Open Journal of Soil Science
Vol. 2 No. 4 (2012) , Article ID: 26190 , 7 pages DOI:10.4236/ojss.2012.24045
Recovery of Soil Test Phosphorus from an Acidic Soil Amended with Organic and Inorganic Phosphorus
![]()
Department of Soil Science, University of Chittagong, Chittagong, Bangladesh.
Email: *kashem00@yahoo.com
Received August 1st, 2012; revised September 5th, 2012; accepted September 17th, 2012
Keywords: Extraction Methods; Organic Amendments; Phosphorus; Incubation
ABSTRACT
Information on soil test phosphorus (P) in soil treated with organic amendments is important to a sound management of manure additions to agricultural fields. This study compared the recovery of cow manure, chicken manure, city compost P relative to triple super phosphate P (TSP) for an acidic soil with different antecedent soil test P (STP). Phosphorus was added at rates of 0, 100, 200, 400 and 800 mg P·kg−1 soil based on total P. The soil was incubated at field capacity for 1, 4, 8 and 16 weeks (wk) after which they were extracted using NaHCO3 (Olsen) Mehlich-3, Kelowna and Bray & Kurtz-1 extractants. Regardless of extractants, after 1 wk incubation, the highest STP source was the TSP and the least was the city compost. Soil Test P increased with the addition of amendments from different P sources. Among the amendments, soil test P in TSP amended soil gradually decreased but in the city compost amended soil slightly increased with incubation time, whereas the changes of soil test P with time in the cow and chicken manures amended soil was very negligible. Across the amendments and rates of P additions, the value of extractable P with Olsen was of 55 mg·kg−1 (16%), with Mehlich-3 was of 112 mg·kg−1 (32%), with Kelowna was of 88 mg·kg−1 (24%) and with Bray & Kurtz was of 104 mg kg−1 (29% of total added P). The P extraction efficiency was in the order: NaHCO3 < Kelowna < Bray & Kurtz-1 < Mehlich-3. This study indicates that P in organic amendments reflects plant available P through the entire incubation period but P in the TSP are likely to under estimate after 8 wk of incubation.
1. Introduction
Phosphorus is generally added to agricultural soils as inorganic fertilizer or as organic manures. As there are generally no obvious adverse effects of high soil P concentrations on plant growth, farmers have frequently added P in amounts which exceed crop removal. This is particularly in region with intensive pig, poultry or cattle production systems. As a consequence, P has been accumulated in many intensively farmed agricultural soils [1].
Organic fertilizer such as, animal manure, green manure, compost and sewage sludge may be added to cultivated soil [2]. The increasing demand of chicken meat has prompted more poultry farming with consequent effects on increased utilization of organic waste (e.g. chicken manure) as fertilizers. Organic wastes contain varying amounts of water, mineral nutrients, organic matter [3]). While the use of organic wastes as manure has been in practice for centuries world-wide and in the recent times [4-5], there still exists a need to assess the potential impacts of chicken manure on soil chemical properties and crop yield and in particular evaluating the critical application levels. Furthermore, chicken manure is preferred amongst other animal wastes because of its high concentration of macro-nutrients [6,7].
Several soil test phosphorus (STP) methods are used to estimate extractable soil P. The higher the level of STP, the higher is the risk of P exports from the soil. STP methods can be divided into two broad categories: agronomic P tests and environmental P tests. Agronomic tests such as Olsen, Kelowna and Mehlich-3 have been developed to estimate the amount of P that will be available to a crop throughout the growing season [8-10]. However environmental soil P tests extract a portion of soil P that is easily lost through surface runoff or subsurface flow and therefore use mild extractrants, for example, distilled water [11], CaCl2 [12] and NH4Cl [13]. Recently, agronomic soil P tests have been used to derive indices of environmental risk such as the degree of P saturation [14] and P index [15]. Several studies have shown that agronomic soil P tests are often correlated with soil P measured by environmental P tests as well as P in the runoff and drainage waters [16,17].
Application of city waste compost and livestock manures can benefit crop production as a valuable source of nitrogen and phosphorus. The efficiency of added mineral fertilizer P for increasing soil test P levels is typically <20%. Furthermore, the availability of city waste, chicken-and cattle-manure P to crops and it impact on soil P pools may differ from that of inorganic fertilizer. Biosolids P was 4 to 7 times less available to plants than triple super phosphate P [18]. Phosphorus in pig slurry was at least 90% as effective as P in the fertilizer because it contains 70% to 90% inorganic P [19]. However some studies suggested that manure P may be equally or more available than fertilizer P [20]. These results indicate that P availability with organic amendments is variable and not understood. A sustainable amount of effort has been put into understanding soil P chemistry specifically as it relates to P fixation P availability from inorganic fertilizer applied to soil [21]. By comparison, far less research has been devoted to understanding P availability from organic sources of P from manures and compost. Understanding city waste compost P and animal manure P chemistry in soil and trying to predict P availability is an important component for making nutrient management plans that maximize economic benefits and minimize environmental risks. Different organic amendments contain varying amounts of P. The tendency is to manage these amendments based on total P in the amendment or total loading of P. This strategy will be appropriate only if the P in these amendments behaves similarly in the soil. Therefore, the objectives of this study were 1) to compare the soil test P in soil treated with cow manure, chicken manure, city compost and TSP fertilizer P sources;
2) to determine the changes in soil test P with time treated with either organic or inorganic P sources and 3) to investigate the relationship among the soil test P extraction methods.
2. Materials and Methods
2.1. Characteristics of Soil and Organic Amendments
An acidic mineral soil, Parhartoli Sandy Loam (Aeric Endoaquepts) was used in this study. A composite soil sample from a crop producing field collected from the surface layer (0 - 15 cm) was air dried and crushed to pass through 2-mm sieve. Soil properties and some characteristics of organic amendments are presented in Table 1. The soil texture was measured by hydrometer method [22]. Soil pH was measured using 1:2.5 soil to water ratio and pH of organic amendments at 1:10 ratio. Organic carbon was measured by Walkley and Black [23] and CEC was measured by extraction with 1 M NH4OAc (pH 7.0) [24]. Cow manure was collected from Chittagong University Campus, city compost from Halishore of Chittagong City and chicken manure was from the Veterinary and Animal Sciences University of Chittagong, Bangladesh. Total P in soil and organic amendments was determined by H2O2-H2SO4 wet oxidation methods [25].
2.2. Incubation Experiment
The experiment was carried out using three organic amendments: cow manure, chicken manure, and city compost in comparison with TSP to measure soil test P in different incubation time. Cow manure was collected from the Chittagong University Campus, chicken manure from the Veterinary and Animal Sciences University of Chittagong and city compost was collected from the composting plant of Chittagong City Corporation, Halishahar, Chittagong, Bangladesh. After collection, these composts were air dried, ground and sieved to analysis and incubation study.
One hundred gram of soil was mixed with cow manure, chicken manure, city compost and TSP at rates equivalent to 0, 100, 200, 400 and 800 mg P kg−1 soil based on total P on oven dry weight basis. Each treatment was replicated three times. The soil was incubated at field capacity for 1, 4, 8 and 16 weeks at room temperature in a 250 ml plastic jar with perforated lids to allow gas exchange. Total number of jars was 240 [4 (amend) × 5 (rate) × 4 (time) × 3 (rep)]. Samples were weighed weekly and additional water was added as required. After each incubation time, soils of 60 jars were collected, air-dried, ground and passed through a 2 mm sieve for analysis. Soil P was extracted using four different extractants [8-10,26] (Table 2). Phosphorus in the various extracts and digests was measured by ascorbic acid blue color method [27]. A UV/Vis Double Beam Spectrophotometer (T80) was used to measure absorbance at wave length of 882 nm.
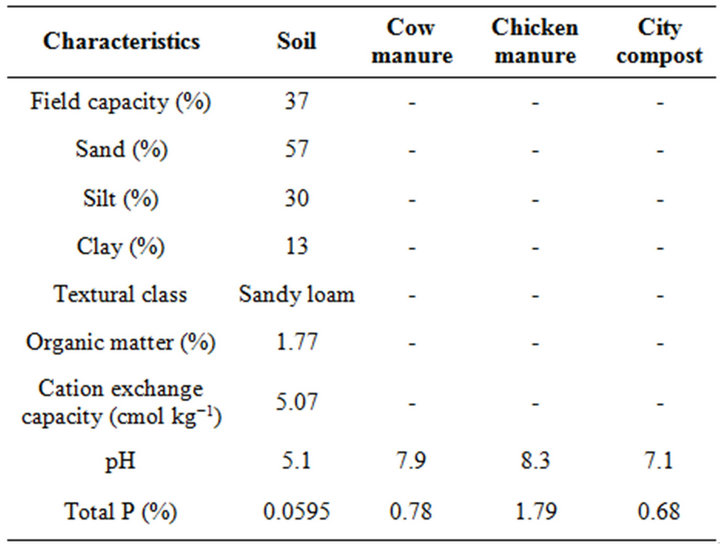
Table 1. Characteristics of the soil and organic amendments.
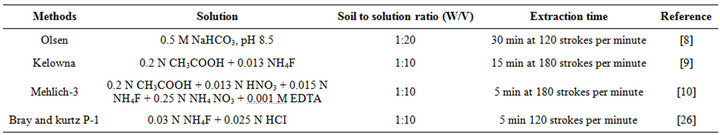
Table 2. Different methods used for extracting P from soil.
2.3. Net Phosphorus Concentration and Extraction Efficiency
To remove the effect of changes in extractable P in the control soil, we calculated the net P concentration as the difference between P in the amended soil and the control soil at each time of incubation (1, 4, 8 and 16 wk).
2.4. Statistical Analyses
The experiment was statistically analyzed as a completely randomized factorial design with three factors, amendments, rate of P additions and incubation time using analysis of variance (ANOVA). Data from each extractant was analyzed independently. Minitab statistical software, Released 11 [28] was used for analysis.
3. Results and Discussion
The results are discussed on the basis of net P concentration in amended soils. The analysis of variance (ANOVA) of the data was computed to evaluate the effect of different amendments, incubation time and rate on soil test P. The analysis of variance showed extractable P was significantly influenced by amendments, incubation time, rates of P addition and their interactions. Positive values of net P indicate that additions from organic and inorganic P sources increased extractable P (Table 3).
3.1. Effects of Amendment on Soil Test P
There were significant differences in the soil test P among the amendments regardless of soil test P extraction methods. The differences obtained among the amendments on soil test P were rate and time dependent (Table 3). The results obtained after 1 wk incubation, as extracted by NaHCO3 (Olsen-P) are used to illustrate the amendment effect of soil test P. The trend in the data obtained with other three extractants was similar to that of the NaHCO3-extractable P (Olsen-P) (Figure 1).
After 1 wk incubation, the highest soil test P source was the TSP and the least was the city compost amended soil. The regression with NaHCO3, Mehlich-3, Kelowna and Bray & Kurtz-1 extractable P against the rate of P additions were significant (p < 0.001), considering amendment and incubation time combination (Table 3). Slope of the regression reflects the increase in extractable soil P with each increment of P added by the amendments (Table 4). The slope of NaHCO3-extractable P was 0.08 for cow manure, 0.11 for chicken manure, 0.06 for city compost and 0.25 for TSP after 1 wk incubation. The corresponding slopes after 16 wk incubation were 0.09 for cow manure, 0.12 for chicken manure, 0.14 for city compost and 0.15 for TSP. The slopes of STP after 1, 4 and 8 wk incubation for cow and chicken manures were similar to those after 16 wk incubation remained relatively unchanged. However, the slopes for city compost gradually increased with incubation time, on the other hand, the slopes of extractable P decreased in the TSP amended soil (Table 4). The slope of Olsen P after 1 wk incubation was in the order of: TSP > chicken manure > cow manure > city compost. A similar order of slopes of extractable P in soils was found for the other three extractants (Table 4). Slopes indicate that city compost had the smallest STP compared to other two organic amendments. The highest extractable P obtained by TSP after 1 wk incubation is not surprising due to the high solubility of the inorganic P fertilizer. These results indicate that P extractability does not depend on the total amounts of added P, but on the characteristics of P sources. The smaller slopes of extractable P in the organic amended soils compared to inorganic fertilizer is consistent with the results of other investigators [18,29]. McCoy et al. [18] reported that city waste compost (Biosolids) P was four to seven times less available to plants than TSP fertilizer P source. These results indicate that there would be less environmental risk with cow manure and city compost P compared to other amendments at equal loading of total P.
3.2. Effects of Incubation Time on Soil Test P
The effects of incubation time on the soil test P in the amended soils depend on the nature of amendment and application rate (Tables 1 and 3). Olsen P is taken into account to represent the time effect on soil test P. In general, regardless of extractants and rates of P addition, soil test P in the TSP amended soils decreased and city compost amended soils increased with incubation time. For example, Olsen P at the lowest rate of P addition (100 mg·kg−1) varied from 9.0 to 10, 23 to 26, 8 to 15 and 39 to 8 mg·kg−1 from 1 to 16 wk incubation in the cow manure
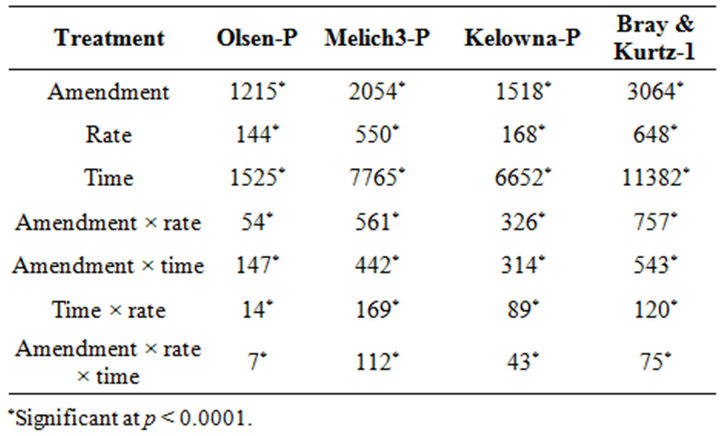
Table 3. The F-values of the analysis of variance of the effects of amendments, rate and time on the net extractable P.
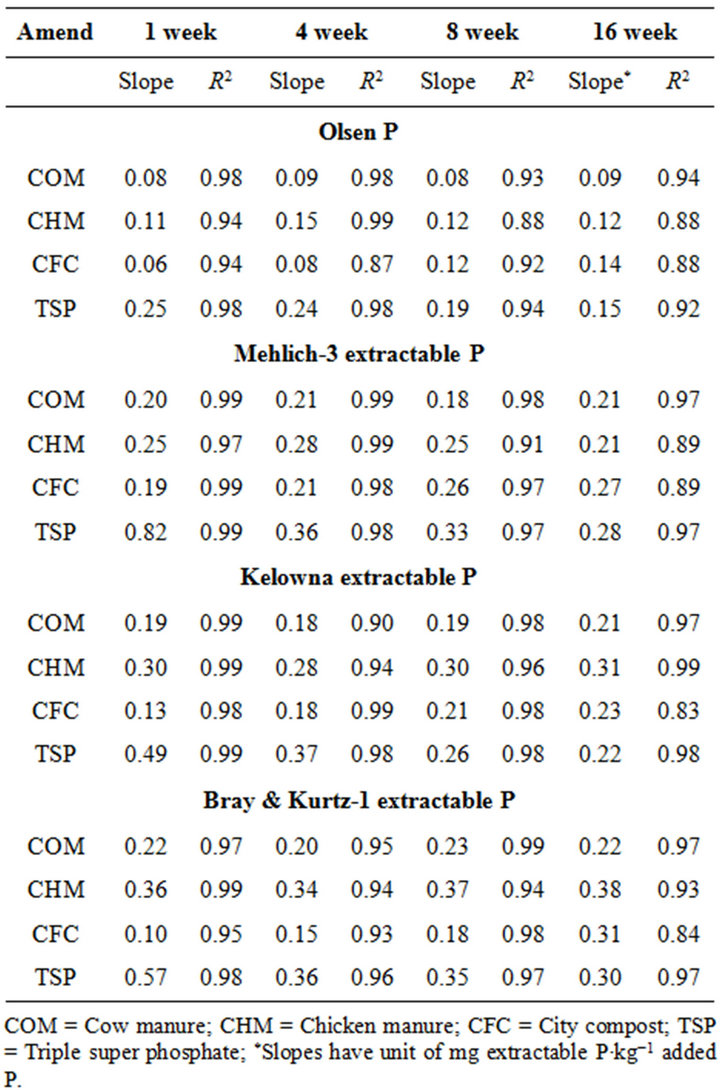
Table 4. Slope and coefficient of determination (R2) of the regression of soil extractable P at 1, 4, 8 and 16 weeks of incubation.
chicken manure, city compost and in the soil test P amended soils, respectively. The corresponding values in the same amended soils at the highest rate of P addition (800 mg·kg−1) varied from 70 to 77, 106 to 103, 55 to 88 and 220 to 122 mg·kg−1, respectively (Figure 1). The trend of soil test P changes was similar with other extractants in different amended soils but the magnitude of extractability was higher than the 0.5 M NaHCO3 extractant.
The differences in magnitude of declined in soil test P between TSP and manure suggest the P retention mechanisms. The fertilizer P was mixed with the soil in the form of granules, high P concentration around the granules would favour the precipitation of P probably as dicalcium phosphate dehydrate [30]. In contrast, with chicken and cow manures, low concentration of P will favor sorption and desorption rather than precipitation. The increase of city compost P with incubation time indicating net mineralization of city compost P. The behavior of city compost P was comparable with biosolids P. Extractable and labile (H2O plus NaHCO3 extractable P) biosolids P increased with incubation time [29,31].
3.3. Comparison of the Phosphorus Extractability in Soil
Tukey’s test for multiple means comparison showed that the means (n = 192) of Olsen, Mehlich-3, Kelowna and Bray & Kurtz-1 extractable P were significantly different (p < 0.05; Figure 2). The average net value of extractable P with Olsen was of 55 mg·kg−1 (16% of total added P), with Mehlich-3 was of 112 mg·kg−1 (32% of added total P), with Kelowna was of 88 mg·kg−1 (24% of total added P) and with Bray & Kurtz was of 104 mg·kg−1 (29% of total added P). The amount of P extracted from amended soils increased in the order: NaHCO3 < Kelowna < Bray & Kurtz-1 < Mehlich-3 (Figure 2). Sims [16] noted that Olsen extractant has less ability to remove P from the soil than the acidic extractants. In order to ascertain whether or not the several soil extracting methods considered could provide a comparable and consistent evaluation of extractable P pool, values of soil extractable P as determined by different procedures were related to each other using regression analysis. The differences among the P extraction methods probably arose from the fact that plant available P in the soil is not from a discreet fraction but from a continuum of fractions: extracting agents preferentially extract from different fractions depending on their reactions with soil components involved in P sorption [32]. Further more, each extracting solution has a different ability to extract varying portions of soil P because they were targeted at different pools of soil P [33]. The mean of Olsen P is significantly small than the means of other three extractants. This is an indication that other extracting agents extracted some forms of labile P that are not immediately available to 0.5 M NaHCO3 extractant. Though, NaHCO3 is used for calcareous soil, however, it has been commonly used as a soil P extractant in acid and neutral soils [8]. The coefficient of determination (R2) varied from 0.74 to 0.91 indicating that any of these extractants can be used to estimate P
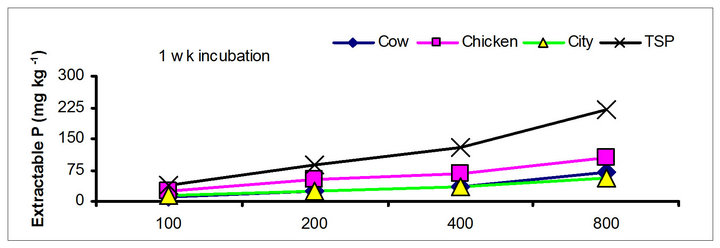
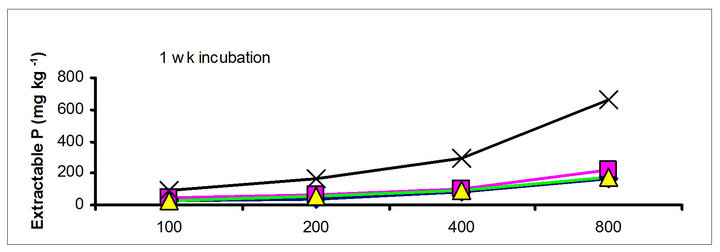
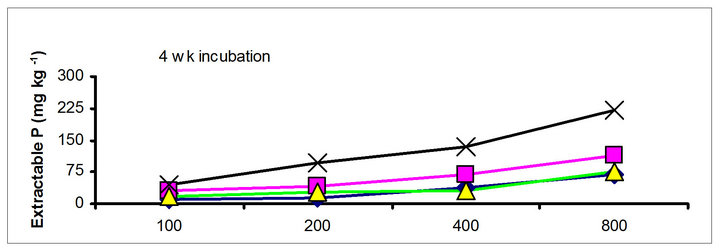
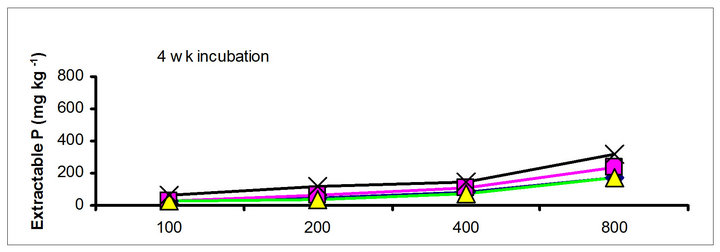
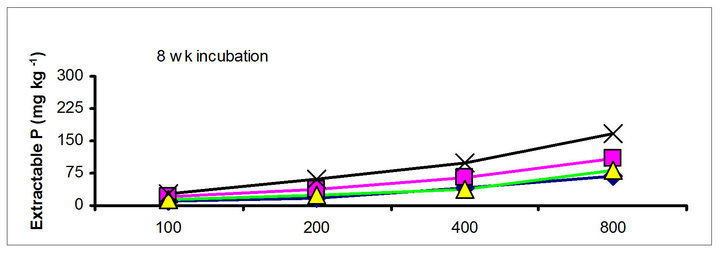
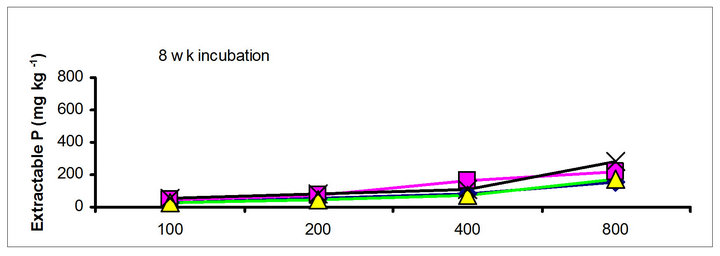
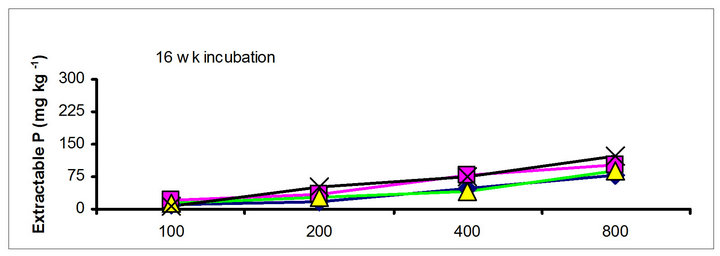
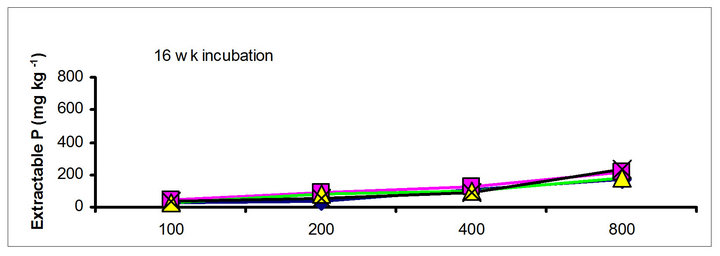
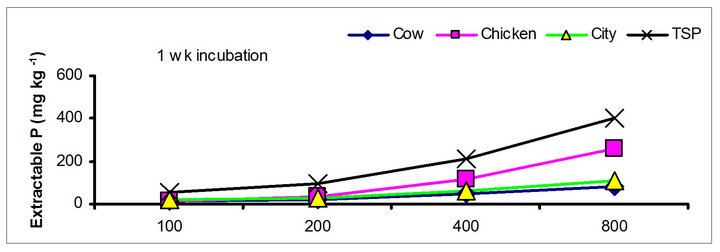
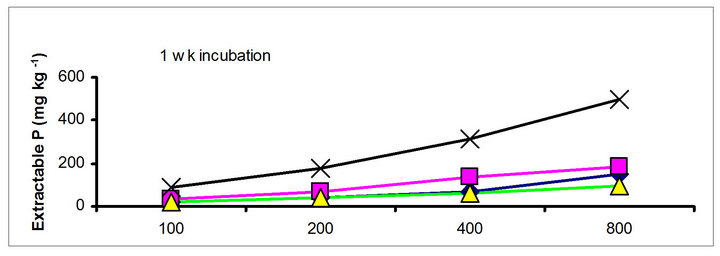
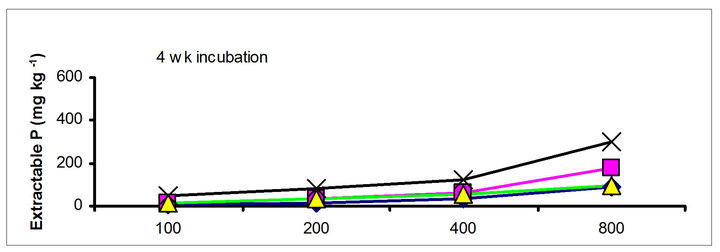
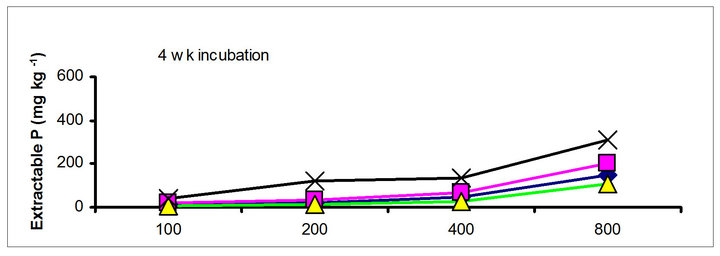
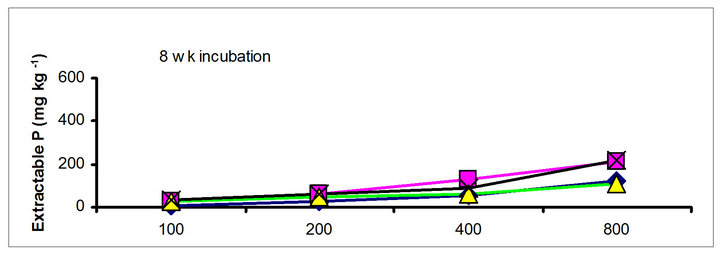
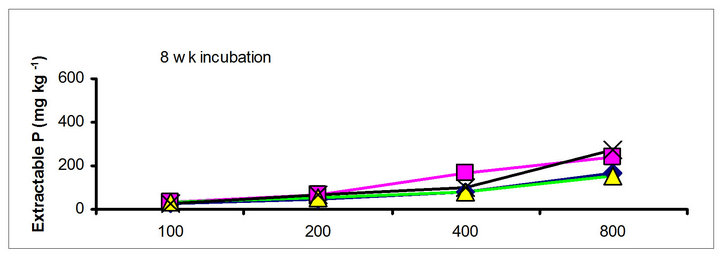
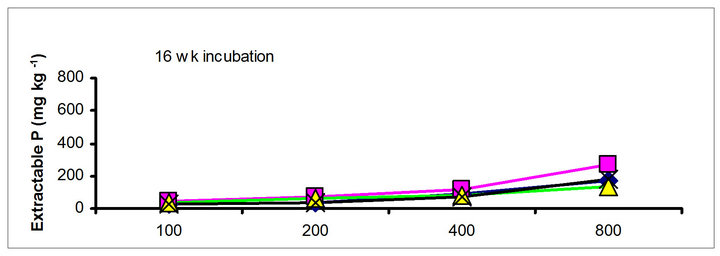
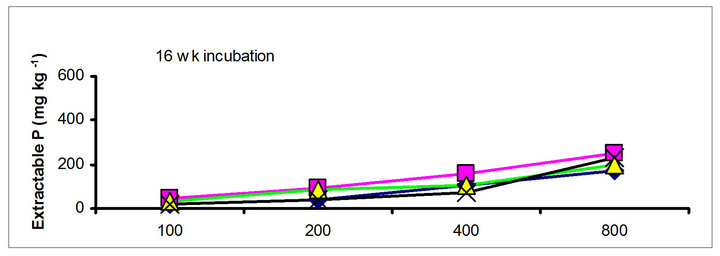
Figure 1. Net extractable P concentration (mg·kg-1) following incubation of amended soil extracted with Olsen, Mehlich-3, Kelowna and Bray & Kurtz-1.
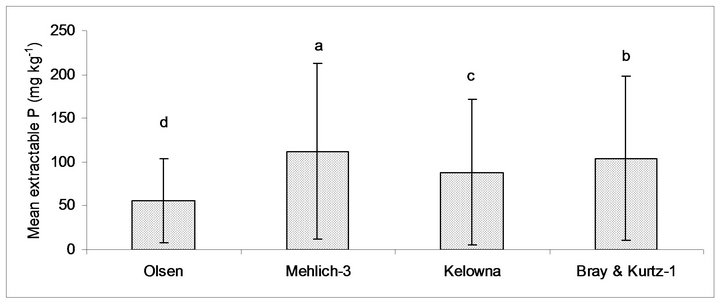
Figure 2. Mean of P extracted by the different extractable methods. Means indicated with the same letter were not significantly different (p < 0.05).
extractability (available P) in soils. It is possible to predict Mehlich-3, Kelowna and Bray & Kurtz extractable using the following regression equation.
1) Mehlich 3 extractable P = 1.817 × Olsen P + 10.94;
2) Kelowna extractable P = 1.494 × Olsen P + 4.86;
3) Bray & Kurtz extractable P = 1.702 × Olsen P + 9.54.
4. Conclusion
Soil test P increased in soils amended with organic and inorganic P sources, but the increase varied with P sources of different amendments. Extractable soil was the least with city compost and the highest with the TSP while cow and chicken manures P was intermediate between city compost and TSP at 1 wk of incubation. Among the amendments, soil test P in TSP amended soil decreased and increased in city compost amended soil with increasing incubation time. The high correlation coefficient between the various extractants, shows that all of them will provide a good index of P labiality in this soil. Our results indicate that the P in cow and chicken manures behaved almost similarly but city and TSP behaved differently upon addition to the soil and hence should be take into consideration in managing their land application for optimum plant growth and environmental point of view.
REFERENCES
- H. Tunney, “A Note on a Balance Sheet Approach to Estimating the Phosphorus Fertilizer Needs of Agriculture,” Irish Journal of Agricultural Research, Vol. 29, No. 2, 1990, pp. 149-154.
- W. E. Splittstosser, “Vegetable Growing Handbook: Organic and Traditional Method,” 3rd Edition, New York, USA, 1998.
- D. R. Edwards and T. C. Daniel, “Environmental Impacts on Farm Poultry Waste Disposal: A Review,” Bioresource Technology, Vol. 41, No. 1, 1992, pp. 9-33. doi:10.1016/0960-8524(92)90094-E
- D. C. Clay, V. Kelly, E. Mpyisi and T. Readon, “Input Use and Conservation Investments and Determinants,” In: C. B. Barrett, F. Place and A. A. Aboud, Eds., Natural Resource Management in African Agriculture: Understanding and Improving Current Practices, CABI Publishing, Oxon and New York, 2002, pp. 103-114.
- M. E. Lopez-Masquera, F. Cabaleiro, M. S. Sainz, A. Lopez-Fabal and E. Carral, “Fertilizing Value of Boiler Litter: Effect of Drying and Palletizing,” Bioresource Technology, Vol. 99, No. 13, 2008, pp. 5626-5633. doi:10.1016/j.biortech.2007.10.034
- P. R. Warman, “The Effect of Fertilizer, Chicken Manure on Timothy Yield, Tissue, Composition and Soil Fertility,” Agric Wastes, Vol. 18, No. 4, 1986, pp. 289-298. doi:10.1016/0141-4607(86)90074-0
- J. Duncan, “Composting Chicken Manure,” WSU Cooperative Extension, King County Master Gardener and Cooperative Extension Livestock Advisor, Washington State University, Pullman, 2005.
- S. R. Olsen, C. V. Cole, F. S. Watanabe and L. A. Dean, “Estimation of Available Phosphorus in Soils,” USDA, Washington, 1954.
- A. Mehlich, “Mehlich 3 Soil Extractant: A Modification of Mehlich 2 Extractant,” Communications in Soil Science and Plant Analysis, Vol. 15, No. 12, 1984, pp. 1409- 1416. doi:10.1080/00103628409367568
- W. Van Lierop, “Determination of Available Phosphorus in Acid and Calcareous Soils with Kelowna MultipleElement Extractant,” Soil Science, Vol. 146, No. 4, 1988, pp. 284-291. doi:10.1097/00010694-198810000-00009
- F. Van der Paaw, “An Effective Water Extraction Method for the Determination of Plant Available Phosphorus,” Plant and Soil, Vol. 34, No. 1, 1971, pp. 467-481. doi:10.1007/BF01372799
- M. L. Self-Davis, P. A. Moore and B. C. Joern, “Determination of Water or Dilute Salt-Extractable Phosphorus in Soils,” In: G. M. Pierzynski, Ed., Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters, Kansas State University, Manhattan, 2000, p. 24.
- G. J. Racz, “Release of Phosphorus in Organic Soils under Aerobic and Anaerobic Conditions,” Canadian Journal of Soil Science, Vol. 59, No. 3, 1979, pp. 337-339. doi:10.4141/cjss79-038
- M. Zhou and Y. Li, “Phosphorus Sorption Characteristics of Calcareous Soils and Limestone from the Southern Everglades and Adjacent Farmlands,” Soil Science Society of America Journal, Vol. 65, No. 5, 2001, pp. 1404- 1412. doi:10.2136/sssaj2001.6551404x
- A. N. Sharpley, J. L. Weld, D. B. Beegle, P. J. A. Kleinman, W. J. Gburek, P. A. Moor Jr. and G. Mullins, “Development of Phosphorus Indices for Nutrient Management Planning Strategies in the United States,” Journal of Soil and Water Conservation, Vol. 58, No. 3, 2003, pp. 137-152.
- J. T. Sims, “Soil Test Phosphorus: Olsen P,” In: G. M. Pierzynski, Ed., Methods for Phosphorus Analysis for Soils, Sediments, Residuals and Water, Kansas State University, Manhttan, 2000, pp. 20-21.
- R. W. McDowell and A. N. Sharpley, “Phosphorus Losses in Subsurface Flow Bfore and after Manure Application to Intensively Farmed Land,” Science of the Total Environment, Vol. 278, No. 1-3, 2001, pp. 113-125. doi:10.1016/S0048-9697(00)00891-3
- J. L. McCoy, L. J. Sikora and R. R. Weil, “Plant Availability of Phosphorus in Sewage Sludge Compost,” Journal of Environmental Quality, Vol. 15, No. 4, 1986, pp. 403-409. doi:10.2134/jeq1986.00472425001500040016x
- H. Tunney and B. Pommel, “Phosphorus Uptake by Ryegrass from Monocalcium Phosphate and Pig Manure on Two Soils in Pots,” Irish Journal of Agricultural & Food Research, Vol. 26, No. 2-3, 1987, pp. 189-198.
- P. M. Gale, M. D. Muller, C. Cieslik, D. D. Tyle, B. N. Duck, M. Kirchner and J. McClure, “Phosphorus distribution and Availability in Response to Diary Manure Applications,” Communications in Soil Science and Plant Analysis, Vol. 31, No. 5-6, 2000, pp. 553-565. doi:10.1080/00103620009370459
- E. F. Khasawneh, E. C. Sample and E. J. Kamprath, “The Role of Phosphorus in Agriculture,” ASA, CSSA and SSSA, Madison, 1980.
- G. J. Bouyoucos, “Hydrometer Method Improved for Making Particle Size Analysis of Soils,” Agronomy Journal, Vol. 54, No. 5, 1962, pp. 464-465. doi:10.2134/agronj1962.00021962005400050028x
- A. Walkley and I. A. Black, “An Examination of Degtjareff Method for Determining Soil Organic Matter and a Proposed Modification of the Chromic Acid Titration Method,” Soil Science, Vol. 37, No. 1, 1934, pp. 29-38. doi:10.1097/00010694-193401000-00003
- Soil Survey Laboratory Staff, “Soil Survey Laboratory Methods Manual,” USDA-SCS, Washington, 1992.
- O. O. Akinremi, N. Armisen, M. A. Kashem and H. H. Janzen, “Evaluation of Analytical Methods for Total Phosphorus in Organic Amendments,” Communications in Soil Science and Plant Analysis, Vol. 34, No. 19-20, 2003, pp. 2981-2991.doi:10.1081/CSS-120025220
- R. H. Bray and L. T. Kurtz, “Determination of Total, Organic and Available form of Phosphorus in Soil,” Soil Science, Vol. 59, No. 1, 1945, pp. 39-45. doi:10.1097/00010694-194501000-00006
- J. Murphy and J. P. Riley, “A Modified Single Solution Methods for the Determination of Available Phosphate in Natural Water,” Acta Analytica, Vol. 27, 1962, pp. 31-36. doi:10.1016/S0003-2670(00)88444-5
- Minitab Inc., “Minitab User Guide Release II,” Minitab, State College, Pennsylvania, 1996.
- M. A. Kashem, O. O. Akinremi and O. J. Racz, “Phosphorus Fractions in Soil Amended with Organic and Inorganic Phosphorus Sources,” Canadian Journal of Soil Science, Vol. 84, No. 1, 2004, pp. 83-90. doi:10.4141/S03-018
- G. J. Racz and R. J. Soper, “Reaction Products of Orthophosphates in Soils Containing Varying Amounts of Calcium and Magnesium,” Canadian Journal of Soil Science, Vol. 47, No. 3, 1967, pp. 223-230. doi:10.4141/cjss67-035
- M. A. Kashem, O. O. Akinremi and G. J. Racz, “Extractable Phosphorus in Soil Amended with High Rates of Organic Amendments,” Canadian Journal of Soil Science, Vol. 84, No. 4, 2004, pp. 459-467. doi:10.4141/S03-085
- Council for Agricultural Science and Technology, “Relevance of Soil Testing to Agriculture and the Environment,” CAST, Ames, 2000, pp. 1-12.
- M. Zhang, R. Wright, D. Heaney and D. Vanderwel, “Comparison of Different Phosphorus Extraction and Determination Methods Using Manured Soils,” Canadian Journal of Soil Science, Vol. 84, No. 4, 2004, pp. 469-475. doi:10.4141/S02-023
NOTES
*Corresponding author.

