Advances in Infectious Diseases
Vol. 3 No. 2 (2013) , Article ID: 32633 , 6 pages DOI:10.4236/aid.2013.32014
Presence of Staphylococcus aureus in the Nasal Cavity of Children Attending a Public Daycare Center in Francisco Beltrão—Paraná—Brazil*
![]()
Department of Biological Sciences and Pharmacy, Faculty of Educational of Dois Vizinhos, União de Ensino do Sudoeste do Paraná, Dois Vizinhos, Brazil.
Email: #becker@unisep.edu.br
Copyright © 2013 Sideney Becker Onofre, Géssica Araceli Costa. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received December 18th, 2012; revised January 19th, 2013; accepted February 19th, 2013
Keywords: Nasal Cavity; Frequency; Susceptibility; Antimicrobials
ABSTRACT
The objective of this study was to evaluate the presence of Staphylococcus aureus in the nasal cavity of children attending a daycare center in the town of Francisco Beltrão—PR, by comparing the frequency between age groups and genders. Antimicrobial susceptibility testing with different drugs was also carried out in the bacterial isolates. A prospective study involving 200 children aged 2 and 4 years, grouped by gender, was carried out. Samples were collected from the nasal vestibules of children, incubated and identified. Antimicrobial susceptibility testing was carried out in 30% of the S. aureus colonies. The results showed that 35% of children (10% female and 25% male) were colonized by S. aureus. The frequency of colonization in both male age groups was similar, with 201.93 ± 13.10 CFU/plate for 2- year-old and 266 ± 12.60 CFU/plate for 4-year-old. However, colonization averages for females were different, with 30.43 ± 1.17 CFU/plate for 2-year-old and 394.38 ± 10.70 CFU/plate for 4-year-old. In the antimicrobial susceptibility testing, some strains were resistant to oxacillin, erythromycin and tetracycline, in addition to showing intermediate susceptibility to cephalothin.
1. Introduction
Humans interact with the many microorganisms, because they are a definitive and intermediate host of various species. The main interaction occurs with endogenous microorganisms, mostly bacteria of the normal human microbiota [1]. The human body represents several environmental niches for the bacteria, providing warmth, moisture and food necessary for their development.
One of the most important bacteria in the normal human microbiota is Staphylococcus sp., commonly transmitted from person to person through direct or indirect contact [2]. The transmission depends on a source (carriers or patients) and the rate of microorganisms released, as well as the microorganism survival ability, pathogenicity and frequency of contact between healthy and infected individuals [3].
Staphylococcus sp. are usually found in the human skin and mucous membranes; however, the main species Staphylococcus aureus has been isolated from various body parts, including hands and mouth [4]. The nasal cavity is an ecosystem suitable for the growth and inter-relationship of this microorganism, showing colonization with different phagotypes [5]. The nostrils provide an environment in which S. aureus can propagate and remain for extended periods [6]. S. aureus has a prevalence of 20% to 40% in the nasal cavity of adults [4], but in children this percentage can reach 100% by the age of four and declines thereafter [7]. However, carriage is a long-term condition, with its incidence decreasing from the age of one, ranging between 10% and 40% for both immunocompromised and healthy children. Carriage is a transient condition variable for each patient [8], which plays a key role as a risk factor for infections in hemodialysis and surgery patients, as well as those with catheters, intravascular devices and infected by HIV [9].
This species is extremely important in the nasal cavity, because it can act as secondary microbiota and acquire pathogenic character, overcoming physical and chemical barriers, as well as the host’s immune system, to cause infections [5]. Staphylococcal infections can be superficial, affecting the skin and subcutaneous tissue, or deep, which originate from the focus of the superficial infections [4]. The consequences of S. aureus infections can be serious [10], because the pathogen is considered the main cause of surgical and skin infections (pimples and boils), being also responsible for the toxic shock syndrome and severe illnesses, including pneumonia, meningitis, endocarditis and septicemia [11]. Furthermore, it is considered an important pathogen in children for commonly causing pharyngitis [12], because of its pathogenic mechanisms, such as the production of virulence factors.
Colonization by S. aureus is currently a concern due to the increasing number of carriers and colonized patients in the general population, as infections can result in additional complications, thus requiring antimicrobial therapy [13]. Children who attend daycare centers and other community care premises can carry strains resistant to multiple antimicrobials, which cause frequent infections in immune immature individuals [14].
However, this profile is most commonly found in hospitalized children, because the strains do not have their growth affected by the maximum level of antibacterial tolerated by the host [15]. The main resistance developed by S. aureus is to methicillin. This is currently becoming a serious problem because of the related high morbidity and mortality indices, as well as a high prevalence in community-acquired infections [16].
The microorganism has developed resistance to this beta-lactam antibiotic after having acquired resistance to penicillin. Methicillin-resistant strains, also known as MRSA, are not inhibited by any of the beta-lactam antibiotics [17]. This can be related to the presence of the protein PBP2 or ß-lactamases, which are enzymes produced by S. aureus capable of hydrolyzing the beta-lactam ring of some antibiotics. The production of ß-lactamases is mediated by plasmids, which confer resistance to such antibiotics by breaking the beta-lactam ring, and thus inactivating the drug [18].
In recent decades, MRSA has become a serious public health problem because of the threat of developing resistance to vancomycin, the only antimicrobial currently effective against MRSA. CA-MRSA or community-acquired MRSA is also a cause of concern, as it can cause fatal infections in individuals who are not in the riskgroup for S. aureus, such as healthy children, teenagers and adults [19,20].
Therefore, the main concern lies with the healthy carrier, who is considered the most silent, yet the most dangerous source of infection-causing microorganisms [21]. The pathogen can become a serious health problem especially for children living in collective environments, such as daycare and community centers, because transmission can occur by close contact, generating pathological events [12] due to the immature immune system of these individuals, consequently making them more susceptible to the bacteria [21].
However, it is suggested that in the absence of risk factors, colonization does not characterize a worse prognosis. Still, effective strategies to prevent transmission through nasal carriage are important measures against S. aureus infections [11]. It is necessary to highlight the value of identification and determination of this microorganism susceptibility to indicate the first-line empiric therapy to assist in choosing an effective alternative antimicrobial.
In this context, the present study aimed to evaluate the presence of S. aureus in the nasal cavity of 2 and 4-yearold children who attend a public daycare center in the town of Francisco Beltrão—PR.
2. Material and Methods
The study was carried out from May to July 2010, with 200 children divided into two groups. Group 1 had a total of 100 females (50 aged 2 years and 50 aged 4 years) whereas group 2 was formed by 100 males (50 aged 2 years and 50 aged 4 years). Children attended the Delfo Fregonese public daycare center in Francisco Beltrão-PR.
Inclusion criteria were children of both genders who regularly attended the daycare center, aged 2 and 4 years, with no obvious signs of airway infection.
A total of three collections were made from each group. Each sampling was performed in triplicate, generating 300 plates per group at each collection and, thus, totaling 900 plates per group. Sample collection was performed with a sterile swab, with 15-day intervals between collections. The material was collected from both nostrils of each individual, then inoculated onto Petri plates containing Baird Parker medium. The plates were incubated at 36˚C for 48 h.
After incubation, colonies with S. aureus characteristics were stained by the Gram method and evaluated in micromorphological tests. Strains staining as gram-positive cocci were subjected to identification through the catalase and coagulase tests.
Colonies with S. aureus characteristic morphology were selected and suspended in a 0.85% NaCl solution to reach a 0.5 McFarland turbidity standard, corresponding to approximately 1.5 × 108 CFU. Inoculation was then carried out on Mueller-Hinton agar (Merck) using a sterile swab. After, paper disks with the following antimicrobials were applied: oxacillin (1 μg), ciprofloxacin (5 μg), erythromycin (15 μg), gentamicin (10 μg), cephalothin (30 μg) and vancomycin (30 μg). Plates were incubated at 35˚C for 24 h. Susceptibility was determined by measuring the growth inhibition zone (mm). The results were interpreted according to the CLSI [22].
After the antimicrobial susceptibility testing, strains resistant to oxacillin were analyzed for the presence of blactamase through the iodometric method, which consists of iodine tapes that acquire a light coloring when reacting with the enzyme.
The obtained data underwent statistical evaluations following the Analysis of Variance (ANOVA) rules, the mean and standard deviation were determined and compared by the Tukey’s test at 5% significance.
This study was conducted in compliance with the provisions of Resolution 196/96 of the National Committee for Ethics in Research at the Brazilian Ministry of Health, and it was approved by the Human Research Ethics Committee. It is noteworthy that the children involved did not suffer any kind of risk, and an informed term of consent was signed by the parents and the institution responsible.
3. Results and Discussion
It was found that 70 children were colonized by S. aureus strains, as follows: 30 children aged 2 years and 40 children aged 4 years, of which 20 were females and 50 males. Results related to the quantification of S. aureus found in the nasal cavity of these children are presented in Table 1.
Table 1 shows that S. aureus frequency was different for females from both age groups, because 2-year-old had an average of 30.43 ± 11.17 CFU/plate, whereas 4- year-old had an average of 394.38 ± 10.70 CFU/plate, thus being considered the most colonized group, with significant differences between the two groups.
Both age groups showed different colonization frequencies for males: 2-year-old had an average of 201.93 ± 13.10 CFU/plate, whereas 4-year-old had an average 266 ± 12.60 CFU/plate.
The colonies isolated and identified as S. aureus were evaluated for antimicrobial resistance. Of the total colonies observed in the male and female groups, 30% were randomly selected: 10 and 118 colonies from the female group, aged 2 and 4 years, respectively; and 60 and 80 colonies from the male group, aged 2 and 4 years, respectively. The results are summarized in Tables 2 and 3.
Table 2 shows a difference in antimicrobial susceptibility between the female age groups (Group 1). Erythromycin-resistant strains were found in three isolated colonies from the 2-year-old group. Remaining colonies from the same group were susceptible to other antim
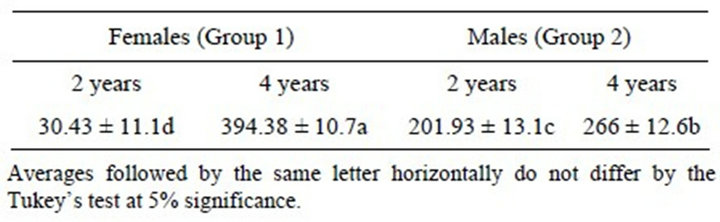
Table 1. Comparison of Staphylococcus aureus CFU/plate across three samplings from both genders, aged 2 and 4 years.
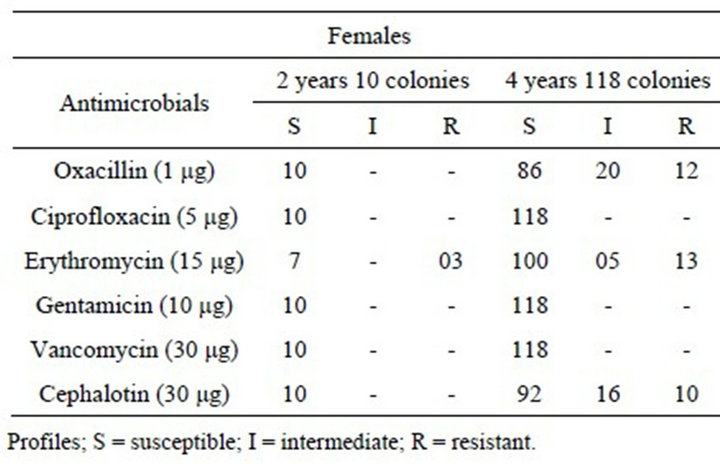
Table 2. Antimicrobial susceptibility testing in S. aureus strains isolated from the nasal cavity of females aged 2 and 4 years.
icrobials. However, the 4-year-old group had 12 oxacillin-resistant strains and 20 colonies of intermediate susceptibility, five erythromycin-resistant and 13 intermediate colonies, and 10 cephalothin-resistant and 16 intermediate colonies. The remaining colonies showed susceptibility to the other antimicrobials evaluated.
Table 3 shows differences in antimicrobial susceptibility between the two male age groups, with 2-year-old having 5 erythromycin-resistant strains and 15 intermediaries, whereas the remaining colonies proved susceptible to the other antibiotics. However, the 4-year-old group had eight oxacillin-resistant colonies and 22 intermediate, eight erythromycin-resistant colonies and 12 intermediate, and two oxacillin-resistant colonies and 13 intermediate. The remaining colonies are susceptible to the other antibiotics.
It was verified that all S. aureus colonies (268) were susceptible to ciprofloxacin, gentamicin and vancomycin. The 20 colonies that showed resistance to oxacillin, from Groups 1 and 2, age Group 4 years, were tested for the production of ß-lactamase, which resulted negative.
In recent years, there has been an increased concern with healthy nasal S. aureus carriers, especially children, regarding the high rate of infections and acquired resistance to this pathogen in the community. Considering the epidemiology, the profile of children in this study is similar to other studies conducted with the same age group, involving children who attended a community en-

Table 3. Antimicrobial susceptibility testing in S. aureus strains isolated from the nasal cavity of males aged 2 and 4 years.
vironment [14,23,24].
In the study, S. aureus nasal colonization was observed in 7 (35%) children. Other authors have reported higher incidence of S. aureus in the nasal cavity of healthy children [14,25]. The highest prevalence was found in males, being this predominance also common in other research with children [26] and adults [27,28].
The difference between the colonization average in samples collected from both groups is observed in Table 1. The data obtained show that 4-year-old had a higher frequency of colonization than 2-year-old. These inequalities can be attributed to characteristics, such as gender and age, as well as immunity, risk factors, genetics, among others.
Several authors have reported that antimicrobial resistance in S. aureus strains over the years as a worrying fact [19,20,29]. This argument could be confirmed through the susceptibility tests conducted in this research, as strains resistant to different antibiotics were found. According to the literature [30], this characteristic can be attributed mostly to the excessive and indiscriminate use of antimicrobials.
The results found in this work show that only colonies from 4-year-old, in both groups, were resistant to oxacillin. This profile was also observed in a study with healthy newborns [7] and in a research on communityacquired infections in children [24].
In a general analysis, the use of antibiotics like ciprofloxacin and gentamicin for infections caused by this organism in the community continue to be the most effective method, because of antimicrobial susceptibility found in both age groups and genders. Vancomycin is an alternative only when S. aureus strains are not susceptible to any other antimicrobial, often being used in hospital treatments.
The iodometric test performed on oxacillin-resistant strains for the assessment of b-lactamase resulted negative for the presence of this enzyme. Considering this resistance pattern, it can be suggested that these strains have a different resistance mechanism, such as the production of PBP2 protein, which generates a low affinity for this beta-lactam antimicrobial [18].
The results obtained in this study can contribute in the creation of programs for the development of healthy children and strategies to prevent S. aureus transmission, considering the increased presence of community-acquired resistant strains.
4. Conclusions
With the results obtained in this study, it was concluded that S. aureus strains are frequently found in the nasal cavity of children aged 2 to 4 years, both males and females. However, higher frequency is found among 4- year-old males.
Oxacillin-resistant S. aureus strains were only found among 4-year-old from both groups, but they were considered b-lactamase negative. All S. aureus strains from the 268 colonies evaluated were susceptible to ciprofloxacin, gentamicin and vancomycin.
5. Acknowledgements
We thank the Secretary of Health and the Department of Education of Francisco Beltrão, and especially the children and staff at Delfo Fregonese daycare center in Francisco Beltrão—PR, who enabled the development of this research.
REFERENCES
- M. A. Neves, “Colonização das Fossas Nasais de Acadê- micos de Medicina Por Staphylococcus aureus Resistente à Meticilina, Relacionada ao Tempo de Exposição no Ambiente Hospitalar,” Tese, Faculdade de Ciências Mé- dicas da Santa Casa de São Paulo, São Paulo, 2007.
- P. R. Murray, K. S. Rosenthal and M. A. Pfaller, “Microbiologia Médica,” In: P. R. Murray, K. S. Rosenthal and M. A. Pfaller, Eds., Mecanismos da Patogênese Bacteriana, Elsevier, Rio de Janeiro, 2006, pp. 187-195.
- B. M. Santos and A. L. C. Darini, “Colonização por Staphylococcus aureus em Portadores Sãos Relacionados de Uma Creche de Hospital Universitário,” Medicina, Ribeirão Preto, Vol. 35, No. 2, 2002, pp. 160-172.
- E. W. Koneman, S. D. Allen, S. M. Janda, P. C. Schereckenberger and W. C. Winn, “Diagnóstico Microbiológico,” In: E. W. Koneman, S. D. Allen, S. M. Janda, P. C. Schereckenberger and W. C. Winn, Cocos Gram-Positivos, Parte I: Estafilococos e Microrganismos Relacionados, Medsi, Rio de Janeiro, 2001, pp. 551-588.
- C. Mims, H. M. Dockrell, R. L. V. Goering, I. Roitt, D. Wokelin and M. Zuckerman, “Microbiologia Médica,” 3ª Edition, Elsevier, Rio de Janeiro, 2005.
- G. R. W. Burton and P. G. Engelkirk, “Microbiologia para Ciências da Saúde,” In: G. R. W. Burton and P. G. Engelkirk, Eds., Ecologia Microbiana, Guanabara Koogan, Rio de Janeiro, 2005, pp. 197-213.
- M. P. Cardoso, “Aquisição Nasal de Staphylococcus aureus Por Recém-Nascidos Saudáveis,” Dissertação de Mestrado, Universidade Estadual de Maringá, Maringá, 2007, 127 Pages.
- A. Lebon, J. A. M. Labout, H. A. Verbrugh, V. W. V. Jaddoe, A. Hofman and W. V. W. Van Wamel, “Dynamics and Determinants of Staphylococcus aureus Carriage in Infancy: The Generation R Study,” Journal of Clinical Microbiology, Vol. 46, No. 5, 2008, pp. 3517-3521. doi:10.1128/JCM.00641-08
- J. R. Boelaert, “Staphylococcus aureus Infection in Haemodialysis Patients. Mupirocin as a Topical Strategy against Nasal Carriage: A Review,” Journal of Chemotherapy, Vol. 2, No. 1, 1994, pp. 19-24.
- C. V. Eiff, K. Becker, K. Machka, H. Stammer and G. Peters, “Nasal Carriage as a Source of Staphylococcus aureus Bacteremia,” The New England Journal of Medicine, Vol. 34, No. 4, 2001, pp. 11-16. doi:10.1056/NEJM200101043440102
- A. L. Santos, D. O. Santos, C. C. Freitas, B. L. A. Ferreira, I. F. Afonso and C. R. Rodrigues, “Staphylococcus aureus: Visitando uma Cepa de Importância Hospitalar,” The Jornal Brasileiro de Patologia e Medicina Laboratorial, Vol. 43, No. 2, 2007, pp. 413-426. doi:10.1590/S1676-24442007000600005
- A. Braios, R. Oliveira, I. B. S. Lima and E. Kendrew, “Crianças Portadoras Assintomáticas de patóGenos,” Colloquium Vitae, Vol. 1, No. 3, pp. 25-29.
- C. Carvalho, E. M. Berezin, I. P. Pistelli, L. Mímica and M. R. A. Cardoso. “Monitoramento Microbiologico Sequencial da Secreção Traqueal em Pacientes Intubados Internados em Unidade de Terapia Intensiva Pediátrica,” Journal of Pediatrics, Vol. 81, No. 1, 2005, pp. 29-33. doi:10.1590/S0021-75572005000100007
- M. F. Bernardo and M. Ueno, “Incidence of Staphylococcus aureus Colonization in Children Attending DayCare Centers,” Revista Panamericana de Infectología, Vol. 10, No. 3, 2008, pp. 20-23.
- H. Paganini, M. P. D. Latta, B. M. Opet, G. Ezcurra, M. Uranga and C. Aguirre, “Infecciones por Staphylococcus aureus Resistente a Meticilina Adquiridas en la Comunidad en Niños Antes Sanos y en Niños Relacionados al Hospital en la ARGENTINA,” Revista Chilena de Infectología, Vol. 26, No. 2, 2009, pp. 406-412.
- M. M. Baddour, M. A. Abuelkheir and A. Fatani, “Trends in Antibiotic Susceptibility Patterns and Epidemiology of MRSA Isolates from Several Hospitals in Riyadh, Saudi Arabia,” Annals of Clinical Microbiology and Antimicrobials, Vol. 5, No. 5, 2006, pp. 1-11.
- G. Remonatto, C. M. Cardoso, C. G. Marques, A. E. B. Silva, L. C. Gelatti and C. F. M. Leite, “CA-MRSA: Um Patógeno Emergente,” NewsLab, Vol. 80, 2007, pp. 92- 96.
- M. Ueno and A. O. C. Jorge, “Caracterização de Staphylococcus aureus Resistentes à Meticilina, Envolvidos em Infecções Nosocomiais, Por Meio de técnicas Fenotípicas e Análise de Perfil Plasmidial,” Revista Biociências, Vol. 7, No. 3, 2001, pp. 15-22.
- F. Vandenesch, T. Naimi, M. D. Enright, G. Lina, G. R. Nimmo and H. Heffernan, “Community-Acquired Methicillin-Resistant Staphylococcus aureus Carrying PantonValentine Leukocidin Genes: Worldwide Emergence,” Emerging Infectious Disease, Vol. 9, No. 8, 2003, pp. 978- 984. doi:10.3201/eid0908.030089
- H. F. L. Wertheim, D. C. Melles, M. C. Vos, W. V. Leeuwen, A. V. Belkum and H. A. Verbrugh, “The Role of Nasal Carriage in Staphylococcus aureus Infections,” The Lancet Infectious Diseases, Vol. 5, No. 3, 2005, pp. 751- 762. doi:10.1016/S1473-3099(05)70295-4
- CLSI—Clinical and Laboratory Standards Institute— NCCLS, “Normas de Desempenho para Testes de Sensibilidade Antimicrobiana,” 15º Suplemento Informativo, M100-S15, Vol. 25, No. 1, 2005, pp. 1-117.
- J. L. Cardoso, “Prevalência e Tipagem Molecular de Staphylococcus aureus Isolados da Nasofaringe de Crianças No Município de Goiânia—Goiás,” Dissertação de Mestrado, Universidade Federal de Goiás, Goiás, 2005.
- H. Paganini, M. P. D. Latta, B. M. Opet, G. Ezcurra, M. Uranga and C. Aguirre, “Estudio multicéNtrico Sobre las Infecciones Pediátricas por Staphylococcus aureus Meticilino-Resistente Provenientes de la Comunidad en la Argentina,” Archivos Argentinos de Pediatría, Vol. 106, No. 5, 2008, pp. 397-403.
- I. S. Faria and M. Ueno, “Estudo Comparativo da Colonização por Staphylococcus aureus em Crianças Saudá- veis e Crianças de Uma Unidade Pediátrica de um Hospital Universitário,” Revista Biociências, Vol. 15, No. 1, 2009, pp. 34-38.
- C. A. Z. Pereira, S. C. G. P. Rocio, M. F. R. Ceolin, A. P. N. B. Lima, F. Borlot and R. S. T. Pereira, “Achados Clínico-Laboratoriais de uma série de Casos Com Endocardite Infecciosa,” Journal of Pediatrics, Vol. 79, No. 5, 2003, pp. 423-428. doi:10.2223/JPED.1075
- W. P. Spiandorello, F. Morsh, S. Sebben and F. S. A. Spiandorello, “A Resistência do Staphylococcus aureus à Oxacilina em Hospital de Caxias do Sul,” Revista Amrigs, Vol. 44, No. 3-4, 2000, pp. 120-125.
- S. M. M. Cavalcanti, E. R. França, M. A. Vilela, F. Montenegro, C. Cabral and A. C. R. Medeiros, “Estudo Comparativo da Prevalência de Staphylococcus aureus Importado Para as Unidades de Terapia Intensiva de Hospital Universitário, Pernambuco, Brasil,” Revista Brasileira de Epidemiologia, Vol. 9, No. 4, 2006, pp. 436-46. doi:10.1590/S1415-790X2006000400004
- O. H. Telechea, M. N. Speranza, P. L. Lucas, G. A. Santurio, L. G. Giachetto and R. G. Algorta, “Evolución del Consumo de Antibióticos y de la Susceptibilidad Antimicrobiana en el Centro Hospitalario Pereira Rossell en la era de Staphylococcus aureus Resistente a Meticilina,” Revista chilena de infectología, Vol. 26, No. 5, 2009, pp. 413-419. doi:10.4067/S0716-10182009000600003
- R. A. Mota, K. P. C. Silva, M. F. L. Freitas, W. J. N. Porto and L. B. C. Silva, “Utilização Indiscriminada de Antimicrobianos e Sua Contribuição a Multirresitência Bacteriana,” Brazilian Journal Veterinary Research Animal Science, Vol. 42, No. 6, 2005, pp. 465-470.
- D. M. Livermore, “Bacterial Resistance: Origins, Epidemiology, and Impact,” Clinical Infectious Diseases, Vol. 36, Suppl. 1, 2003, pp. 11-23. doi:10.1086/344654
NOTES
*Conflict of interest: the authors declare no conflict of interest.
#Corresponding author.

