World Journal of Cardiovascular Diseases
Vol.4 No.6(2014), Article ID:46492,9 pages DOI:10.4236/wjcd.2014.46041
Idiopathic Giant Cell Myocarditis: State of the Art
Alessia Veia1, Chiara Cavallino2, Sara Bacchini1, Fabio Pastore1, Alessandro Lupi1, Andrea Rognoni1*, Francesco Rametta2, Angelo Sante Bongo1
1Coronary Care Unit, A.O.U. Maggiore della Carità, Novara, Italy
2Division of Cardiology, A.S.L., Vercelli, Italy
Email: *arognoni@hotmail.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 9 April 2014; revised 14 May 2014; accepted 21 May 2014
ABSTRACT
Giant cell myocarditis (GCM) is a rare, rapidly progressive and highly lethal disease in young and middle-aged adults. It is attributed to an inflammation of the heart muscle, and mediated by T lymphocytes and anti-myosin autoantibodies. Making diagnosis of GCM with multiple noninvasive imaging modalities is possible in a small percentage of patients, so myocardial tissue diagnosis is often required. An early diagnosis is very important, because immunosuppressive treatment may significantly improve clinical course and survival of these patients. GCM often escapes diagnosis until autopsy or transplantation and has defied proper treatment trials for its rarity and deadly behavior. This review will focus on the diagnostic approach to patients with suspected GCM and currently evidence-based treatment strategy for this disease.
Keywords:Giant Cell, Myocarditis Endomyocardial Biopsy, Heart Failure, Immunosuppression

1. Introduction
Myocarditis is a non-familial form of heart muscle disease [1] , defined as an inflammation of the heart muscle and identified by clinical or histopathologic criteria [2] . Several insults have been implicated as causes of myocarditis: infectious, autoimmune, toxic, drug-induced, hypersensitive and vasculitic diseases. Histologic patterns of myocarditis are usually characterized by predominant inflammatory cells and can be divided into lymphocytic (including viral and autoimmune forms); neutrophilic (bacterial, fungal and early forms of viral myocarditis), eosinophilic (hypersensitivity myocarditis or hypereosinophilic syndrome) and granulomatous (cardiac sarcoidosis and giant cell myocarditis). Among several categories of myocarditis, a significant overlap is present and no finding is specific for a single etiology.
Giant cell myocarditis (GCM) is a rare cardiac inflammatory disorder that is characterized by diffuse ventricular myocardium infiltration, by lymphocytes, abundant multinucleated giant cells, mainly eosinophilis, necrosis and fibrosis. Functional consequences of myocardial injury are similar to those seen in other kind of myocarditis, including ventricular dysfunction and ventricular arrhythmias, although usually much more are severe in GCM.
2. Historical View
In 1905, Saltykow [3] published the first case report of idiopathic GCM: he described a case of fatal myocarditis characterized by giant cells associated with widespread inflammation and myocyte necrosis, unassociated with tubercolosis, syphilis or other known causes. Since then, additional reports of this lesion have been published infrequently [4] . In 1939, Jonas [5] recorded 5 typical cases, in which myocardium and other tissue were involved by a granulomatous process characterized by tubercle-like foci with giant cells, necrosis and mononuclear cell infiltration. In Magner’s [6] report, too, giant cells were preeminent among the mononuclear cells and proliferating fibroblasts in the myocardium.
Until the late 1950s, GCM and cardiac sarcoidosis (CS) were grouped together: the terms “giant cell myocarditis” and “granulomatous myocarditis” were interchangeable and they were used to describe myocardial disease in which multinucleated giant cells were present, either as granulomas (CS) or as diffuse inflammatory myocardial infiltrates (GCM) [7] . Since the late 1960s, however, most publication distinguishes the well-organized granulomatous lesions of CS from the diffuse nongranulomatous inflammatory infiltrates of GCM [8] . Okura et al. [9] showed that GCM and CS have different histologic features: CS specimens had significant more granulomas and fibrosis, while GCM had more necrosis and eosinophils; the number of giant cells was similar in both disorders.
3. Epidemiology, Etiology and Pathophysiology
The GCM incidence is low and it varies with the studied population and diagnostic method used. GCM incidence is known primarily from autopsy studies. From a recent autopsy case series, the incidence of GCM in India is evaluated at 0.051% [10] . In England and in Japan the incidence at autopsy is reported at 0.023% and 0.007% respectively [11] [12] . The incidence of giant cell myocarditis was similarly low (3 of 12,815 necropsies) in the period from 1959 to 1963 at the Oxford Infirmary [13] . A clinical report of endomyocardial biopsies carried out for native heart disease reported 2 of 462 biopsied had GCM [14] . Since autopsies are not routinely performed on an unselected population, the true incidence is likely lower than these estimates.
The aetiology of GCM is unknown, likely GCM has multiple causes. Viral infection may occasionally trigger GCM: single case reports have suggested that infection with Human Herpes virus [15] , coxsackie B2 virus [16] [17] and parvovirus [18] may each play a role. Autoimmune disorders are associated with approximately 20% of cases of GCM: systemic lupus erythematosus [19] , Sjogren syndrome [20] , myositis [21] , Hashimoto’s thyroiditis, rheumatoid arthritis, autoimmune hepatitis [19] , myastenia gravis [21] , Takayasu’s arteritis, pernicious anemia. Also inflammatory bowel disease has been described as being associated with GCM [22] [23] and tumors, mostly thymoma [21] and lymphoma [24] [25] . GCM has also been described as a manifestation of drug hypersensitivity [26] ; this link is important because early offending drug interruption and treatment with steroids can lead to improving outcomes.
Myocarditis that resembles human Giant Cell Myocarditis can be induced in the Lewis rat by immunization with cardiac myosin; thus supporting hypotesis of an autoimmune mechanism [27] [28] , although these anticardiac myosin antibodies are not specific for GCM [29] . Both human and experimental giant cell myocarditis are characterized by an infiltrated of T lymphocites producing interferon gamma and macrophages producing tumor necrosis factor (TNF) [27] . There is an early infiltrated of CD4-positive T cells with a T helper type 1 response, secreting Il-2 and interferon gamma and a later stage of lesion evolution, in which a dominant T helper 2 response may lead to fibrosis [30] [31] . Recent studies focused on the role of Il-17 and Th17 lymphocytes [32] .
Genetic factors influence susceptibility to GCM: Shioji et al. [33] studied histocompatibility characteristics of 5 inbred strains of rats in which myocarditis was induced with porcine cardiac myosin. Immune-mediated GCM was induced in Lewis and Fisher rats but not in brown Norway rats and the disease was most severe in former. Kittleson et al. [34] examined left ventricular samples from two GCM patients and six unused donor hearts: they found 115 differentially expressed genes between GCM and nonfailing hearts. The majority of upregulated genes were involved in the immune response, primarly the Th1 pathway.
Altered desmosomal proteins may also play a role in the pathogenesis of GCM, especially in the pathogenesis of arrhythmias [35] . Plakoglobin, from intercalated disks, was found reduced in patients with GCM (similarly to patient with arrhythmogenic right ventricular dysplasia and sarcoidosis, disorders associated to ventricular arrhythmias), while it was found normal in patients with lymphocytic myocarditis and in control tissue. Neonatal rat myocytes were incubated with various concentrations of cytokines: Il-9, Il-12, Il-4 and INF gamma, which have been implicated in nongranulomatous inflammation, had no apparent effect on plakoglobin distribution. In contrast, Il-17 and TNFa, both of which are thought to mediate granulomatous myocarditis, caused a marked loss of plakoglobin signal from myocardial cell-cell junction.
Sex differences may play a role in GCM; Fairweather et al. [36] recently found that testosterone promotes myocarditis, including GCM, through the soluble ST2 pathway. Soluble ST2 levels are higher in male versus female with GCM and increased soluble ST2 levels in male mice correlate with poorer heart function and a more severe myocarditis. Further investigation is needed to determine whether differences in pathogenesis will impact prognosis or treatment.
4. Clinical Presentation and Diagnosis
Heart failure is the widespread symptom in the majority of cases (approximately 75%). Unfortunately it often progresses to death or cardiac transplantation despite optimal treatment [9] . Other common presentation symptoms are ventricular tachycardia (14%), chest pain with ECG signs of acute myocardial infarction (6%) and complete heart block (5%) [37] [38] . In less than 10% of patients, heart failure symptoms progress more slowly or may even be self-limited [9] [39] [40] .
Classic echocardiography findings in patients with acute GCM include wall thickening, normal left ventricular size and poor left ventricular systolic function in early phases, whereas left ventricle usually dilates as disease progresses. Right ventricular function often deteriorates after left ventricular function worsens and it is a powerful independent death predictor or heart transplantation in patients with myocarditis [41] . Sometimes segmental wall motion abnormalities can mimic myocardial infarction. Although the echocardiographic features of myocarditis are often non-specific, a careful review of findings may be helpful in suggesting a diagnosis and to rule out other causes of heart failure, such as valvular and congenital heart disease. Echocardiographic features are also important to help distinguish between fulminant and acute myocarditis [42] : patients with fulminant myocarditis have near normal left ventricular diastolic dimensions and increased septal thickness, secondary to acute myocardial edema, whereas patients with acute myocarditis have a dilated left ventricle.
Cardiac magnetic resonance (MRI) offers various imaging sequences that target acute and chronic myocarditis [43] .
MRI has the unique potential to visualize tissue changes and can detect the typical changes in myocarditis including intracellular and interstitial edema, capillary leakage, hyperemia and, in more severe cases, cellular necrosis and fibrosis. Tissue edema can be demonstrated by T2-weighted imaging. Hyperemia and capillary leak can be detected by contrast-enhanced fast spin echo T1-weighted MR and early gadolinium enhancement. Normally gadolinium contrast material is excluded from the intracellular space of the myocytes by the sarcolemmal membranes, whereas in acute myocarditis, rupture of myocyte membranes enables gadolinium to diffuse into the cells, resulting in an increased tissue concentration and subsequent contrast enhancement. Necrosis and fibrosis, as irreversible tissue damage, are demonstrated by late gadolinium enhancement. MRI can also play a role in discriminating myocarditis from myocardial infarction: in myocarditis, the infiltrates are characteristically located in the mid-wall and tend to spare the sub-endocardium, whereas in myocardial infarction the sub-endocardium is first involved.
Compared with histopathology, currently available MRI techniques allow non invasive diagnosis of myocarditis with a high specificity, but suboptimal sensitivity [44] -[46] . Presence of myocarditis is visualized by cardiac MRI if at least one area of myocardial inflammation approaches the typical values of in-plane resolution for MRI techniques (1.4 × 1.4 mm in case of T1-weighted LGE or T2-weighted imaging). Such a “confluent hot spot” of myocardial inflammation is present at advanced and severe phase of disease; therefore diagnosis of myocarditis is more frequently made by cardiac MRI in patient with active myocarditis than those with borderline disease [47] . Another drawback of the use of cardiac MRI in myocarditis diagnosis is the potential therapeutic options if the exact cause (such as viral, bacterial, giant cell or eosinophilic) is known (Figure 1).
Data regarding cardiac MRI in GCM diagnosis are limited because the majority of patients are instable and can’t undergo cardiac MRI, so myocardial tissue diagnosis is required to making the diagnosis of GCM on initial clinical presentation.
Right ventricular endomyocardial biopsy (EMB) has a high sensitivity because GCM is characterized by a diffuse involvement of the endocardium. EMB sensitivity was found to be 82% - 85% in patients who present early in the course of fulminant disease, compared to the gold standard of surgical pathology [48] . Because of the possibly life-threatening complications associated with GCM and the potential benefit from treatment, early percutaneous or surgical myocardial biopsy is recommended. The 2007 AHA/ACC/ESC scientific statement on the role of EMB recommended that biopsy should be performed in the clinical scenario of new onset heart failure of 2 weeks to 3 months duration associated with dilated left ventricle and new ventricular arrhythmias, second or third degree heart block, or failure to respond to usual care within 1 - 2 week [49] . In up to 20% of cases a false negative EMB may occur and dictate a need for a second biopsy if the clinical course strongly suggests GCM [50] . Repeated endomyocardial biopsies are frequently needed to diagnose GCM, as recently attested by Kandolin et al. [51] . They reviewed diagnostic procedures in 32 patients with histologically verified GCM: repeated procedures improved the yield of EMB from 68% to 93%. In Table 1, we summarized the current indications for EMB.
In patients with a fulminant clinical course, EBM is useful to differentiate between GCM and lymphocytic myocarditis [48] . Differential diagnosis is important because the transplant-free survival with GCM is significantly lower than for lymphocytic myocarditis. Further different combinations of immunosuppressive agents may be needed to treat GCM.
GCM is characterized by serpiginous areas of myocyte necrosis with mixed inflammatory infiltrate composed of lymphocytes, plasma cells, histiocytes, eosinophils and multinucleated giant cells, in absence of well-formed granulomas [52] .
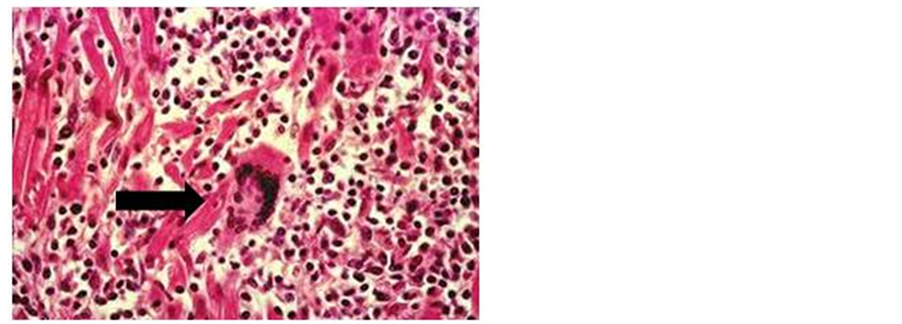
Figure 1. Infiltrated myocarditis with multinucleated giant cells.
Table 1. Indications of endomyocardial biopsy.
It may be difficult to differentiate cardiac sarcoidosis (CS) from GCM on EMB. In GCM, giant cells are often located at the edges of the inflammation and are associated with myocyte destruction and active inflammation [9] . CS is characterized by noncaseating granulomas with limited lymphocyte infiltrate and patchy fibrosis; giant cells, if present, are generally in the centre of follicular granulomas [9] . Eosinophils are significantly more common in GCM, while fibrosis is more common in CS. In addition in GCM most lymphocytes are of the CD8+ phenotype, whereas in sarcoidosis they are predominantly CD4+ type [53] . Therefore we can say that the main differences between GCM and CS can be summarized: the absence of well formed granulomata in GCM as opposed to cardiac sarcoidosis, the lack of involvement of the epicardial fat in GCM, while in most cases of cardiac sarcoidosis, granulomata were seen in the epicardial fat, the prominent eosinophilia in the inflammatory infiltrate seen in GCM is in general absent in cardiac sarcoidosis.
EBM has proven to be useful for excluding other causes for cardiac disease, included hypersensivity myocarditis and myocarditis associated with systemic lupus erythematosus or Takayasu’s aortitis.
EBM is an invasive procedure with a 1:1000 risk of death and 1:2450 risk of perforation in adults [54] , so the risk and cost must be weighed against the potential benefit of early initiating treatment. If EMB seems indicated based on clinical presentation, it should be performed by an experienced operator in a medical centre with a low procedural complication rate, surgical back-up and timely expert cardiac pathology consultation available. EMB has other method-specific limitations [46] , such as the “sampling error” and false negative results due to the “patchy” distribuition of myocarditis and the inability to perform serial biopsies or even no biopsies in patient with preserved left ventricular function as guidelines do not support this in such patients. MRI non invasive imaging strategy allows the diagnosis of myocarditis without risk of complications and allows repeating the procedure at any time and follow-up changes in the extent and degree of inflammation. So it may be reasonable to initially perform non invasive cardiac MRI (when it is possible) in patients with clinically suspected myocarditis and, when MRI study is not conclusive, if symptoms are persistent, invasive biopsy can be employed as a second step. Baccouche et al. [47] demonstrated that a combined approach of cardiac MRI and EMB was superior to each single technique regarding the final diagnosis of myocarditis. A combined approach could be superior for future risk stratification and implementation of specific therapies, but the exact algorithm of this combined approach is still not available. MRI may also be useful to guide tissue sampling of an endomyocardial biopsy [44] .
5. Treatment
Patients with GCM should receive the same guideline-based treatment for heart failure and arrhythmias as patients with left ventricular dysfunction and symptomatic heart failure from other causes [55] , including the administration of diuretics and ACE inhibitors or Angiotensin Receptor II blockers. Beta blockers can be used cautiously in the acute setting. Digoxin should be avoided for the risk for heart block and arrhythmias in the setting of acute inflammation, whereas amiodarone may be useful to treat ventricular tachyarrhythmias in GCM.
Immunosuppressive therapy is a well established treatment in GCM, in contrast with other types of myocarditis [56] . Treatment with cyclosporine and corticosteroids is associated with a median transplant-free survival of 12.3 months compared with 3 month for those not treated with immunosuppressive agents [57] , while corticosteroids alone had no effect [57] . More recently, the addition of CD-3 muromonad was also tried successfully in these patients [58] : an observation study of biopsy-proven GCM patients (excluding those with fulminant presentation) reported that treatment with cyclosporine and corticosteroids for one year, with/without CD-3 muromonad pretreatment for ten days dramatically improved survival (64%) without cardiac transplantation.
On contemporary immunosuppression, two thirds of patients reach a partial clinical remission characterized by freedom for severe heart failure and need of transplantation but remained subject to ventricular tachyarrhythmias [51] . There are no good data to guide immunosuppression for long-term maintenance of remission in GCM. Yet, continued treatment appears important because cessation of immunosuppression may lead to a fatal disease relapse [59] . Recurrence of GCM has been associated with a decrease or discontinuation of immunosuppression up to 8 years after initial diagnosis [60] .
Mechanical circulatory support with intra-aortic balloon pumps, extracorporeal membrane oxygenation and ventricular assist devices (VADs) has been used in GCM patients as a bridge to transplantation [61] [62] or occasionally recover [63] . The use of mechanical circulatory support as a bridge to transplantation in GCM is usually successful, but has been associated with higher risk of GCM recurrence in the allograft [64] . GCM is known to recur in the transplanted heart, but most histological recurrences in adults occur during routine survellaince biopsies. Of the 34 patients of the international GCM Registry that underwent cardiac transplantation, 9 patients had a diagnosis of GCM by EBM after transplantation and only 3 of 9 had signs and symptoms of heart failure. One symptomatic patient died 3.5 years after transplantation, whereas in the remaining 2 patients, GCM infiltrates and symptoms resolved with heightened immunosuppressive therapy [37] . The 3-year mortality rate was 15% which at the time was similar to the overall survival rates for ischemic or dilated cardiomyopathy [37] . Therefore, the possibility of GCM recurrence should not be a controindication to transplantation.
Although the outlook of GCM on combined immunosuppression looks like more favorable than usually thought, it still remains many deficiencies in our knowledge about GCM.
6. Future Recommendations
Future challenges include the optimal immunosuppressive regimens for acute phase and maintenance therapy, the most informative markers for disease follow-up and the best methods to control and prevent tachyarrhythmias. We trust in the publication of new and larger case studies that can make clarity on these topics.
Conflict of Interest
The authors have no conflict of interest regarding the opinion expressed in this manuscript and did not receive grant or financial support from industry or from any other source to prepare this review.
References
- Elliott, P., Andersson, B., Arbustini, E., et al. (2008) Classification of the Cardiomyopathies: A Position Statement from the European Society of Cardiology Working Group on Myocardial and Pericardial Disease. European Heart Journal, 29, 270-276. http://dx.doi.org/10.1093/eurheartj/ehm342
- Lindenfeld, J., Albert, N.M. and Boehmer, J.P. (2010) HFSA 2010 Comprehensive Heart Failure Practice Guideline. Journal of Cardiac Failure, 16, e1-e2. http://dx.doi.org/10.1016/j.cardfail.2010.04.004
- Saltykow, S. (1905) Uber Diffuse Myokarditis. Virchows Archiv fur Pathologische Anatomie, 182, 1-39.
- Baumgartner, H. (1915) Uber Spezifische Diffuse Produktive Myocarditis. Frankfurt. Ztschr.f.Path., 16, 91-120.
- Jonas, A.F. (1939) Granulomatous Myocarditis. Bulletin of the Johns Hopkins Hospital, 15, 749-756
- Magner, D. (1939) A Case of Fatal Subacute Myocarditis of Unknown Etiology. American Journal of the Medical Sciences, 198, 246-252 http://dx.doi.org/10.1097/00000441-193908000-00016
- Palmer, H. and Michael, I. (1965) Giant-Cell Myocarditis with Multiple Organ Involvement. Archives of Internal Medicine, 116, 444-447. http://dx.doi.org/10.1001/archinte.1965.03870030124022
- Tesluk, H. (1956) Giant cell vs Granulomatous Myocarditis. American Journal of Clinical Pathology, 26, 1326.
- Okura, Y., Dec, G.W., Hare, J.M., et al. (2003) A Clinical and Histopatologic Comparison of Cardiac Sarcoidosis and Idiopathic Giant Cell Myocarditis. Journal of the American College of Cardiology, 41, 322-328. http://dx.doi.org/10.1016/S0735-1097(02)02715-8
- Vaideeswar, P. and Cooper, L. (2013) Giant Cell Myocarditis: Clinical and Pathological Disease Characteristics in an Indian Population. Cardiovascular Pathology, 22, 70-74.
- Whitehead, R. (1965) Isolated Myocarditis. British Heart Journal, 27, 220-230. http://dx.doi.org/10.1136/hrt.27.2.220
- Okada, R. and Wakafuji, S (1986) Twenty Year Autopsy Statistics of Myocarditis Incidence in Japan. Japanese Circulation Journal, 50, 1288-1293.
- December, G.W. (2003) Introduction to Clinical Myocarditis. In: Cooper Jr., L.T., Ed., Myocarditis from the Bench to the Bedside, Humana Press, Totowa, 257-281.
- Winters, G. and Costanzo-Nordin, M. (1991) Pathological Findings in 2300 Consecutive Endomyocardial Biopsies. Modern Pathology, 4, 441-448.
- Drut, R.M. and Drut, R. (1986) Giant Cell Myocarditis in a Newborn with Congenital Herpes Simplex Virus (HSV) Infection: an Immunohistochemical Study on the Origin of the Giant Cells. Fetal & Pediatric Pathology, 6, 431-437. http://dx.doi.org/10.3109/15513818609041557
- Meyer, T., Grumbach, I.M., Kreuzer, H. and Morguet, A.J. (1997) Giant Cell Myocarditis Due to Coxackie B2 Virus Infection. Cardiology, 88, 296-299. http://dx.doi.org/10.1159/000177346
- Lee, M., Kwon, G.Y., Kim, J.S. and Jeon, E.S. (2010) Giant Cell Myocarditis Associated with Coxsackievirus Infection. Journal of the American College of Cardiology, 56, Article ID: e19.
- Dennert, R., Schalla, S., Suylen, R.J., Eurlings, L. and Heymans, S. (2009) Giant Cell Myocarditis Triggered by Parvovirus B19 Infection. International Journal of Cardiology, 134, 115-116. http://dx.doi.org/10.1016/j.ijcard.2007.12.020
- Shariff, S., Straatman, L., Allard, M. and Ignaszewski, A. (2004) Novel Associations of Giant Cell Myocarditis: Two Case Reports and a Review of the Literature. Canadian Journal of Cardiology, 20, 557-561.
- Schumann, C., Faust, M., Gerharz, M., Ortmann, M., Schubert, M. and Krone, W. (2005) Autoimmune Polyglandular Syndrome Associated with Idiopathic Giant Cell Myocarditis. Experimental and Clinical Endocrinology & Diabetes, 113, 302-307. http://dx.doi.org/10.1055/s-2005-837551
- Burke, J.S., Medline, N.M. and Kratz, A. (1969) Giant Cell Myocarditis and Myositis: Associated with Thymoma and Myastenia Gravis. Archives of Pathology, 88, 359-366.
- Nash, C.L., Panaccione, R., Sutherland, L.R. and Meddings, J.B. (2001) Giant Cell Myocarditis, in a Patient with Crohn’s Disease, Treated with Etanercept—A Tumor Necrosis Factor-Alpha Antagonist. Canadian Journal of Gastroenterology, 15, 607-611.
- Ariza, A., Lopez, M.D., Mate, J.L., Curos, A., Villagrasa, M. and Navas-Palacios, J.J. (1995) Giant Cell Myocarditis: Monocytic Immunophenotype of Giant Cells in a Case Associated with Ulcerative Colitis. Human Pathology, 26, 121- 123. http://dx.doi.org/10.1016/0046-8177(95)90124-8
- Hales, S.A., Theaker, J.M. and Gatter, K.C. (1987) Giant Cell Myocarditis Associated with Lymphoma: An Immunocytochemical Study. Journal of Clinical Pathology, 40, 1310-1313. http://dx.doi.org/10.1136/jcp.40.11.1310
- Anderson, K., Carrier, M., Romeo, P., Pelletier, G.B. and Ducharme, A. (2013) An Unusual Case of Giant Cell Myocarditis Missed in a Heartmate-2 Left Ventricle Apical-Wedge Section: A Case Report and Review of the Literature. Journal of Cardiothoracic Surgery, 8, 12. http://dx.doi.org/10.1186/1749-8090-8-12
- Daniles, P.R., Berry, G.J., Tazelaar, H.D., Berry, G.J. and Cooper, L.T. (2002) The Role of Right Ventricular Endomyocardial Biopsy for Idiopathic Giant Cell Myocarditis. Journal of Cardiac Failure, 8, 74-78. http://dx.doi.org/10.1054/jcaf.2002.32196
- Kodama, M., Matsumoto, Y., Fujiwara, M., Masani, F., Izumi, T. and Shibata, A. (1990) A Novel Experimental Model of Giant Cell Myocarditis Induced in Rats by Immunization with Cardiac Myosin Fraction. Clinical Immunology and Immunopathology, 57, 250-262. http://dx.doi.org/10.1016/0090-1229(90)90039-S
- Kodama, M., Matsumoto, Y. and Fujiwara, M. (1992) In Vivo Lymphocyte-Mediated Myocardial Injuries Demonstrated by Adoptive Transfer of Experimental Autoimmune Myocarditis. Circulation, 85, 1918-1926. http://dx.doi.org/10.1161/01.CIR.85.5.1918
- Caforio, A.L., Goldman, J.H., Haven, A.J., Baig, K.M., Libera, L.D., McKenna, W.J. and Myocarditis Treatment Trial Investigators (1997) Circulating Cardiac-Specific Autoantibodies as Markers of Autoimmunity in Clinical and BiopsyProven Myocarditis. European Heart Journal, 18, 270-275. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a015230
- Fuse, K., Kodama, M., Aizawa, Y., Yamaura, M., Tanabe, Y., Takahashi, K., Sakai, K., Miida, T., Oda, H. and Higuma, N. (2001) Th1/Th2 Balance Alteration in the Clinical Course of a Patient with Acute Viral Myocarditis. Japanese Circulation Journal, 65, 1082-1084. http://dx.doi.org/10.1253/jcj.65.1082
- Hirono, S., Oslam, M.O., Nakazawa, M., Yoshida, Y., Kodama, M., Shibata, A., Izumi, T. and Imai, S. (1997) Expression of Inducible Nitric Oxide Synthase in Rat Experimental Autoimmune Myocarditis with Special Reference to Changes in Cardiac Hemodynamics. Circulation Research, 80, 11-20. http://dx.doi.org/10.1161/01.RES.80.1.11
- Chang, H., Hanawa, H., Yoshida, T., et al. (2008) Alteration of IL-17 Related Protein Expression in Experimental Autoimmune Myocarditis and Inhibition of IL-17 by IL-10-Ig Fusion Gene Transfer. Circulation Journal, 72, 813-819. http://dx.doi.org/10.1253/circj.72.813
- Shioji, K., Kishimoto, C., Nakayama, Y. and Sasayama, S. (2000) Strain Differences in Rats with Experimental Giant Cell Myocarditis. Japanese Circulation Journal, 64, 283-286. http://dx.doi.org/10.1253/jcj.64.283
- Kittlesson, M., Minhas, K., Irizarry, R., Ye, S.Q., Edness, G., Breton, E., Conte, J.V., Tomaselli, G., Garcia, J.G. and Hare, J.M. (2005) Gene Expression in Giant Cell Myocarditis: Altered Expression of Immune Response Genes. International Journal of Cardiology, 102, 333-340. http://dx.doi.org/10.1016/j.ijcard.2005.03.075
- Asimaki, A., Tandri, H., Duffy, E.R., et al. (2011) Altered Desmosomal Proteins in Granulomatous Myocarditis and Potential Pathogenic Links to Arrhythmogenic Right Ventricular Cardiomyopathy. Circulation: Arrhythmia and Electrophysiology, 4, 743-752. http://dx.doi.org/10.1161/CIRCEP.111.964890
- Fairweather, D., Petri, M.A., Coronado, M.J. and Cooper, L.T. (2012) Autoimmune Heart Disease: Role of Sex Hormones and Autoantibodies in Disease Pathogenesis. Expert Review of Clinical Immunology, 8, 269-284. http://dx.doi.org/10.1586/eci.12.10
- Cooper Jr., L.T., Berry, G.J., Shabetai, R. and Multicenter Giant Cell Myocarditis Study Group Investigators (1997) Idiopathic Giant-Cell Myocarditis-Natural History and Treatment. New England Journal of Medicine, 336, 1860-1866. http://dx.doi.org/10.1056/NEJM199706263362603
- Cooper Jr., L.T. and Blauwet, L.A. (2011) When Should High-Grade Heart Block Trigger a Search for a Treatable Cardiomyopathy? Circulation: Arrhythmia and Electrophysiology, 4, 260-261. http://dx.doi.org/10.1161/CIRCEP.111.963249
- Ren, H., Poston, R., Hruban, R.H., Baumgartner, W.A., Baughman, K.L. and Hutchins, G.M. (1993) Long Survival with Giant Cell Myocarditis. Modern Pathology, 6, 402-407.
- Davies, R.A., Veinot, J.P., Smith, S., Struthers, C., Hendry, P. and Masters, R. (2002) Giant Cell Myocarditis: Clinical Presentation, Bridge to Transplantation with Mechanical Circulatory Support, and Long-Term Outcome. Journal of Heart and Lung Transplantation, 21, 674-679. http://dx.doi.org/10.1016/S1053-2498(02)00379-0
- Mendes, L.A., Dec, G.W., Picard, M.H., Palacios, I.F., Newell, J. and Davidoff, R. (1994) Right Ventricular Dysfunction: An Independent Predictor of Adverse Outcome in Patients with Myocarditis. American Heart Journal, 128, 301-307. http://dx.doi.org/10.1016/0002-8703(94)90483-9
- Felker, G.M., Boehmer, J.P., Hruban, R.H., Hutchins, G.M., Kasper, E.K., Baughman, K.L. and Hare, J.M. (2000) Echocardiographic Findings in Fulminant and Acute Myocarditis. Journal of the American College of Cardiology, 36, 227-232. http://dx.doi.org/10.1016/S0735-1097(00)00690-2
- Friedrich, M.G., Sechtem, U., Schulz-Menger, J., Holmvang, G., Alakija, P., et al. (2009) Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. International Consensus Group on Cardiovascular Magnetic Resonance in Myocarditis. Journal of the American College of Cardiology, 53, 1475-1487.
- Mahrholdt, H., Goedecke, C., Wagner, A., Meinhardt, G., Athanasiadis, A., Vogelsberg, H., Fritz, P., Klingel, K., Kandolf, R. and Sechtem, U. (2004) Cardiovascular Magnetic Resonance Assessment of Human Myocarditis: A Comparison to Histology and Molecular Pathology. Circulation, 109, 1250-1258.
- Abdel-Aty, H., Boyé, P., Zagrosek, A., Wassmuth, R., Kumar, A., Messroghli, D., Bock, P., Dietz, R., Friedrich, M.G. and Schulz-Menger, J. (2005) Diagnostic Performance of Cardiovascular Magnetic Resonance in Patients with Suspected Acute Myocarditis: Comparison of Different Approaches. Journal of the American College of Cardiology, 45, 1815-1822.
- Yilmaz, A., Ferreira, V., Klingel, K., Kandolf, R., Neubauer, S. and Sechtem, U. (2013) Role of Cardiovascular Magnetic Resonance Imaging (CMR) in the Diagnosis of Acute and Chronic Myocarditis. Heart Failure Reviews, 18, 747-760. http://dx.doi.org/10.1007/s10741-012-9356-5
- Baccouche, H., Mahrholdt, H., Meinhardt, G., Merher, R., Voehringer, M., Hill, S., Klingel, K., Kandolf, R., Sechtem, U. and Yilmaz, A. (2009) Diagnostic Synergy of Non-Invasive Cardiovascular Magnetic Resonance and Invasive Endomyocardial Biopsy in Troponin-Positive Patients without Coronary Artery Disease. European Heart Journal, 30, 2869-2879. http://dx.doi.org/10.1093/eurheartj/ehp328
- Shields, R.C., Tazelaar, H.D., Berry, G.J. and Cooper Jr., L.T. (2002) The Role of Right Ventricular Endomyocardial Biopsy for Idiopathic Giant Cell Myocarditis. Journal of Cardiac Failure, 8, 74-78.
- Cooper, L.T., Baughman, K.L., Feldman, A.M., et al. (2007) The Role of Endomyocardial Biopsy in the Management of Cardiovascular Disease: A Scientific Statement from the American Heart Association, the American College of Cardiology and the European Society of Cardiology. Circulation, 116, 2216-2233. http://dx.doi.org/10.1161/CIRCULATIONAHA.107.186093
- Kandolin, R., Lehtonen, J. and Kupari, M. (2011) Cardiac Sarcoidosis and Giant Cell Myocarditis as Causes of Atrioventricular Block in Young and Middle-Aged Adults. Circulation: Arrhythmia and Electrophysiology, 4, 303-309. http://dx.doi.org/10.1161/CIRCEP.110.959254
- Kandolin, R., Lehtonen, J., Salmenkivi, K., Raisanen-Sokolowski, A. and Kupari, M. (2013) Diagnosis, Treatment and Outcome of Giant Cell Myocarditis in the Era of Combined Immunosoppression. Circulation: Heart Failure, 6, 15-22. http://dx.doi.org/10.1161/CIRCHEARTFAILURE.112.969261
- Davies, M.J., Pomerance, A. and Teare, R.D. (1975) Idiopathic Giant Cell Myocarditis a Distinctive Clinico-Pathological Entity. British Heart Journal, 37, 192-195. http://dx.doi.org/10.1136/hrt.37.2.192
- Litovsky, S.H., Burke, A.P. and Virmani, R. (1996) Giant Cell Myocarditis: An Entity Distinct from Sarcoidosis Characterized by Multiphasic Myocyte Destruction by Cytotoxic T Cells and Histiocytic Giant Cells. Modern Pathology, 9, 1126-1134.
- Wu, L. and Cooper, L. (2004) Potential of the Right Ventricular Endomyocardial Biopsy to Diagnose and Assist in the Management of Congestive Heart Failure: Insights from Recent Cinical Trials. Congestive Heart Failure, 10, 133-139. http://dx.doi.org/10.1111/j.1527-5299.2004.03362.x
- Dickstein, K., Cohen-Solal, A., Filippatos, G., et al. (2008) ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in Collaboration with the Heart Failure Association of the ESC (HFA) and Endorsed by the European Society of Intensive Care Medicine (ESICM). European Heart Journal, 29, 2388-2442. http://dx.doi.org/10.1093/eurheartj/ehn309
- Mason, J.W., O’Connel, J.B., Herskowitz, A., Rose, N.R., McManus, B.M., Billingham, M.E. and Moon, T.E. (1995) A Clinical Trial of Immunosuppressive Therapy for Myocarditis. The Myocarditis Treatment Trial Investigators. New England Journal of Medicine, 333, 269-275. http://dx.doi.org/10.1056/NEJM199508033330501
- Cooper, L.T., Berry, G.J. and Shabetai, R. (1997) Idiopathic Giant Cell Myocarditis—Natural History and Treatment. Multicenter Giant Cell Myocarditis Study Group Investigators. New England Journal of Medicine, 336, 1860-1866. http://dx.doi.org/10.1056/NEJM199706263362603
- Cooper, L.T., Hare, J.M., Tazelaar, H.D., Edwards, W.D., Starling, R.C., Deng, M.C., Menon, S., Mullen, G.M., Jaski, B., Bailey, K.R., Cunningham, M.W., Dec, G.W. and Giant Cell Myocarditis Treatment Trial Investigators (2008) Usefulness of Immunosuppression for Giant Cell Myocarditis. American Journal of Cardiology, 102, 1535-1539. http://dx.doi.org/10.1016/j.amjcard.2008.07.041
- Menghini, V.V., Savcenko, V., Olson, L.J., Tazelaar, H.D., Dec, G.W., Kao, A. and Cooper, J.T. (1999) Combined Immunosuppression for the Treatment of Idiopathic Giant Cell Myocarditis. Mayo Clinic Proceedings, 74, 1221-1226. http://dx.doi.org/10.4065/74.12.1221
- Cooper, L.T., Orellana, V., Kuhl, U. and Schultheiss, H.P. (2012) Long Term Risk of Death, Transplantation, and Disease Recurrence in Giant Cell Myocarditis. Journal of the American College of Cardiology, 59, E1547.
- Stoica, S.C., Goddard, M., Tsui, S., Dunning, J., McNeil, K., Parameshwar, J. and Large, S.R. (2003) Ventricular Assist Surprise: Giant Cell Myocarditis or Sarcoidosis? Journal of Thoracic and Cardiovascular Surgery, 126, 2072- 2074.
- Eid, S.M., Schamp, D., Haluschka, M.K. and Barouch, L.A. (2009) Resolution of Giant Cell Myocarditis after Extended Ventricular Assistance. Archives of Pathology & Laboratory Medicine, 133, 138-141.
- Marelli, D., Kermani, R., Bresson, J., Fishbein, M.C., Hamilton, M., Moriguchi, J., Fonarow, G.C., Cohen, B., Kobashigawa, J. and Laks, H. (2003) Support with the BVS 5000 Assist Device during Treatment of Acute Giant Cell Myocarditis. Texas Heart Institute Journal, 30, 50-56.
NOTES

*Corresponding author.


