Journal of Materials Science and Chemical Engineering
Vol.03 No.08(2015), Article ID:58658,7 pages
10.4236/msce.2015.38006
Extraction and Modelling of Oil from Eucalyptus camadulensis by Organic Solvent
Khalid M. Abed1*, Badoor M. Kurji2, Basma A. Abdul-Majeed1
1Chemical Engineering Department, College of Engineering, University of Baghdad, Baghdad, Iraq
2Chemical Engineering Department, College of Engineering, University of Anbar, Ramadi, Iraq
Email: *Khalid.chemical82@gmail.com
Copyright © 2015 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 5 July 2015; accepted 4 August 2015; published 7 August 2015
ABSTRACT
This work was conducted to study the extraction of eucalyptus oil from natural plants (Eucalyptus camadulensis leaves) by organic solvents. the effects of the main operating parameters were studied; type of solvent (n-hexane and ethanol), time to reach equilibrium, the temperature (45˚C to 65˚C) for n-hexane and (45˚C to 75˚C) for ethanol, solvent to solid ratio (5:1 to 8:1 (v/w)), agitation speed (0 to 900 rpm) and the particle size (0.5 to 2.5 cm) of fresh leaves to find the best processing conditions for the achieving maximum oil yield. The concentration of eucalyptus oil in solvent was measured by using UV-spectrophotometer. The results (for n-hexane) showed that the agitation speed of 900 rpm, temperature 65˚C with solvent to solid ratio 7:1 (v/w) of particle size 0.5 cm for 210 minute give the highest value of oil (68.5 wt%). Similar conditions for ethanol with the exception of the temperature (75˚C) give the highest yield of oil (65.07 wt%).
Keywords:
Extraction, Eucalyptus Oil, Modeling

1. Introduction
Eucalyptus oil C10H18O, is one of the most important essential oil. Oil is extracted from fresh and dried leaves, in addition to branch tips [1] . Eucalyptus oil has biological effects, antibacterial, antiviral and antifungal components and the long history of use against the effect of cold, influenza, other respiratory infection, rhinitis and sinusitis [2] . Essential oils chemistry is very complex; in nature essential oils, they have many chemical ingredients. Some of them play a major part and others a minor part. The ingredients found in essential oils are organic due to their molecular structure which is based on carbon atoms held together by hydrogen atoms. Oxygenatoms and sometimes nitrogen and sulphur atoms are also present [3] . They can be essentially classified into two groups:
A. Volatile fraction: Essential oil constituting of 90% - 95% of oil in weight.
B. Nonvolatile residue: This comprises 1% - 10% of oil, containing, fatty acids, sterols, carotenoids, waxes, and flavonoids.
The properties of eucalyptus oil are shown below [4] :
Description: Colourless to pale yellow liquid with an aromatic and camphoraceous. Odour and pungent, cooling, spicy taste.
Molecular formula: C10H18O.
1.1. Extraction
Solid liquid extraction, leaching, refers to the extraction of soluble constituent from a solid by means of a solvent. The method used for the extraction will be determined by the proportion of soluble constituent present, its distribution throughout the solid, the nature of the solid and the particle size.
If the solute is uniformly dispersed in the solid, the material close to the surface will first dissolve, leaving a porous structure in the solid residue. The solvent will then have to penetrate this outer layer before it can reach further solute, and the process will become progressively more difficult and the extraction rate will fall. Generally the process can be considered in three parts: first the change of phase of the solute as it dissolves in the solvent, secondly its diffusion through the solvent in the pores of the solid to the outside of the particle, and thirdly the transfer of the solute from the solution in contact with the particles to the main bulk of the solution, the factor influencing the rate of extraction is particle size, the type of solvent used, temperature and agitation of the fluid [5] .
1.2. Extractionby Organic Solvent
Organic solvent extraction is the most common and most economic important technique for extracting aromatics in the modern perfume industry. Raw materials are submerged and agitated in a solvent that can dissolve the desired aromatic compounds as well as wax and pigments [6] . Generally a good solvent should; dissolve the desired compounds, have an appropriate boiling point, easily removed at the end of the extraction. Solvents such as benzene, hexane, ethyl alcohols, acetone and petroleum ether, etc. may be used.
The principle of solvent extraction is that when a solid material comes in contact with a solvent, the soluble components in the solid materials move to the solvent. Thus, solvent extraction of plant material results in the mass transfer of soluble active principle (medicinal ingredient) to the solvent, and this takes place in a concentration gradient. The rate of mass transfer decreases as the concentration of the active principle in the solvent increases, until equilibrium is reached. Thereafter, there will no longer be a mass transfer of the active principle from plant material to the solvent [3] .
2. Experimental
Fresh leaves of Eucalyptus camaldulensis was collected from the gardens of Baghdad University, the leaves were taken to laboratory and cut out by a pair of scissors to a small part. Figure 1 is the schematic diagram of the equipment used in this research.
Fifty gram of fresh leaves of E. camaldulensis and n-hexane were placed into the three necked extraction flask equipped with a stirrer. It was placed in a water bath as shown in Figure 1. Samples (1 ml) were taken every 10 minutes, until the equilibrium was reached. Concentration of essential oil in the solvent was determined by using UV-VIS.
3. Results and Discussion
3.1. Effect of Extraction Time
The effect of extraction time on oil extraction was studied until the equilibrium was reached, the particle size 0.5 cm at 45˚C with solvent to solid ratio 7:1 (ml/g) and agitation speed 900 rpm for both solvent (ethanol and n- hexane). The obtained results are plotted in Figure 2.
It can be seen from this figure, that using n-hexane gave more oil yield than that of ethanol.
Figure 1. Schematic diagram of the experimental unit of organic solvent extraction: (1) Water bath, (2) Heating coil, (3) Thermometer, (4) Stirrer, (5) Stirrer motor and (6) Condenser.
Figure 2. The effect of extraction time on oil extraction by solvent extraction Operating conditions (P.S. = 0.5 cm, S.R. = 7/1 (ml/g) and A.S. = 900 rpm, T = 45˚C).
3.2. The Effect of Temperature
The effect of extraction temperature was examined at different temperature (45˚C, 55˚C and 65˚C) and (45˚C, 55˚C and 75˚C) for n-hexane and ethanol respectively, under the condition of particle size 0.5 cm with solvent to solid ratio 7:1 (ml/g) and agitation speed 900 rpm until the equilibrium was reached. The obtained results are plotted in Figure 3 for n-hexane and ethanol respectively.
As seen in Figure 3 the oil yield increase by increasing temperature. Figure 3(a), shows the oilyield was 45.41 wt% at 45˚C after 225 minutes and it has reaches 68.50% at 65˚C after the same time. Figure 3(b), shown the oil yield was 40.59 wt% at 45˚C after 225 minutes. And it has reach 65.07 wt% at 75˚C after the same time. From above result the maximum yield of oil, by both solvent, obtained at 65˚C by n-hexane and 75˚C by ethanol. Increasing in temperature causes the increase in both the diffusion coefficient and the solubility of the oil in the solvent are enhanced, thus improve the extraction rate. The best extraction temperature for both solvents would be the boiling point to ensure the maximum recovery of oil [7] . These results are in agreement with the results which obtained by Sayyar et al. (2009) [7] .
3.3. The Effect of Agitation Speed
The effect of agitation speed on oil extraction was examined in the range of 300 to 900 rpm and compare it with the yield without mixing, for both solvent (ethanol and n-hexane) under the condition of particle size 0.5 cm

Figure 3. The effect of temperature on oil extraction by (a) n-hexane and (b) Ethanol; operating condition: (P.S. = 0.5 cm, S.R. = 7/1 (ml/g) and A.S. = 900 rpm).
with solvent to solid ratio 7:1 (ml/g) at 65˚C for n-hexane and 75˚C for ethanol until the equilibrium was reached. The obtained results are plotted in Figure 4 for n-hexane and ethanol respectively.
As seen in Figure 4 the oil yield increase by increasing the stirring speed and there are obvious difference between use mixing and without use.
Figure 4 show oil yield by n-hexane and ethanol respectively. The maximum oil yield without use mixing was 37 wt% by n-hexane and 32.9 wt% by ethanol for 225 minute, but the stirring speed of 300 rpm gave yield 53.96 wt% by n-hexane and 47.71 wt% by ethanol for 225 minute and it has reached 68.5 wt% and 65.07 wt% with n-hexane and ethanol respectively for 225 minute. From these results, the maximum yield of oil with both solvent 68.5 wt% by n-hexane and 65.07 wt% by ethanol with stirring speed 900 rpm for 225 minute.
Agitation of the solvent is important because it increases the eddy diffusion and therefore increases the transfer of material from the surface of the particle to the bulk of the solution [8] . Otherwise agglomerations, of dense material will settle on the bottom and become thermally degraded [3] .
3.4. The Effect of Solvent to Solid Ratio
The effect of solvent to solid ratio on oil extraction by n-hexane and ethanol was examined in the range of 5:1 to 8:1 (ml/g). The obtained results are plotted in Figure 5 for hexane and ethanol respectively under the condition of particle size 0.5 cm with agitation speed 900 rpm at 65˚C for n-hexane and 75˚C for ethanol until the equilibrium was reached.
Figure 5 show that oil extraction increases with increase in S.R. from 5:1 to 8:1 for both solvents. In case of ethanol, yield of essential oil was found to be 48.50, 55.06, 65.07 and 66.29 wt% at S.R. 5:1, 6:1, 7:1 and 8:1 (ml/g), respectively while in the case of n-hexane, it was 50.5, 60.17, 68.50, and 70.67 wt%, at S.R. 5:1, 6:1, 7:1 and 8:1 (ml/g), respectively.
Increasing solvent to solid ratio up to a specific limit will increase the yield since the concentration gradient between the solid and the liquid phase becomes greater which favors good mass transfer [7] . Based on the results, the solvent to solid ratio of 7:1 would be sufficient for both solvents to extract the maximum amount of oil and only marginal increment of oil amount can be obtained by increasing the ratio to 8:1. Therefore ratio 7:1 is the best in agreement with the results which obtained by Saxena et al. (2011) [9] .
3.5. The Effect of Particle Size
The effect of particle size on oil extraction was examined in three different size (0.5, 1.5, 2.5 cm) for each solvent (ethanol and n-hexane) under the condition of solvent to solid ratio 7/1 (ml/g) with agitation speed 900 rpm at 65˚C by n-hexane and 75˚C by ethanol until the equilibrium was reached. The obtained results are plotted in Figure 6.
As seen in Figure 6, less oil is extracted from the larger particles compared to the smaller size particles. Further it could be seen that the maximum oil extraction using hexane is 68.50 wt% from P.S. 0.5 cm compared to 32.60 wt% from 2.5 cm particle size. The similar trend is also found with ethanol. Thus percentage extraction with 0.5cm particle is nearly double of that of the particle 2.5 cm by two solvents.

Figure 4. The effect of agitation speed on oil extraction by (a) n-hexane and (b) Ethanol; operating condition: (P.S. = 0.5 cm, S.R. = 7/1 (ml/g) and temp. = 65˚C and 75˚C for n-hexane and ethanol respectively).

Figure 5. The effect of solvent to solid ratio oil extraction by (a) n-hexane and (b) Ethanol; operating conditions: (P.S. = 0.5 cm, A.S. = 900 rpm and temp. = 65˚C and 75˚C for n-hexane and ethanol respectively).

Figure 6. The effect of solvent to solid ratio oil extraction by (a) n-hexane and (b) Ethanol; operating conditions: (S.R = 7:1, A.S. = 900 rpm and temp. = 65˚C and 75˚C for n-hexane and ethanol respectively).
From these results, the maximum yield of essential oil was 68.50 wt% and 65.07 wt% for n-hexane and ethanol respectively at 0.5 cm of particle size. These results are in agreement with the results which obtained by Saxena et al. (2011) [9] and Sayyar et al. (2009) [7] .
The reason is that The smaller the size the greater is the interfacial area between the solid and liquid and therefore the higher is the rate of transfer of material; further, the smaller is the distance the solute must diffuse within the solid as already indicated [5] . Therefore, the less amount of oil will be transferred from inside the larger particles to the surrounding solution in comparison with the smaller ones [7] .
4. Mass Transfer Coefficient
In solid-liquid extraction, the solute mass transfer from solid to liquid occurs in two stages of the process [10] , namely:
1) Diffusion from the inside of the solid material to its surface.
2) Mass transfer from the surface of the solid material to liquid.
Because the size of the solid material is small, it is assumed that the solute concentration within the solid material is always homogeneous. Thus there is no gradient concentration within the solid material. In other words, the effective diffusivity within the solid material can be neglected. Therefore mass transfer between phases controls the overall mass transfer [10] .
From the overall material balance of the essential oil in the liquid phase:
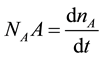 (1)
(1)
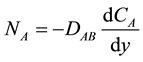 (2)
(2)
 (3)
(3)
Equation (2) is referred to Fick’s law, where NA is the molar rate of diffusion per unit area (mole/cm2・s), DAB is the diffusivity of essential oil in liquid (cm2/s), CA is the molar concentration of essential oil in the liquid phase (mole/cm3),
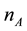 is the number moles of essential oil (mole), y is the distance in the direction of diffusion, t is the time(s), V is the volume of solvent (cm3), A is interfacial area of the solid (cm2).
is the number moles of essential oil (mole), y is the distance in the direction of diffusion, t is the time(s), V is the volume of solvent (cm3), A is interfacial area of the solid (cm2).
By substituting the Equation (2) and (3) in to the Equation (1) we get:
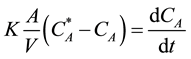 (4)
(4)
With;
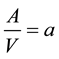
 (5)
(5)
Ka = volumetric mass transfer coefficient (1/min).
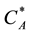 = Oil concentration in the liquid phase which in equilibrium with the oil concentration in the solid material, (mole/cm3).
= Oil concentration in the liquid phase which in equilibrium with the oil concentration in the solid material, (mole/cm3).
 (6)
(6)
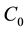 = initial concentration of essential oil in liquid (mole/cm3).
= initial concentration of essential oil in liquid (mole/cm3).

Pure solvent is used initially,


A plot of

Table 1 and Table 2 show the effect of temperature and agitation speed on the volumetric mass transfer coefficient.
Table 1. Values of volumetric mass transfer coefficient, show the effect of temperature.
Table 2. Values of volumetric mass transfer coefficient, show the effect of agitation speed.
5. Conclusions
In solvent extraction method, hexane gives slightly better oil yield compared to ethanol at 65˚C with stirring speed 900 rpm for particle size 0.5 cm and solvent to solid ratio 7:1 (v/w) for 210 min.
The maximum yield of eucalyptus oil produced by solvent extraction, under the best conditions, was 68.5 wt%.
The value of mass transfer coefficient for hexane (3.93 *(1/s)) is greater than the value when using the ethanol as solvent.
Cite this paper
Khalid M.Abed,Basma A.Abdul-Majeed,Badoor M.Kurji, (2015) Extraction and Modelling of Oil from Eucalyptus camadulensis by Organic Solvent. Journal of Materials Science and Chemical Engineering,03,35-42. doi: 10.4236/msce.2015.38006
References
- 1. Abdul-Majeed, B.A., Hassan, A.A. and Kurji, B.M. (2013) Extraction of Oil from Eucalyptus Camadulensis Using Water Distillation Method. Iraqi Journal of Chemical and Petroleum Engineering, 14, 7-12.
- 2. Sadlon, A.E. and Lamson, D.W. (2010) Immune-Modifying and Antimicrobical Effects of Eucalyptus Oil and Simple Inhalation Devices. Alternative Medicine Review, 15, 33-47.
- 3. Handa, S., Khanuja, S.P., Longo, G. and Rakesh, D.D. (2008) Extraction Technologies for Medicinal and Aromatic Plants. United Nations Industrial Development Organization and the International Centre for Science and High Technology, 260 p.
- 4. Jiangxi Province Jishui County Hongda Natural Perfume Co. (2010) Data sheet of Eucalyptol.
- 5. Coulson, J.M. and Richardson, J.F. (2002) Chemical Engineering. 5th Edition.
- 6. http://en.wikipedia.org/wiki/Fragrance_extraction
- 7. Sayyar, S., Abidin, Z.Z., Yunus, R. and Muhammad, A. (2009) Extraction of Oil from Jatropha Seeds-Optimization and Kinetics. American Journal of Applied Sciences, 6, 1390-1395.
http://dx.doi.org/10.3844/ajassp.2009.1390.1395 - 8. Abdul-Nabi, M. (2011) Extraction of Valuable Metals from Spent Hydrodesulfurization Catalyst by Two Stage Leaching Method. MSc. Thesis, Chemical Engineering Department of the College of Engineering, University of Baghdad, Baghdad.
- 9. Saxena, D.K., Sharma, S.K. and Sambi, S.S. (2011) Comparative Extraction of Cottonseed Oil by N-Hexane and Ethanol. ARPN Journal of Engineering and Applied Sciences, 6, 84-89.
- 10. Mindaryani, A. and Rahayu, S. (2007) Essential Oil from Extraction and Steam Distillation Ocimum Basillicum. Jour- nal of WCECS.
Abbreviation
NOTES
*Corresponding author.





