4.66 ± 0.33). Individual application of chirata extract also showed good protection of cucumber leaves (1.67 ± 0.19) from insect attack. A smaller number of leaves perforations were found in the combined treatment of mahogany and chirata (3.44 ± 0.29) compared to control (14.22 ± 1.05). Chirata extract also showed good performance (4.00 ± 0.19) against leaves perforation of insect. Besides the pest control, botanical pesticides also have enormous effect on plant growth. The tallest cucumber plant was observed in the combined treatment of mahogany and chirata (469.00 ± 63.51 cm) and shortest in garlic treatment (84.56 ± 15.24 cm). The cucumber production was also high in combined treatment of mahogany and chirata (1863.33 ± 196.32 g) compared to control (1260.00 ± 501.63 g). From this study, it is found that combined application of mahogany and chirata extract not only showed good protection of cucumber plant from insect attack but also increased the cucumber production. Therefore, we conclude that farmers should use botanical pesticides from mahogany seeds and chirata leaves instead of toxic chemical insecticides for controlling pest in cucumber field.
1. Introduction
Cucumber (Cucumis sativus L.) is an important vegetable and one of the most popular members of the Cucurbitaceae family [1,2]. It is thought to be one of the oldest vegetables cultivated by man with historical records dating back 5000 years [3]. The crop is the fourth most important vegetable after tomato, cabbage and onion in Asia [4], the second most important vegetable crop after tomato in Western Europe [5]. In tropical Africa, its place has not been ranked because of limited use.
The cucumber is a creeping vine that roots in the ground and grows up trellises or other supporting frames, wrapping around supports with thin, spiraling tendrils.
The plant has large leaves that form a canopy over the fruit. The fruit of the cucumber is roughly cylindrical, elongated with tapered ends, and may be as large as 60 centimeters (24 in) long and 10 centimeters (3.9 in) in diameter. Having an enclosed seed and developing from a flower, botanically speaking, cucumbers are classified as accessory fruits. Much like tomatoes and squash they are often also perceived, prepared and eaten as vegetables. Cucumbers usually have more than 90% water content.
1.1. Brief History
The cucumber originated in India, where a great many varieties have been observed [6-8], from Cucumis hystrix [6,9]. It has been cultivated for at least 3000 years, and was probably introduced to other parts of Europe by the Greeks or Romans. Records of cucumber cultivation appeared in France in the 9th century, England in the 14th century, and in North America by the mid-16th century.
Cucumber is a long, green and cylinder-shaped edible fleshy fruit of a creeping plant (Cucumis sativus). A native to India, these plants have been cultivated for thousands of years. This fruit is used primarily for pickling and for slicing as a salad. In India, salad is incomplete without this green fruit. Cucumber raita, (mixture of cucumber and curd) is another popular recipe in India. Besides being widely used for culinary purposes, cucumbers are also used in facial creams, lotions, and cleansers. This anti-inflammatory agent is known for its astringent and soothing properties.
Cucumber is a warm season annual vining plant that produces stiff hairs on the leaves and stems which can be irritating to human skin when touched. Since this plant is herbaceous, it is easily susceptible to moisture stress. Its triangular leaves are simple, alternate and lobed, located at the base of the main axils. Perfect flowers are rare in cucumbers. Most cultivars produce separate male and female flowers on the same plant. For pollination, insects are required. Honey bees are the primary pollinators in the field.
Cucumber plant requires a well drained light friable soil for maximum yield. It prefers hot climate, i.e. a daily temperature is 65˚F - 75˚F. The plant requires proper irrigation for its vigorous growth. It needs water during blossoming and fruiting. The fruit is roughly cylindrical, elongated, with tapered ends, and may be as large as 60 cm long and 10 cm in diameter.
1.2. Nutritional Value
Cucumber, the edible fruit of Cucumis sativus is a member of the gourd family. It contains 95% water; a 50-g portion provides 0.3 g of dietary fibre and supplies 5 kcal (20 kJ). It is very good for skin and contains anti-inflammatory properties.
1.3. Importance of Eco-Friendly Pesticides
The use of conventional insecticides has raised some concern about their threat to the environment and development of insecticide resistance in insects [10], there is an imperative need for the development of safer, alternative crop protectants such as botanical insecticides. Current pest control technology is based largely on imported synthetic insecticides, which are frequently priced beyond easy reach of small farmers, who constitute a very large proportion of the farming population in Bangladesh.
Moreover, many insects have been reported to be resistant to chemical insecticides like malathion, DDT, lindane, demeton methyl, pyrethroids etc. [11,12]. The problems caused by pesticides and their residues have increased the need for effective, biodegradable pesticides with greater selectivity. Alternative strategies include the search for new types of insecticides and the re-evaluation and use of traditional botanical-pest control agents [13]. Bangladesh and many other Asian countries are rich in plant products and traditionally used by the rural inhabitants for medicinal purpose and in some instance as preparations for insect control [14]. Botanical insecticides tend to have broad spectrum activity, are relatively specific in their mode of action, and easy to process and use in farm levels. They are also safe for higher animals and the environment [15]. Botanical insecticides can often be easily produced by farmers and small-scale industries, indigenous plant materials are cheaper and hazard free in comparison to chemical insecticides [16]. Plants are rich sources of natural substances that can be utilized in the development of environmentally safe methods for insect control [17]. Crude plant extracts often consist of complex mixtures of active compounds, they may show greater overall bioactivity compared to the individual constituents [18,19]. The deleterious effects of crude plant extracts on insects were manifested in several ways, including toxicity [20], feeding inhibition [21,22]. Certain plant families, particularly Meliaceae, Rutaceae, Asteraceae, Labiatae, Piperaceae and Annonaceae were viewed as exceptionally promising sources of plant-based insecticides [23-25].
The research in botanical pesticides has a good scope of study. Bangladesh has a rich plant biodiversity. Therefore, this study was conducted to find out a suitable botanical pesticide from indigenous herbs, shrubs and plants of Bangladesh for eco-friendly pest management in cucumber field.
2. Materials and Methods
2.1. Experimental Plot Preparation
A field experiment was conducted at IES experimental plot, Rajshahi University, during 12th April 2011 to 15th June 2011. Grasses and weeds of experimental plot were removed and ploughed the land properly with spade. Appropriate amount of manure (cow-dung) and chemical fertilizers (triple super phosphate, muriate of potash and urea) were applied into soil during field preparation. Then, the seeds were planted in the experimental plot. The plot was irrigated with tap water when necessary.
There were nine treatments in this experiment and each treatment has three replications. Each replication has only one cucumber plant.
2.2. Cucumber Cultivation
Cucumber seeds were sown in 12th April, 2011 and fruits harvest completed by 15th June, 2011.
2.3. Botanicals and Control Treatments
Four individual botanical-extracts and four combined botanical extracts were tested in this experiment. Individual botanicals are mahogany seeds (Swietenia mahagoni); chirata leaves (Swertia chirata); jute seeds (Corchorus olitorius L.); garlic (Allium sativum L.), whereas combined botanicals are mahogany seeds and chirata leaves; jute seeds and chirata leaves; garlic and jute seeds, and marigold leaves and carrot leaves.
One control treatment without botanicals was maintained in this experiment. Only water was sprayed in this treatment.
2.4. Extraction of Botanical Pesticides
Leaves were dried at room temperature for seven days before grinding or cutting into small pieces. 100 g of grinding or cutting leaves and seeds were dissolved in one liter distilled water. The plant leaves and seed samples were kept in water for three days. Then solutions were filtered with plastic filter. The filtrated water-extracts were kept in refrigerator with plastic bottles until use.
2.5. Spraying Method
The botanical extracts were sprayed on experimental plants twice a week.
2.6. Pest Attack Counting
The pest attack was monitored every day and the damages were counted every 3 days in a week. The number of plants attacked, leaves attacked and the number of leaves perforation were recorded.
2.7. Data Processing and Statistical Analysis
Data on plant length, total leaves, insect attack, leaves perforation and cucumber production were received from the monitoring data of three plants that means three replications of each treatment. The data of three replications were first averaged treatment-wise and statistical analysis was done using one way ANOVA (P ≤ 0.05) followed by Duncan’s Multiple Range Test (DMRT) to identify the significant differences between the treatments. Those data were presented in table in results and discussion part.
3. Results and Discussion
3.1. Effect of Botanical Pesticides on Pest Control in Cucumber Leaves
Insect attacks on cucumber leaves were found to decrease significantly (P ≤ 0.05) with applications of botanical pesticides. Some researcher found that the use of botanicals such as neem and other bio-pesticides to control insect of vegetables are gaining much attention [26,27]. In this study, we found a less number of insect attacks on cucumber leaves in combined treatment of mahogany and chirata (1.33 ± 0.33), whereas a high number of insect attacks were found in the combination of garlic and jute seed treatment (5.87 ± 0.69) and control (4.66 ± 0.57). The individual application of chirata extract also showed good protection of cucumber leaves (1.67 ± 0.33) against pest (Table 1).
Cucumber leaves perforation was also found to decrease significantly with applications of botanical pesticides (P ≤ 0.05). Leaves perforation in cucumber field was regularly monitored. Water extract of combined treatment of mahogany and chirata showed best performance against cucumber leaves perforation (3.44 ± 0.51) caused by pests (Table 1). Individual application of water extract of chirata was also found very effective against leaves perforation (4.00 ± 0.33) compared to control (14.22 ± 1.83). Plant oils, neem oil (3%), C. citratus (0.05%), M. indica oil (3%) are reported to be effective against insect [28]. These observations are similar with the findings of some reports [29-32].
3.2. Effect of Botanical Extracts on Cucumber Plant Growth
The number of leaves per plant was significantly influenced by botanical pesticides (P ≤ 0.05). The highest number of leaves was found in mahogany seed treatment (33.67 ± 3.84) while the least number of leaves was in garlic treatment (11.11 ± 3.56) (Table 2). Some researcher reported that nutrients from mineral fertilizers and bio-pesticides enhanced the establishment of crops while those from the mineralization of organic matter promoted yield [33].
The plant length of cucumber was significantly enhanced by the application of botanical pesticides (P ≤ 0.05). The tallest cucumber plant was observed in the treatment of combination of mahogany and chirata (469.00 ± 110.01 cm) while the shortest plant was in the treatment of garlic (84.56 ± 26.41 cm). The vigorous growth in cucumber was experienced during growing period as evidenced in the vine length and number of leaves produced per plant (Table 2). Nutrients from mineral fertilizers and botanical pesticides enhanced the establishment of crops while those from the mineralization of organic matter promoted yield [34,35].
3.3. Effect of Botanical Pesticides on Pest Control
Pest attack was monitored regularly in cucumber field. We observed that the treatment of control was affected by many kinds of pests and insects (Epilachna beetle, red pumpkin beetle, striped cucumber beetle (Acalymma vittatum), ants, grasshoppers, and white mite) compared to less number of insect attack in botanical pesticides applied treatments. During this study, only one insect (black ants) was found in the treatment of combination of mahogany and chirata (Table 3).
Some studies reported that fruit borer populations were consistently lower with the application of eco gold, garlic extract and neem extract. Similar results from other studies in Asia and parts of Africa support these findings [36-38].
3.4. Effect of Botanical Extracts on Fruit of Cucumber
The fruit length of cucumber was significantly increased by the application of botanical pesticides (P ≤ 0.05). From this result, it is very clear that among the tested plant extracts, the combined extract of mahogany and chirata seeds treatment produced highest amount of cucumber (1863.33 ± 340.05 g), whereas a lowest cucumber production was found in garlic treatment (591.67 ± 41.93). The second highest production was observed in individual mahogany seed treatment (Table 4).
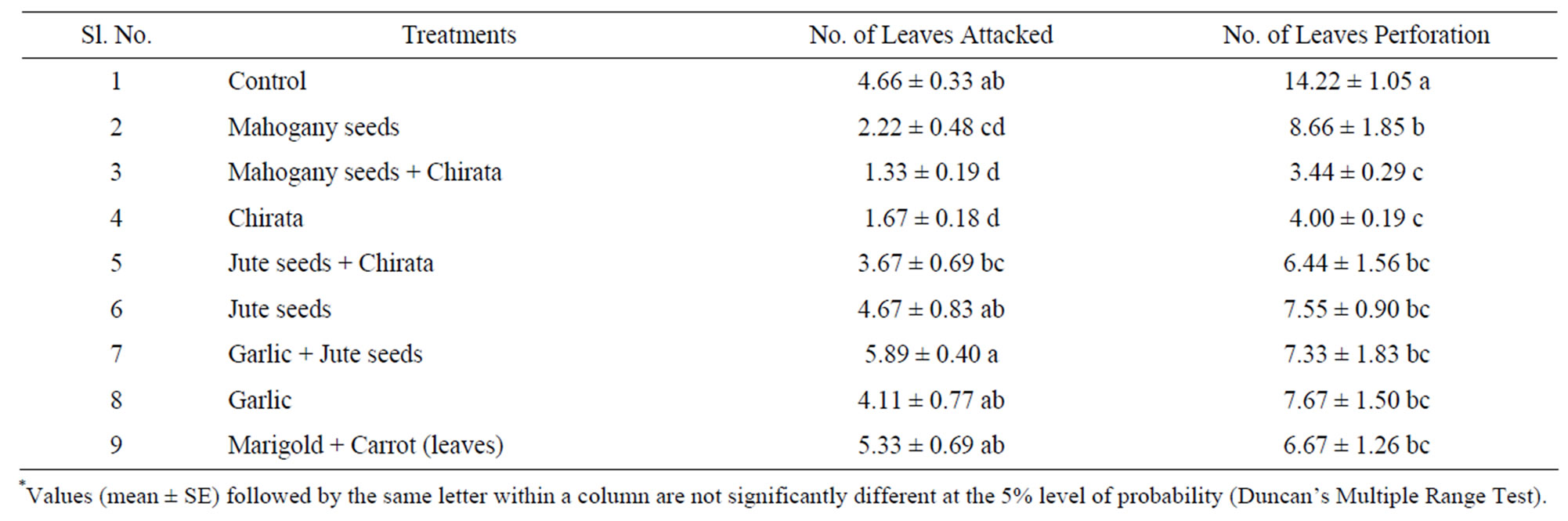
Table 1. Effect of botanical extracts against pest attack on cucumber leaves.

Table 2. Effect of botanical extracts on cucumber plant growth.

Table 3. Observed pests in cucumber field.
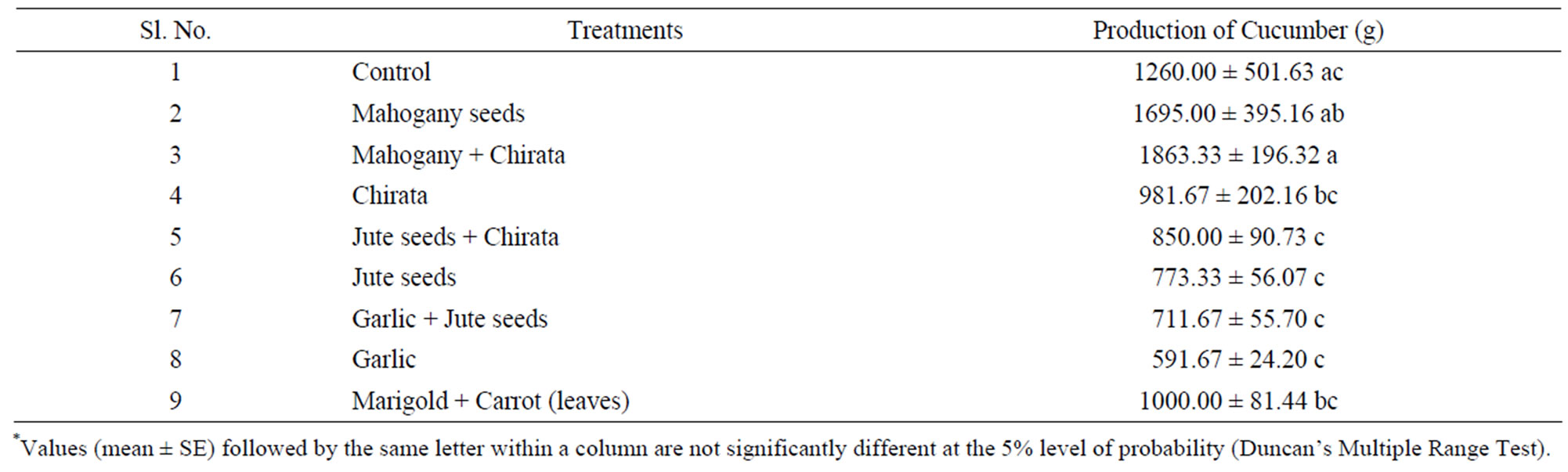
Table 4. Effect of botanical extract on production of cucumber (gm.)
4. Conclusion
Eight botanical pesticides were sprayed on cucumber experimental plot. Out of these botanicals, application of combined extract of mahogany seeds and chirata leaves showed good results for pest control in cucumber field. The highest production of cucumber was also found in this treatment. Therefore, farmers of Bangladesh should use botanical pesticides from mahogany seeds and chirata in cucumber field instead of toxic chemical and synthetic pesticides.
REFERENCES
- R. L. Lower and M. D. Edwards, “Cucumber Breeding,” In: M. J. Basset, Ed., Breeding Vegetables Crops, AVI Publishing Co., Westport, 1986, pp. 173-203.
- D. K. Thoa, “Cucumber Seed Multiplication and Characterization,” AVRDC/ARC Training Thailand, 1998.
- T. C. Wehner and N. Guner, “Growth Stage, Flowering Pattern, Yield and Harvest Date Prediction of Four Types of Cucumber Tested at 10 Planting Dates,” J. D. McCreight and E. J. Ryder, Eds., Proc. XXVI IHC—Advances in Vegetable Breeding, ISHS Acta Horticulturae, 2004.
- T. Tatlioglu, “Cucumber (Cucumis sativus L.),” In: G. Kailov and B. O. Bergh, Eds., Genetic Improvement of Vegetable Crops, Pergamon Press, Oxford, 1997, pp. 197-227.
- N. T. Phu, “Nitrogen and Potassium Effect on Cucumber Yield,” AVI 1996 Report, ARC/AVRDC Training Thailand, 1997.
- S. D. Doijode, “Seed Storage of Horticultural Crops,” Haworth Press, Philadelphia, 2001.
- S. S. Renner, H. Schaefer and A. Kocyan, “Phylogenetics of Cucumis (Cucurbitaceae): Cucumber (C. sativus) belongs in an Asian/Australian Clade Far from Melon (C. melo),” BMC Evolutionary Biology, Vol. 7, 2007, pp. 58-69. doi:10.1186/1471-2148-7-58
- Newstrackindia.com, “Cucumis hystrix,” 2010.
- Encyclopaedia Britannica on-line, “Cucumber”.
- H. S. Huang, N. T. Hu, Y. E. Yao, C. Y. Wu, S. W. Chiang and C. N. Sun, “Molecular Cloning and Heterologous Expression of a Glutathione S-Transferase Involved in Insecticide Resistance from the Diamond Back Moth, Plutella xylostella,” Insect Biochemistry and Molecular Biology, Vol. 28, No. 9, 1998, pp. 651-658. doi:10.1016/S0965-1748(98)00049-6
- B. R. Champ and J. W. Cribb, “Lindane Resistance in Sitophilus oryzae (L.) and Sitophilus zeamais Motsch. (Coleoptera, Curculionidae) in Queensland,” Journal of Stored Products Research, Vol. 1, No. 1, 1985, pp. 9-24. doi:10.1016/0022-474X(65)90004-4
- W. R. Halliday, F. H. Arthur and F. H. Zettler, “Resistance Status of Red Flour Beetle (Coleoptera: Tenebrionidae) Infesting Stored Peanuts in South Eastern United States,” Journal of Economic Entomology, Vol. 81, No. 1, 1988, pp. 74-77.
- J. V. D. Heyde, R. C. Saxena and H. Schmutterer, “Neem Oil and Neem Extract as Potential Insecticides for Control of Hemipterous Rice Pest,” Proceedings of the 2nd International Neem Conference, Rauischholzhausen, 25-28 May 1984, pp. 377-390.
- F. A. Talukder and O. E. Howse, “Deterrent and Insecticidal Effects of Extracts of Pithraj, Aphanamixis polystachya (Meliaceae) against Tribolium castaneum in Storage,” Journal of Chemical Ecology, Vol. 19, No. 11, 1993, pp. 2463-2471. doi:10.1007/BF00980683
- Annonymous, “Recommendations of the Symposium on Resources for Sustainable Agriculture: The Use of Neem and Other Plant Materials for Pest Control and Rural Development,” Neem Symposium. XVII Pacific Science Congress, Honolulu, 27-28 May 1991, pp. 1-11.
- R. C. Saxena, N. J. Liqudo and H. B. Justo, “Neem Seed Oil an Antifeedant for Brown Planthopper,” Proceedings of the 1st International Neem Conference, Rottach-Egern, 16-18 June 1980, pp. 171-188.
- M. M. Sadek, “Antifeedant and Toxic Activity of Adhatoda vasica Leaf Extract against Spodopiera littoralis (Lepidoptera: Noctuidae),’’ Journal of Applied Entomology, Vol. 127, No. 7, 2003, pp. 396-404. doi:10.1046/j.1439-0418.2003.00775.x
- M. R. Berenbaum, J. K. Niato and A. R. Zangerl, “Adaptive Variation in the Furanocoumarin Composition of Pastinaca sativa (Apiaceae),” Journal Chemical Ecology. Vol. 17, No. 1, 1991, pp. 207-215. doi:10.1007/BF00994434
- W. Chen, M. B. Isman and S. F. Chiu, “Antifeedant and Growth Inhibitory Effects of the Limonoid Toosendanin and Melia toosendan Extracts on the Variegated Cutworm, Peridroma saucia,” Journal Applied Entomology, Vol. 119, No. 1-5, 1995, pp. 367-370. doi:10.1111/j.1439-0418.1995.tb01302.x
- I. G. Hiremath, A. Young Joon, I. Kim-Soon and S. I. Kim, “Insecticidal Activity of Indian Plant Extracts against Nilaparvata lugens (Homoptera: Delphacidae),” Applied Entomology and Zoology, Vol. 32, No. 1, 1997, pp. 159-166.
- K. D. Klepzig and F. Schlyter, “Laboratory Evaluation of Plant Derived Antifeedants against European Pine Weevil, Hylobius abietis,” Journal of Economic Entomology, Vol. 92, No. 3, 1999, pp. 644-650.
- D. A. Wheeler and M. B. Isman, “Antifeedant and Toxic Activity of Trichilia americana Extract against the Larvae of Spodoptera liura,” Enloinologia Experimen/alis ci Applicala, Vol. 98, No. 1, 2001, pp. 9-16.
- M. Jacobson, “Botanical Insecticides. Past, Present and Future,” In: J. F. Arnason, B. J. R. Philogene, and P. Morand, Eds., Insecticides of Plant Origin, American Chemical Society Symposium Series No. 387, Washington DC, 1989, pp. 1-10.
- H. Schmutterer, “Properties and Potential of Natural Pesticides from the Neem Tree, Azadirachta indica,” Annual Review of Entomology, Vol. 35, 1990, pp. 271-297. doi:10.1146/annurev.en.35.010190.001415
- M. B. Isman, “Leads and Prospects for the Development of New Botanical Insecticides,” In: R. M. Roe and R. J. Kuhr, Eds., Reviews in Pesticide Toxicology, Vol. 3. Toxicology Communications Inc., Raleigh, 1995, pp. 1-20.
- D. Obeng-Ofori I. E. Aidoo and R. K. Akuamoah, “Evaluation of Leaf Extracts of the Siam Weed Chromolaena odorata (L.) and Mahogany Tree Khaya senegalensis (Desi) against the Maize Weevil Sitophilus zeamais (Mot.),” Agricultural and Food Science Journal, Vol. 1, 2002, pp. 95-106.
- O. Coulibaly, A. J. Cherry, T. Nouhoheflin, C. C. Aitchedji and R. Al-Hassan, “Vegetable Producer Perceptions and Willingness to Pay for Biopesticides,” International Journal of Vegetable Science, Vol. 12, No. 3, 2007, pp. 27-42.
- S. Z. Ali, “Integrated Management of Sooty Mould (Capnodium sp.) Disease in Mulberry (Morus Spp),” Master’s Dissertation, Annamalai University, Chidambaram, 2007. p. 78.
- K. N. Saxena and H. Rembold, “Orientation and Ovipositional Response of Heliothis urmigera to Certain Neem Constituents,” Proceeding of 2nd International Neem Conference, Rausichoty Housen, 25-28 May 1984, pp. 199-210.
- A. A. Kareem, R. C. Saxena, M. E. M. Boncodin, V. Krishnaswamy and D. U. Seshu, “Neem as Seed Treatment of Beibre Sowing, Effects on Two Hemipterous Insects,” Journal of Economic Entomology, Vol. 82, No. 4, 1989, pp. 1219-1223.
- A. N. Dash and B. Senapathi, “Efficacy of Neem Derivatives as Rice Seedling Root-Dip against Green Leaf Hopper, Nephotettix viresens (Distant) under Green House Conditions,” Journal of Entomological Research, Vol. 19, No. 1, 1995, pp. 33-38.
- J. A. R. P. Alice and M. S. Venugopal, “Role of Botanicals on the Growth and Development of Rice Brown Planthopper Nilaparvata lugens (Stal.) on Rice with Different Methods of Application,” Pestology, Vol. 24, No. 12, 2000, pp. 11-16.
- W. Fuchs, K. Rauch and H. J. Wiche, “Effect of Organic Fertilizer and Organo Mineral Fertilizing on Development and Yield of Cereals,” Abrecht Thaer Arch, Vol. 14, 1970, pp. 359-366.
- P. Fiscian, “Assessment of Damage Due to Feeding of Leucinodes orbonalis Quen. (Lepidoptera: Pyralidae) on Solanum sp.,” Journal of the Ghana Science Association, Vol. 1, No. 2, 1999, pp. 21-25. doi:10.4314/jgsa.v1i2.17801
- AVRDC, “A Farmer’s Guide to Harmful and Helpful Insects in Eggplant Fields,” AVRDC—The World Vegetable Centre, Taiwan, 2003.
- E. O. Titiloye, “The Chemical Composition of Different Sources of Organic Wastes and Effects on Growth and Yield of Maize,” Ph.D. thesis, University of Ibadan, Ibadan, 1982.
- F. C. Costa, G. C. Hernadez and A. Polo, “Residuos Organicos Urbanicos in Manejoy Utilizacion,” CSIC Munica, 1981, p. 181.
- FAO, “Eggplant Ecological Guide,” FAO Inter-Country Programme for IPM in Vegetables in South and Southeast Asia, Rome, 2003.
NOTES
*Corresponding author.

