Advances in Materials Physics and Chemistry
Vol.3 No.1A(2013), Article ID:30337,6 pages DOI:10.4236/ampc.2013.31A006
Y3Fe5O12 Prepared by Mechanosynthesis from Different Iron Sources
1CIITEC, Instituto Politécnico Nacional, Mexico City, Mexico
2Área Académica de Ciencias de la Tierra y Materiales, UAEH, Hidalgo, Mexico
3Departamento de Materiales Metálicos y Cerámicos, IIM, UNAM, Mexico City, Mexico
4ITODYS, UMR, CNRS 7086, Université de Paris-Diderot, Paris, France
Email: claudia.alicia.cortes@gmail.com
Copyright © 2013 Claudia A. Cortés-Escobedo et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received January 18, 2013; revised March 3, 2013; accepted April 7, 2013
Keywords: Mechanochemistry; YIG; Annealing; Iron Garnet
ABSTRACT
Yttrium iron garnet, Y3Fe5O12 (YIG) powders were synthesized by mechanochemical processing (MCP) from different iron sources (FeO, Fe2O3 and Fe3O4) mixed with Y2O3, followed by a heat treatment. The aim of this work is to demonstrate that MCP followed by annealing at very low temperatures (as compared with the classic solid state reaction) can induce the formation of nanostructured YIG. The effect of iron source on final structure was also studied. X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used to characterize the synthesized powders. The precursors mixed in a stoichiometric ratio to obtain YIG were milled at room temperature in a shaker mixer mill with a ball:powder weight ratio of 10:1. A partial synthesis of YIG was achieved after 9 h of milling time by using the three sources of iron; however, a significant fraction of the product was the perovskite YFeO3. The largest yield of YIG was obtained by using FeO. In all cases a single garnet phase could only be completely obtained after an annealing process at 900˚C, around 400˚C lower than the typical temperatures to prepare the material by solid state reaction. An analysis of the microstrain and lattice parameters associated with peak displacements is discussed.
1. Introduction
Ferrimagnetic garnets have attracted attention as microwave device materials and magneto-optical recording medium for their unique magneto-optical properties [1]. In order to modify these properties, doping with different cations such as bismuth [2,3], lanthanum [4,5], cerium [6], titanium [7], gadolinium [6] and other rare earth cations has been reported.
Garnets are characterized by a compact oxygen array [4] and are assigned to space group Ia3d 8(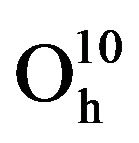 ) where the cations are located at the center oxygen polyhedron [2,8]. Due to the possibility to exchange the positions of the cations in the cell, these materials are the basis for many high-technology devices like telecommunications and microwave. The fundamental magnetic properties of YIG are originated from the magnetic ions and their relationship to the surrounding oxygen ions.
) where the cations are located at the center oxygen polyhedron [2,8]. Due to the possibility to exchange the positions of the cations in the cell, these materials are the basis for many high-technology devices like telecommunications and microwave. The fundamental magnetic properties of YIG are originated from the magnetic ions and their relationship to the surrounding oxygen ions.
Yttrium iron garnet can be synthesized by several methods. The conventional and oldest one is the sintering of the corresponding oxides in a furnace at 1400˚C (solid state reaction) [3,5]. This process produces large particles and consumes a great amount of energy [9]. This method can produce an intermediate phase, YFeO3, which has an antiferromagnetic structure. Iron garnet powders can be produced by wet chemical methods such as sol-gel [6-10], coprecipitation [8], chemical vapor deposition [11], thermal plasma spraying [12], etc. A particular method is mechanochemical synthesis which promotes the formation of a new oxide by mechanical activation of precursor’s oxides or salts [13]. In spinel ferrites, it leads to a change in the distribution of cations in interstitial sites, thereby modifying the magnetic properties [13,14]. Mechanosynthesis of yttrium iron garnet with subsequent heat treatment have been reported in the literature, using annealing temperatures higher than 900˚C. Paesano et al. [15] reported the successful mechanosynthesis of YIG from a mixture of Y2O3 and Fe2O3 followed by an annealing at 1000˚C. Widatallah et al. [16] presented a detailed study of the influence of the milling process on the formation of single phase YIG by mechanochemical synthesis, using similar experimental conditions to those reported by Paesano [15]. Also, M. Niyaifar et al. [2] used high energy milling to mechanically activate the powder mixture of BiO, Y2O3, and Fe2O3 and achieve doped Bi-YIG complete synthesis by subsequent annealing at high temperature. All these authors characterized magnetically the obtained powders.
In mechanochemical synthesis experiments in air involving metallic oxides, the reduction of iron oxide phases has been observed to occur in a closed stainless steel container after prolonged milling, and the reaction occurs in a steady-state manner during milling [14,17-20].
2. Experimental Procedure
Fe2O3 (Sigma Aldrich, 99% purity), FeO (Sigma Aldrich, 99% purity), Fe3O4 (Sigma Aldrich, 99% purity), La2O3 (Sigma Aldrich, 99.9% purity), Y2O3 (Sigma Aldrich, 99.9% purity) and Gd2O3 (Sigma Aldrich, 99.9% purity) powders were used as precursor materials. These powders were mixed in a stoichiometric ratio according to the following equations:
 (1)
(1)
 (2)
(2)
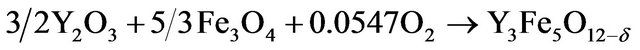 (3)
(3)
The oxygen (0.0547 mol) in these reactions was calculated from the air contained in the milling vial that was used in the milling process. A total of 5 g of the starting mixtures were loaded with steel balls of 1.27 cm in diameter into a steel cylindrical vial (50 cm3) (steel/steel, S/S) at room temperature in air and milled during 9 hours. The charge ratio (CR) was 10:1. To prevent excessive heating of the vials, the experiments were carried out by alternating 90 min of milling followed by 30 min in standby. Annealing treatments were performed from 700˚C to 1000˚C in air atmosphere.
Milled powders were characterized by X-ray diffraction (XRD) using a Siemens D5000 diffractometer with CoKα1 (λ = 1.790300 Å) radiation. Patterns were collected in a 2θ interval of 20˚ - 120˚ with increments of 0.02 (2θ). Rietveld refinement was performed on the Xray patterns. This method takes into account all of the information collected in a pattern, and it uses a least squares approach method [21] to refine the theoretical line profile until it matches the measured profile.
Morphologies of the milling powders were analyzed using a scanning electron microscope, JEOL JSM-6300, working at 15 kV.
3. Results and Discussion
3.1. Mechanosynthesis
Figure 1 shows the X-ray diffraction patterns of powder mixtures after 9 hours of milling time for the three iron sources: FeO, Fe2O3 and Fe3O4. This figure shows the partial formation of perovskite and garnet structures for all the samples.
On the other hand, a widening of the peaks is observed which is associated with a decrease in particle size. In particular, for the mixture FeO + Y2O3, a peak is observed after 9 hours of milling at 2θ = 52.3˚. This reflection is related with the (110) planes of metallic Fe, and suggests the occurrence of the following redox semireactions:
 (4)
(4)
 (5)
(5)
But the presence of metallic Fe due to milling media erosion is also possible, because of the hardness of the FeO, higher than those for the other precursors.
The starting stoichiometry corresponds to the YIG formation; therefore, there is an iron oxide excess in the mixture as far as orthoferrite is present.
In addition, results of Rietveld refinements for powder mixtures milled for 9 hours, presented in Table 1, show the presence of metallic Fe only for the mixture FeO + Y2O3. Nevertheless, it is believed that only with a lack of oxygen the last redox reactions are favored, i.e. with only 0.0547 mol of oxygen in the vial, or by heat treatment in inert atmosphere, as will be discussed below.
Besides, Table 1 shows that YIG formation is favored by starting from Fe2+, instead of Fe3+ or (Fe2+Fe3+); in contrast, the mixture with magnetite (Fe2+Fe3+) privileges the formation of orthoferrite and reactants are undetectable after 9 hours of milling. This perovskite phase is

Figure 1. X-ray powder diffraction patterns of different mixtures milled at 9 h.
more stable than YIG according to Bolarín-Miró, et al. [13].
Cell parameters are reported in Table 2 for milled mixtures obtained from different sources of iron. The values for the orthoferrite phase appear closer to reported values [20,22-27]; these results, together with calculated microstrains, as reported in Table 1, suggest that the stability of orthoferrite structure is higher than that of the garnet phase; this could mean that YIG structure absorbs all the mechanical strain produced by milling, while orthoferrite remains with the same lattice parameters.
Calculations of the Gibbs free energy [28], i.e., the thermodynamic part of the process states that the most spontaneous reaction (presenting the lowest free Gibbs energy) occurs for the FeO + Y2O3 mixture to produce YIG in the presence of oxygen. Less spontaneous reaction, in contrast, corresponds to orthoferrite from Fe2O3 and Fe3O4.
There is however, the kinetic side which has to be considered. Some results [29,30] indicate that, even when starting from Fe3+ sources, Fe2+ ions are formed and have a larger diffusion coefficient than ferric ions. In most cases, the formation of the ferrite phase occurs by simultaneous diffusion of both iron cations. Under air atmosphere, Fe2+ ions are re-oxidized to the ferric state. It seems that in the case of FeO as a source, where ferrous ions are already present, it would be easier to enhance diffusion and formation of the resulting phases.
3.2. Annealing
The obtained results (Figure 1) show that MCP is unable to induce the full formation of YIG as a single phase, independently of the iron source (precursor used). It is therefore necessary to complete the synthesis by means of an annealing process. XRD pattern for mixtures FeO + Y2O3 milled and heat treated at temperatures from 700˚C to 1000˚C are shown in Figure 2. The garnet phase is fully formed at 900˚C; below this temperature the peaks of the orthoferrite phase are clearly observed while the peaks of the garnet phase just begin to appear at 700˚C.
The (110) Fe0 peak reflection disappears when temperature is increased up to 800˚C. It is also observed an increase in relative intensities and narrowing for all the peaks, both indicators of grain and crystal growth.
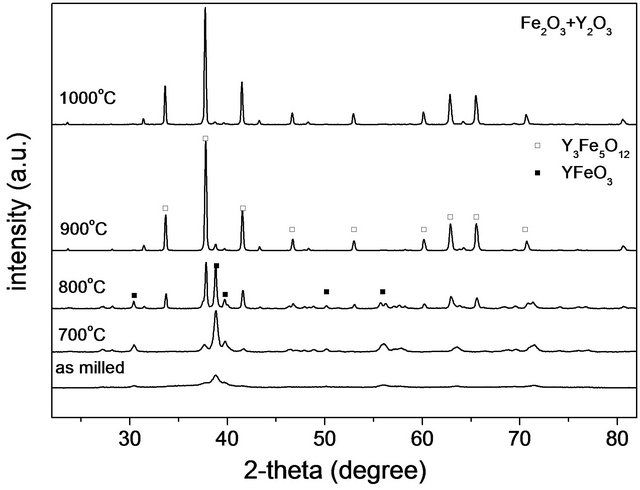
In Figure 3(a) it can be observed for the starting mixture (Fe2O3 + Y2O3), that main peaks of both garnet and perovskite phases reach comparable intensities at 800˚C, while at 900˚C the peak of the perovskite almost completely disappears.
For the Fe3O4 + Y2O3 mixture shown in Figure 3(b), it is only for T > 900˚C that peaks of orthoferrite phase decrease in intensity and disappear; at 800˚C, the perovskite phase still remains and shows a significant proportion in the milled material. Again, the width of the peaks decrease and its height increases for all the peaks, indicating grain and crystal growth.
Table 1. Data from Rietveld refinement of XRD patterns for the mixtures ball-milled 9 h.
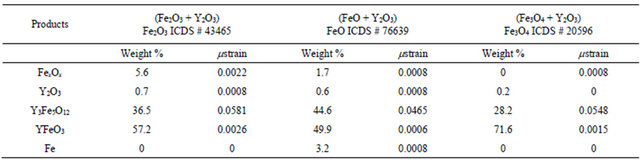
Y2O3 ICDS # 43465; Y3Fe5O12 ICDS # 2012; YFeO3 ICDS# 43260; Fe ICDS # 64998.
Table 2. Lattice parameters for mixtures after mechanosynthesis.
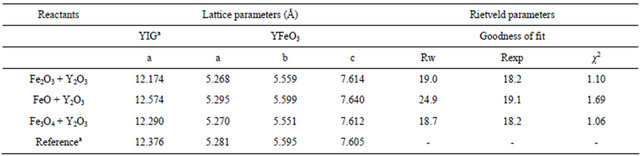
aY3Fe5O12 ICSD #2012 [23]; YFeO3 ICSD #43260 [20].

Figure 2. X-ray powder diffraction patterns of milled powders (FeO + Y2O3) annealed at different temperatures during 3 hours.
 (a)
(a)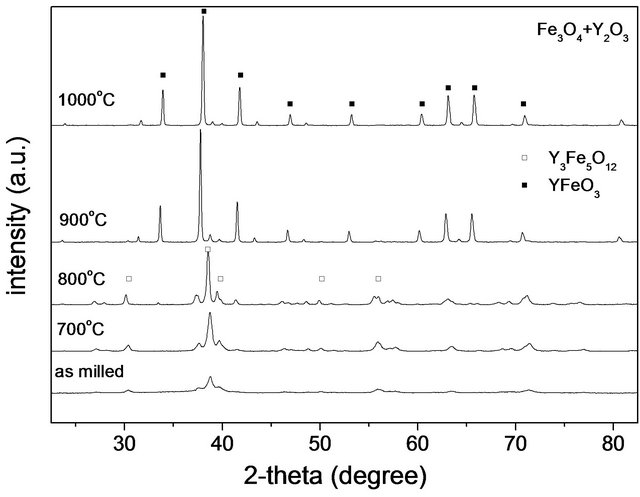 (b)
(b)
Figure 3. X-ray diffraction patterns of milled powders: (a) (Fe2O3 + Y2O3) mixture; (b) (Fe3O4 + Y2O3) mixture, annealed at different temperatures during 3 h.
3.3. Magnetic Characterization
Figure 4 shows the hysteresis loops of powders rich in orthoferrite from different oxide mixtures milled for 9 h.
There are differences in magnetic behaviors for each precursor. The highest saturation magnetization is obtained for FeO + Y2O3 milled powder mixture. This particular sample showed the presence of Fe0 in X-ray diffraction analysis. So, if saturation magnetization is additive in weight proportion to each component (according to the mixture theory), an estimation of the total magnetic saturation for each obtained sample can then be carried out. Results of calculations are presented in Table 3, as well as the experimental results extracted from the hysteresis loops in Figure 4, where is presented the magnetic characterization of different oxides mixtures from different iron oxides after milling for 9 h.
If the experimental and calculated results (Table 3) are compared, we can conclude that the calculated values are always larger than the measured ones. This can be due to two possible reasons: first, the measured value is not strictly the saturation value (at the highest applied field there remains a noticeable slope), and second, the YIG content (which is the main component with high saturation magnetization) could be slightly overestimated. The experimental results for coercive field are in the 120 - 300 Oe range, these are characteristic values of small grains with a large concentration of defects.
Figure 5 is a plot of magnetic characterization of YIG obtained from different iron oxides after heat treatment at
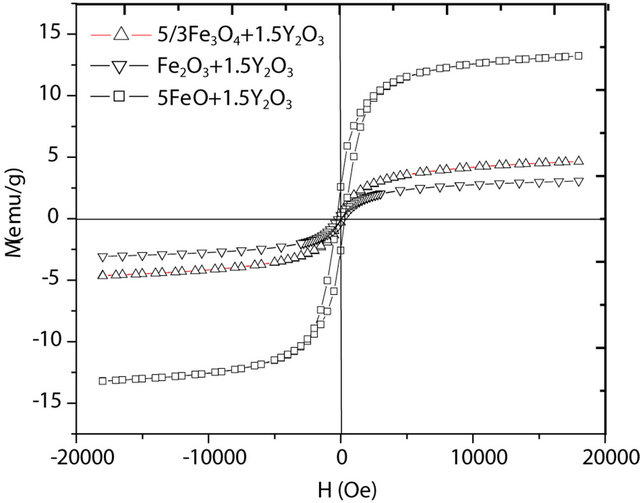
Figure 4. Hysteresis loops at Hmax = 19 kOe measured at room temperature, for oxide powders milled from (FeO + Y2O3), (Fe2O3 + Y2O3) and (Fe3O4 + Y2O3) mixtures.
Table 3. Magnetization results (emu/g at 19 kOe) [29-32].
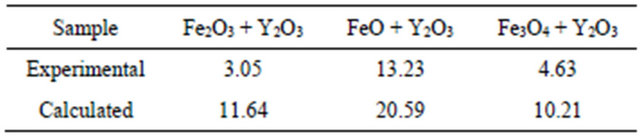

Figure 5. Hysteresis loops measured at room temperature for powders heat-treated at 900˚C.
900˚C. The highest value obtained is for FeO precursor with saturation magnetization of 24.07 emu/g and coercive force (Hc) of 23.93 Oe, for the others starting oxides the values are (Fe2O3) 21.68 emu/g and Hc is 26.8 Oe, and (Fe3O4) showed a magnetization of 23.58 emu/g and Hc of 27.37 Oe.
Diminution in values of coercive field with respect to powder samples indicates a relief of defects due to milling, and an increase of crystallite size, in agreement with XRD results.
4. Conclusion
Mechanosynthesis from mixtures of Y2O3 with Fe2O3, Fe3O4 and Fe2O3 leads to the formation of a mixture of orthoferrite and garnet phases; a single YIG phase is successfully obtained after annealing at 900˚C in all cases. A thermodynamic calculation showed that the (FeO + Y2O3) starting mixture produced a slightly larger fraction of YIG, as compared with the other two precursors. The magnetic properties of YIG samples produced by different sources showed minor differences in magnetic behavior; the YIG obtained from (FeO + Y2O3) exhibited a Ms value only slightly higher and very close to the reported one.
5. Acknowledgements
This project was financially assisted by the National Science and Technology Council of Mexico, CONACyT under grants No. 129910 and 130413. Authors are grateful to Adriana Tejeda-Cruz from Institute for Materials Research, UNAM, for technical support in XR diffraction.
REFERENCES
- Z. Cheng, H. Yang, L. Yu and W. Xu, “Saturation Magnetic Properties of Y3−x RexFe5O12(Re: Gd, Dy, Nd, Sm and La) Nanoparticles Grown by a Sol-Gel Method,” Journal of Materials Science: Materials in Electronics, Vol. 19, No. 5, 2008, pp. 442-447. doi:10.1007/s10854-007-9357-7
- M. N. Ramani, M. C. Radhakrishna, A. Hassnpour, M. Mozaffari and J. Amighian, “The Correlation of Lattice Constant with Superexchange Interaction in Bi-YIG Fabricated by Mechanochemical Processing,” Hyperfine Interactions, Vol. 184, No. 1-3, 2008, pp. 161-166. doi:10.1007/s10751-008-9783-9
- M. N. Ramani, M. C. Radhakrishna, A. Hassnpour, M. Mozaffari and J. Amighian, “Magnetic Studies of BixY3-xFe5O12 Fabricated Using Conventional Method,” Hyperfine Interactions, Vol. 187, No. 1-3, 2008, pp. 137-141. doi:10.1007/s10751-008-9875-6
- Z. Cheng, Y. Cui, H. Yang and Y. Chang, “Effect Lanthanum Ions on Magnetic Properties of Y3Fe5O12,” Journal of Nanoparticle Research, Vol. 11, No. 5, 2009, pp. 1185- 1192. doi:10.1007/s11051-008-9501-1
- S. R. Nimbore, D. R. Shengule, S. J. Shukla, G. K. Bichile and K. M. Jadhav, “Magnetic and Electrical Properties of Lanthanum Substitututed Yttium Iron Garnets,” Journal of Materials Science: Materials in Electronics, Vol. 41, No. 19, 2006, pp. 6460-6464.
- H. Xu and H. Yang, “Magnetic Properties of YIG Doped with Cerium and Gadolinium Ions,” Journal of Materials Science: Materials in Electronics, Vol. 19, No. 7, 2008, pp. 589-593. doi:10.1007/s10854-007-9394-2
- A. G. Arias, C. Torres, C. de Francisco, J. M. Muñoz, P. H. Gómez, O. Alejos, O. Montero and J. I. Iñiguez, “Defect Concentration in Ti-Subtituted YIG from Tg Curves,” Journal of Thermal Analysis and Calorimetry, Vol. 86, No. 1, 2006, pp. 195-198. doi:10.1007/s10973-005-7155-0
- S.-M. Sim, K. A. Keller and T.-I. Mah, “Phase Formation in Yttrium Aluminum Garnet Powders Synthesized by Chemical Methods,” Journal of Materials Science, Vol. 35, No. 3, 2000, pp. 713-717. doi:10.1023/A:1004709401795
- M. Rozman, M. Drofenik and J. Stefan, “Sintering of Nanosized MnZn Ferrite Powders,” Journal of the American Ceramic Society, Vol. 81, No. 7, 1998, pp. 1757-1764. doi:10.1111/j.1151-2916.1998.tb02545.x
- S. H. Vajargah, H. R. M. Hosseini and Z. A. Nemati, “Preparation and Characterization of Yttrium Iron Garnet (YIG) Nanocrystalline Powders by Auto-Combustion of Nitrate-Citrate Gel,” Journal of Alloys and Compounds, Vol. 430, No. 1-2, 2007, pp. 339-343. doi:10.1016/j.jallcom.2006.05.023
- M. Mikami and K. Matsumi, “Epitaxial Growth of Yttrium Iron Garnet by Chemical Vapor Deposition,” Journal of Crystal Growth, Vol. 37, No. 1, 1977, pp. 1-8. doi:10.1016/0022-0248(77)90136-1
- X. Z. Guo, B. G. Ravi, Q. Y. Yan, R. J. Gambino, S. Sampath, J. Margolies and J. B. Parise, “Phase and Microstructure Evolution in Precursor Plasma-Sprayed YIG Coatings,” Ceramics International, Vol. 32, No. 1, 2006, pp. 61-66. doi:10.1016/j.ceramint.2005.02.001
- A. M. Bolarín-Miró, P. Vera-Serna, F. Sánchez-De Jesús, C. A. Cortés-Escobedo and A. Martínez-Luévanos, “Mechanosynthesis and Magnetic Characterization of Nanocrystalline Manganese Ferrites,” Journal of Materials Science: Materials in Electronics, Vol. 22, No. 8, 2011, pp. 1046-1052. doi:10.1007/s10854-010-0257-x
- T. Verdier, V. Nachbaur and M. Jean, “Mechanosynthesis of Zinc Ferrite in Hardened Steel Vials: Influence of ZnO on the Appearance of Fe(II),” Journal of Solid State Chemistry, Vol. 178, No. 11, 2005, pp. 3243-3250. doi:10.1016/j.jssc.2005.07.033
- A. Paesano Jr., S. C. Zanatta, S. N. de Medeiros, L. F. Cótica and J. B. da Cunha, “Mechanosynthesis of YIG and GdIG: A Structural and Mössbauer Study,” Hyperfine Interactions, Vol. 161, No. 1-4, 2005, pp. 211-220. doi:10.1007/s10751-005-9193-1
- H. M. Widatallah, C. Johnson, S. H. Al-harthi, A. M. Gismelssed, A. D. Al-Rawas, S. J. Stewart, M. E. Elzain, I. A. Al-Omari and A. A. Yousif, “A Structural and Mössbauer Study of Y3Fe5O12 Nanoparticles Prepared with High Energy Ball Milling and Subsequent Sintering,” Hyperfine Interactions, Vol. 183, No. 1-3, 2008, pp. 87-92. doi:10.1007/s10751-008-9734-5
- P. M. Botta, P. G. Bercoft, E. F. Aglietti, H. R. Bertorello and J. M. P. López, “Two Alternative Synthesis Routes for MnZn Ferrites Using Mechanochemical Treatments,” Ceramics International, Vol. 32, No. 8, 2006, pp. 857- 863. doi:10.1016/j.ceramint.2005.05.023
- M. Jalaly, M. H. Enayati, F. Karimzadeh and P. Kameli, “Mechanosynthesis of Nanostructured Magnetic Ni-Zn Ferrite,” Powder Technology, Vol. 193, No. 2, 2009, pp. 150- 153. doi:10.1016/j.powtec.2009.03.008
- V. Sepelak, M. Menzel, K. D. Becker and F. Krumeich, “Mechanochemical Reduction of Magnesium Ferrite,” The Journal of Physical Chemistry B, Vol. 106, No. 26, 2002, pp. 20-25.
- P. Coppens and M. Eibschuetz, “Determination of the Crystal Structure of Yttrium Orthoferrite and Refinement of Gadolinium Orthoferrite,” Acta Crystallographica, Vol. 19, 1965, pp. 524-531. doi:10.1107/S0365110X65003833
- D. Balzar and N. C. Popa, “Analyzing Microstructure by Rietveld Refinement,” Rigaku Journal, Vol. 22, 2005, pp. 16-25.
- E. A. Owen and G. I. Williams, “A Low-Temperature XRay Camera,” Journal of Scientific Instruments, Vol. 31, No. 2, 1954, pp. 49-54. doi:10.1088/0950-7671/31/2/305
- M. Bonnet, A. Delapalme, H. Fuess and M. Thomas, “Refinement of the Structure of Yttrium Iron Garnet (YIG). A Case of Severe Extinction and Absorption,” Acta Crystallographica Section B: Structural Crystallography and Crystal Chemistry, Vol. 31, 1975, pp. 2233-2240. doi:10.1107/S0567740875007315
- R. L. Blake, R. E. Hessevick, T. Zoltai and L. W. Finger, “Refinement of the Hematite Structure,” American Mineralogist, Vol. 51, 1966, pp. 123-129.
- M. G. Paton and E. N. Maslen, “A Refinement of the Crystal Structure of Yttria,” Acta Crystallographica, Vol. 19, 1965, pp. 307-310. doi:10.1107/S0365110X65003365
- J. Benard, “Recherches Sur Les Variations de Composition du Protoxyde de Fer,” Bulletin de la Societe Chimique de France, 1949, pp. 109-116.
- G. G. Dvoryankina and Z. G. Pinsker, “The Electron Diffraction Pattern Investigation of Fe3O4,” Doklady Akademii Nauk SSSR, Vol. 132, 1960, pp. 110-113.
- Outokompu, “HSC Chemistry© for Windows,” Pori, 2002.
- R. Valenzuela, “Magnetic Ceramics,” Cambridge University Press, Cambridge, 2005, pp. 68-69.
- C. Kooy, “Material Transport in Solid State Reactions,” Fifth International Symposium on the Reactivity of Solids, Munich, 1965, pp. 21-28.
- H. Danan, A. Herr and A. J. P. Meyer, “New Determinations of the Saturation Magnetization of Nickel and Iron,” Journal of Applied Physics, Vol. 39, No. 2, 1968, pp. 669- 670. doi:10.1063/1.2163571
- M. Mozaffari, M. Gheisari, M. Niyaifar and J. Amighian, “Magnetic Properties of Mechanochemically Prepared IronWüstite (Fe-FeyO) Nanocomposites,” Journal of Magnetism and Magnetic Materials, Vol. 321, No. 19, 2009, 2981-2984. doi:10.1016/j.jmmm.2009.04.034

