Open Journal of Psychiatry
Vol. 2 No. 3 (2012) , Article ID: 21331 , 9 pages DOI:10.4236/ojpsych.2012.23028
Adjunctive pharmacotherapy and treatment patterns among initiators of SSRI therapy for major depressive disorder: A cohort study using a primary care database
![]()
1Lilly Research Laboratories, Eli Lilly and Company, Indianapolis, USA
2School of Medicine, Indiana University, Indianapolis, USA
3Epidemiology and Database Analytics, United BioSource Corporation, London, UK
Email: *classi_peter@lilly.com
Received 24 May 2012; revised 26 June 2012; accepted 6 July 2012
Keywords: SSRIs; antidepressants; major depressive disorder; adjunctive therapy; primary care; GPRD
ABSTRACT
Objective: Adjunctive therapy is often used for treatment of major depressive disorder (MDD) following an inadequate response to an antidepressant. However, there is little information regarding its practice within primary care in the United Kingdom (UK). Objectives of the study were to examine incidence and predictors of adjunctive pharmacotherapy among patients with MDD treated with selective serotonin reuptake inhibitors (SSRIs) by UK general practitioners (GPs). Methods: The General Practice Research Database was used to identify 15,274 MDD patients prescribed first-line treatment with SSRIs from 2006- 2008 (latest patient follow-up towards end of 2010). Treatment trajectories were identified and classified as adjunctive therapy, combination therapy, drug switches, dose increases, and restart of therapy. Incidence and predictors of adjunctive therapy were assessed, and healthcare resource utilization was evaluated. Results: Overall incidence of adjunctive therapy was 3.07/100 person years (95% CI 2.90 - 3.25). Patients prescribed adjunctive therapy were more likely to be female (IRR 1.15, p = 0.03), of higher age (IRRs 1.51 - 2.60, p ≤ 0.001), and had a greater depression severity score (IRR 1.02, p = 0.003). Presence of irritable bowel syndrome (IRR 1.53, p = 0.001), and an increasing Charlson Comorbidity Index (IRR 1.15, p = 0.01) were associated with a higher incidence of adjunctive therapy. MDD-related general practitioner consultations among patients who received adjunctive therapy was lower compared with patients receiving other treatment interventions (IRRs 0.79 - 0.87, p ≤ 0.001). Conclusions: Adjunctive therapy is infrequently utilized relative to other treatment options for management of MDD among patients who are inadequate responders to their SSRI treatments in UK primary care; however some groups are more likely to receive adjunctive therapy than others.
1. INTRODUCTION
Patients with major depressive disorder (MDD) commonly receive first-line pharmacologic treatment with selective serotonin reuptake inhibitors (SSRIs) due to their favourable efficacy, safety, tolerability, and generic availability [1]. However, remission from depression with SSRI therapy can be low, e.g., 37% [2]. Therefore, subsequent treatment interventions are often required for those with an inadequate response to antidepressant treatment. Given the poor outcomes associated with inadequate response, such as increased risk of relapse, chronicity, and poor psychosocial functioning [3-6], the gold standard for MDD treatment has evolved from treatment response to an increasing emphasis on achieving remission [7].
The National Institute for Health and Clinical Excellence (NICE) [1] recommends a sequenced approach in treating suboptimal response to antidepressant therapy, with an initial recommendation of increasing dose to a maximum tolerated amount. Subsequent treatment steps include switching antidepressants and further dose adjustments. Adjunctive treatment can also be undertaken in which a second medication (either an antidepressant or another psychotropic agent) is added to increase clinical efficacy of the initial antidepressant. In guidelines, such as NICE [1], adjunctive therapy is recommended as a secondary or later treatment step for patients whose depression is treatment resistant. The reluctance to use adjunctive therapy earlier in treatment algorithms may be due to off-label considerations, potential increase in adverse events, and specialist knowledge of drug interactions.
Nonetheless, as the gap between response and remission has become increasingly recognized in the treatment of patients with MDD, the role of adjunctive treatment is being considered earlier within clinical decision-making for the patient with partial response to an initial treatment. Therefore, the present study aimed to describe the use of adjunctive treatment in the United Kingdom (UK) among patients with MDD who initiated SSRI therapy. Given that the majority of patients with MDD are treated in a primary care setting, this study was undertaken using a primary care database. The specific study objectives were to: 1) assess incidence of adjunctive therapy, in the context of other MDD pharmacotherapy interventions, including dose increases, drug switches, and restarts of therapy, with respect to lines of treatment; 2) examine predictors of adjunctive therapy; and 3) compare healthcare resource utilization by therapy outcomes and lines of therapy.
2. METHODS
2.1. Data Source
Data were extracted from the General Practice Research Database (GPRD), a UK primary care clinical database. The GPRD currently contains longitudinal data at the individual patient level from 629 UK general practices and covers approximately 8.3% of the UK population, with approximately 5.1 million currently active registrants. Read codes, which involve a standard hierarchical classification system, are used by GPs to record patient medical information [8], such as diagnoses, symptoms, and referrals to secondary care. Demographics, medication prescriptions, and test results (including scores from depression questionnaires) are also included in the database.
2.2. Study Population
Patients were included if they were at least 18 years old and had received a first prescription for an SSRI between June 1st 2006 and December 31st 2008; this period was the enrolment period and the date of the incident SSRI prescription was termed the index date. The enrolment period was selected based on the addition of depression to the UK Quality and Outcomes Framework (QOF) (http://www.qof.ic.nhs.uk) in 2006 and to ensure an adequate follow-up period to assess outcomes. Patients were followed-up up to a maximum date of 25th October 2010 where data was available. To identify patients with a diagnosis in their clinical record in the period from 6 months before to 3 months after the index date, a specific Read code list for MDD was compiled from synonyms, relevant code stems, and the UK Quality and Outcomes Framework (QOF) depression rule set for MDD (http://www.qof.ic.nhs.uk/).
Because GPs do not always enter a specific Read code for a diagnosis for MDD [9,10], records with depression questionnaire scores were also captured to identify patients who scored above the cut-off for MDD in the period from the index date to six months prior to the index date. The recommended questionnaires by the QOF are the Patient Health Questionnaire (PHQ) [11], Hospital Anxiety and Depression Scale (HADS) [12] and the Beck Depression Inventory II (BDI-II) [13], which each have high sensitivity and specificity for detecting MDD. The patient’s record was also required to have at least one day of follow up data after the index date.
Patient records were excluded if they: 1) had a prescription for an SSRI or any other antidepressants in their record prior to the index date; 2) had less than 12 months of computerized data prior to the index date; 3) were not considered as “acceptable” according to GPRD’s data quality criteria; and 4) had an index date prior to the practice becoming a GPRD Up-to-Standard practice (i.e. the date that the practice was deemed to have continuous high quality data fit for use in research).
2.3. Defining Treatment Outcomes
Drug code lists were compiled for antidepressants and other psychotropic medications, including antipsychotics, anxiolytics, antimanics and stimulants, by using the British National Formulary (BNF) as a guide (http://bnf.org/bnf/), and these lists were used to identify the relevant prescriptions. For each patient, the treatment trajectory was tracked for each separate line of treatment, up to four lines. The first (index) line was comprised of the initial SSRI treatment. A new treatment line was defined by a change from the preceding treatment line into one of the treatment regimens defined in Box 1.
The prospective trajectory of each patient’s therapy profile since index date was characterized to indicate the therapy outcome for each line of treatment.
2.4. Healthcare Resource Utilization
MDD-related GP visits were identified using relevant Read code entries for depression, prescriptions for antidepressants, and referrals to specialists, such as psychiatrists and psychologists.
2.5. Covariates
Covariates included patient age, gender, and comorbid conditions, including other psychopathologies (anxiety,

Box 1. Treatment outcomes for defining lines of treatment.
and alcohol/substance misuse) and chronic pain conditions (e.g. trigeminal neuralgia, osteoarthritis, irritable bowel syndrome and migraine, etc.). Additionally, the Charlson Comorbidity Index (CCI) [14,15] was calculated for each patient. The CCI measures 17 medical conditions, such as AIDS, cardiovascular disease, cancer, diabetes, liver and renal disease, and, rheumatologic disease. Weights are assigned according to risk of death from the condition [14,15].
2.6. Statistical Analysis
Incidence of treatment outcomes was calculated using survival analysis methods, and longitudinal trends were examined from the index date through all available data. The incidence rates were calculated using person years at risk, which is defined as the cumulative time contributed in years by the at risk population, and allows for the differing lengths of follow-up among patients. The first event of each treatment outcome was identified, and hazard rates were calculated using Kaplan-Meier methods to examine trends over time. A multivariable analysis of incidence of adjunctive therapy was carried out using Poisson regression, adjusting for patient demographics, index SSRI, depression severity, psychopathologies, chronic pain comorbidities, overall comorbidity burden, and year of enrolment into the study. Wald tests were used for modelling. Annual incidence of MDD-related patient visits to the GP were calculated by line of therapy; the exposure period started from commencement of the line of therapy up to the earlier of either the end of the therapy line or six months after the line start date. The 95% confidence intervals were adjusted to account for the clustered data (i.e. multiple GP visits per patient).
The study was reviewed and approved by the GPRD Independent Scientific Advisory Committee (ISAC).
All data were analysed using Stata 11.2 (StataCorp LP, College Station, Texas).
3. RESULTS
94,932 patients were identified as receiving an incident prescription for an SSRI. Of these patients, 2059 had a recent Read code entry for MDD, and an additional 13,215 scored positively for MDD on a depression screening questionnaire, resulting in a final study cohort of 15,274 patients.
3.1. Patient Characteristics
Median age of patients was 38.0 years (10th to 90th percentiles, 21.0 - 63.0); 9100/15,274 (59.6%) were female; and 13,297/15,274 (91.2%) patients had at least a year of follow-up data after the index date. The initial SSRI prescription was most likely citalopram (N = 7468, 48.9%), followed next by fluoxetine (N = 6477, 42.4%). Relatively fewer numbers of patients received sertraline (N = 612, 4.0%), escitalopram (N = 555, 3.6%), paroxetine (N = 161, 1.1%) or fluvoxamine maleate (N = 1, 0.01%) as their first-line therapy.
3.2. Patterns of Adjunctive Therapy and Other Treatment Outcomes
Table 1 shows the annual incidence of treatment outcomes since index SSRI treatment (first-line therapy), both overall and by lines of therapy (2nd line, 3rd line, 4th line). The overall incidence of adjunctive therapy was 3.07/100 person years (95% CI 2.90 - 3.25), with the incidence being highest in line two of treatment. The annual incidence of adjunctive therapy was lower than that of all other treatment patterns examined except for combination therapy. The incidence of a dose increase for the index SSRI was 6.23/100 person years (95% CI 5.98 - 6.50). Overall incidence of switches in therapy was 8.57/100 person years (95% CI 8.28 - 8.88), and again was highest in the second-line. Restart of therapy after discontinuation was relatively high with an overall incidence of 15.00/100 person years (95% CI 14.61 - 15.40), and was highest in line two of therapy (Table 1).
Figure 1 shows Kaplan-Meier curves for the first event of each treatment outcome after the index date. At 12 months after index, 5% of patients had received adjunctive therapy, and this rate increased slowly over time to 7% of patients at 24 months, 9% at 36 months and 11% at 48 months after index. Combination therapy followed a similar pattern compared with adjunctive therapy. Switch in therapy was a slightly different treatment pattern
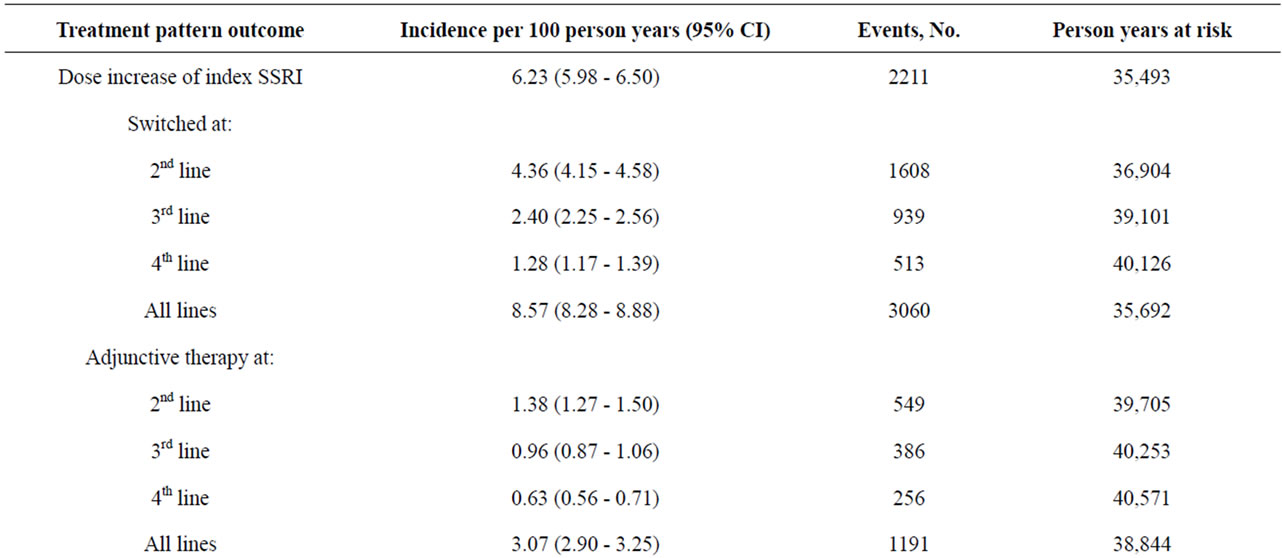
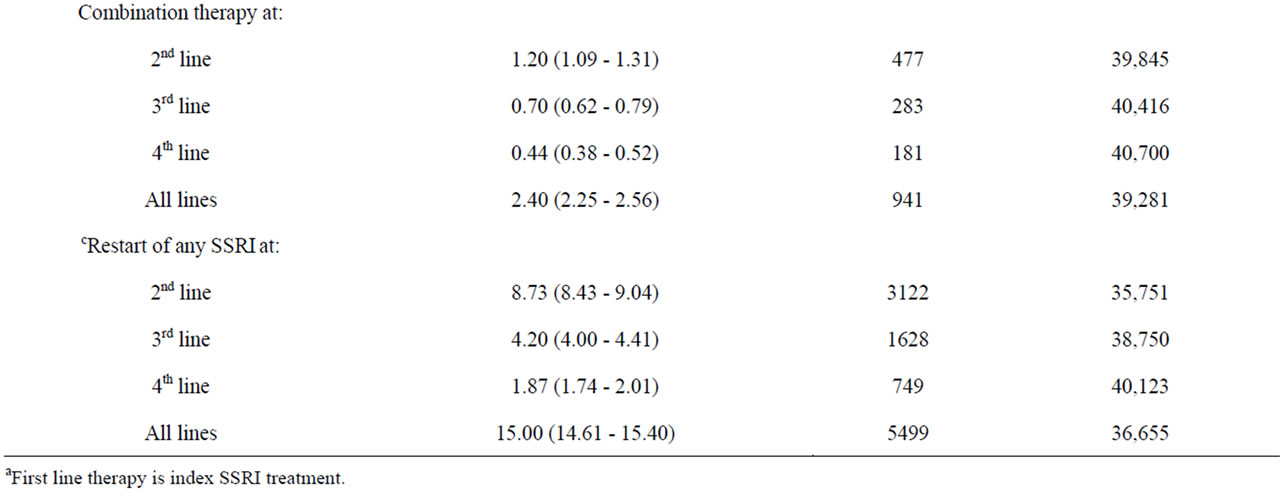
Table 1. Annual incidence of treatment outcomes since index, by lines of therapy (a2nd - 4th).
with a higher proportion of patients receiving a switch earlier than other treatment options: 10% at four months, 14% at 12 months, 17% at 24 months, and 21% at 48 months after index. Patients who received a dose increase for their index SSRI did so relatively early on with 11% with a first dose increase at 3 months after index; this rate did not rise substantially over time with 14% of patients having a dose increase by 48 months. Restarts in antidepressant therapy, in comparison, increased considerably over time, with 45% of patients having restarted therapy at 48 months after index.
3.3. Multivariable Analysis for Predictors of Adjunctive Therapy
Table 2 shows the multivariable analysis for the first incidence of adjunctive therapy after index. Patients who enrolled in the study in 2008 were 29% more likely to have had adjunctive therapy compared with those who enrolled in 2006 (p = 0.002). Females were 16% more likely to have had adjunctive therapy compared with males (p = 0.03). Greater age was strongly associated with adjunctive therapy, with the largest association in the elderly (60+ years) who were approximately twice as likely to have received adjunctive therapy compared with those aged 18 - 29 years (p < 0.001). Increasing depression severity score (p = 0.003) and CCI score (p = 0.01) were each associated with a higher likelihood of adjunctive therapy. Finally, patients with irritable bowel syndrome (IBS) were 46% more likely to have had adjunctive therapy (p = 0.001).
3.4. Healthcare Resource Utilization
Table 3 shows the incidence of MDD-related patient visits to the GP by therapy outcomes and line of treatment.
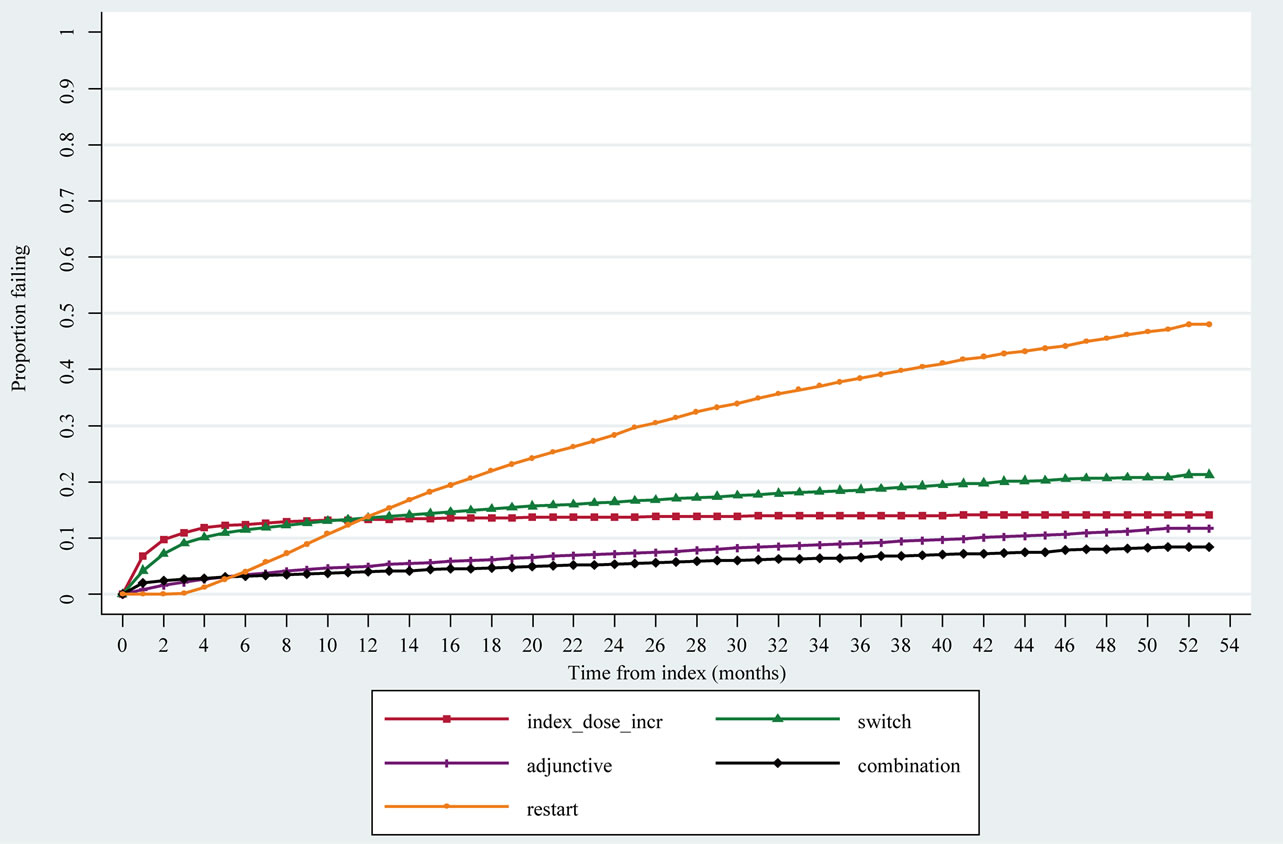
Figure 1. Kaplan-Meier failure curves (hazards) for first event of therapy outcomes after index.
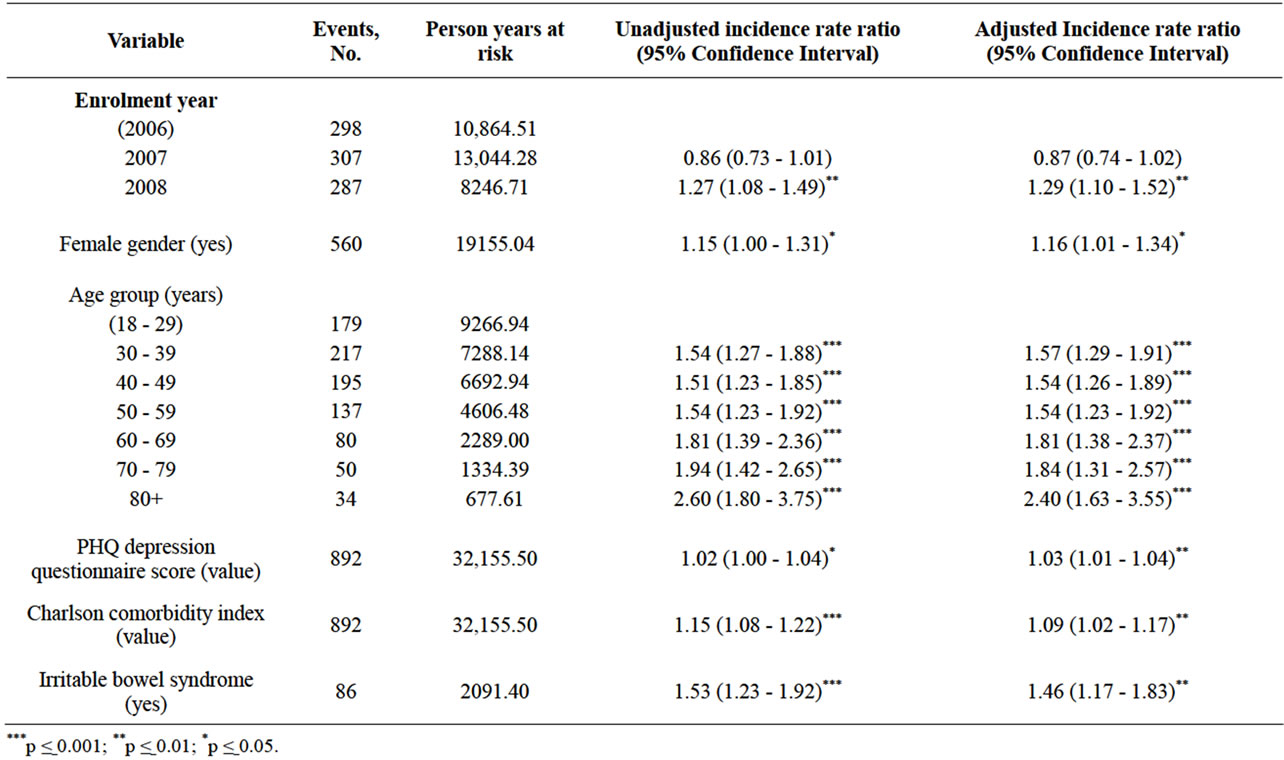
Table 2. Multivariable analysis of first incidence of adjunctive therapy (N = 12,573).
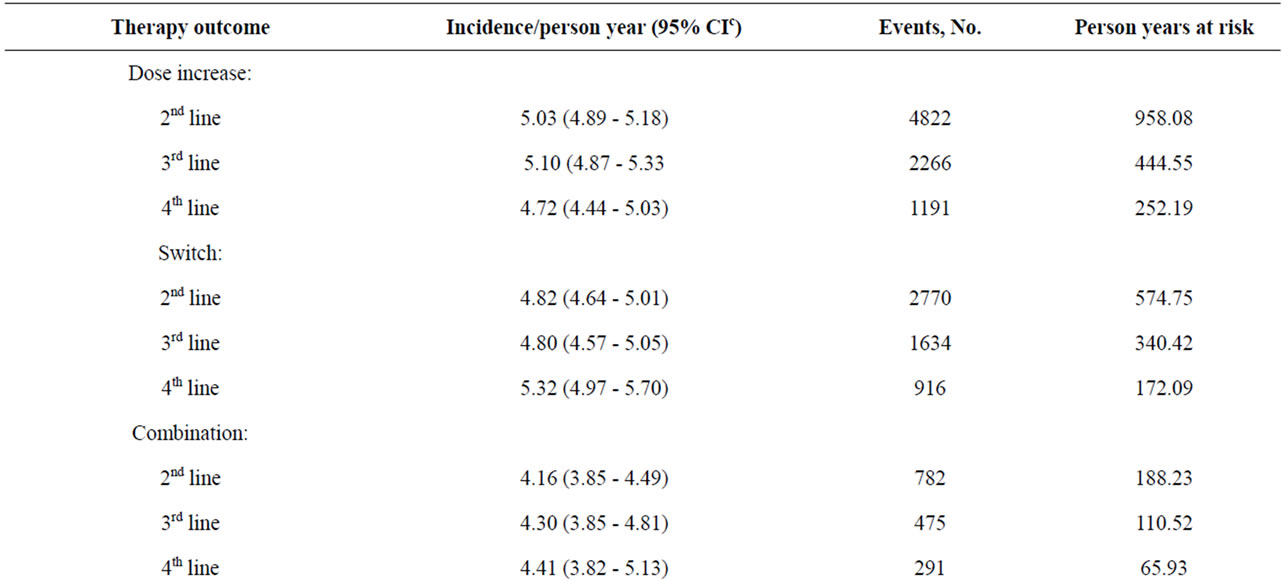
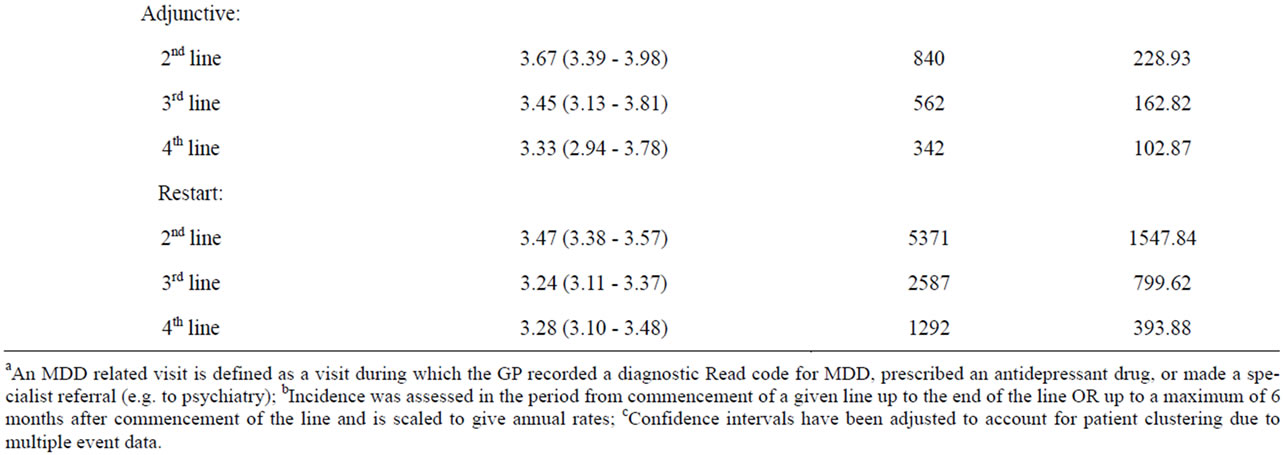
Table 3. Annual incidence of MDD relateda patient visits to GP, by treatment line and by therapy outcomes within linesb.
Across lines of treatment, the incidence of GP visits was consistently lower for adjunctive therapy compared to SSRI dose increase, antidepressant switching, and combination treatment. The incidence of GP visits was lowest for restarts in antidepressant therapy.
4. DISCUSSION
This study assessed the incidence and predictors of adjunctive therapy among patients with MDD in UK primary care in the context of overall treatment patterns and healthcare resource utilisation. Adjunctive therapy was examined by line of therapy along with other outcomes on the treatment trajectory including dose increases, drug switches, combination therapy and restart of antidepressant therapy. As such, it provides a comprehensive description of the current management of MDD patients who are inadequate responders to initial treatment with antidepressants in UK primary care.
The overall incidence for adjunctive therapy (3.07/100 person years) and for combination therapy was low compared with the incidence of either a dose increase or a drug switch. A study of 12 European countries, including the UK, found the prevalence of adjunctive antidepressant therapy to be less than 7% [16]. Similarly, a study in Spain found the prevalence of adjunctive antidepressant therapy to be 8.3% among patients treated for MDD [17]; several other studies have reported a range of adjunctive therapy prevalence of between 2% to 25% [16,18,19]. Reflecting a different health care system, a study based on a US insurance claims database found that 38% of patients who initiated on SSRI monotherapy received adjunctive treatment in the year following the initial index prescription [20].
The relatively low rates of adjunctive therapy observed in the present UK primary care study may be explained by the stepped-care model of treatment recommended by NICE [1] which advocates the initiation and management of depression in primary care, but recommends more complex interventions, including those for treatment-resistant depression, to be initiated only by mental health specialists [1]. This model of recommended treatment steps may also explain the surprising finding of adjunctive therapy most commonly occurring as early as the second-line of treatment. Possibly, the adjunctive therapy patients that were observed within primary care may have been those who were relatively stable with respect to their treatment response and had been transferred from specialist to primary care for maintenance treatment. Hence, it is possible that the database does not reflect the full trajectory of treatment for MDD in primary care. In addition, MDD-related GP consultation rates among those receiving adjunctive therapy in a given line were less frequent than for patients who received other treatment options. This finding may reflect that patients who get adjunctive therapy receive shared care from both a mental health specialist and a GP, therefore these particular patients would be likely to consult their GPs less for their depression than MDD patients on monotherapy who are solely seeking treatment from a GP.
Several factors were associated with the first incidence of adjunctive therapy. First, patients initiating therapy for MDD in 2008 were more likely to receive adjunctive therapy compared with those initiating therapy in 2006. This finding suggests that treatment patterns in the UK are in flux and may indicate an increasing proclivity towards adjunctive therapy prescribing in primary care in recent years. This trend may likely be reinforced with the recent approval in 2010 of the atypical antipsychotic Seroquel XL® (quetiapine prolonged release) as an adjunctive agent for MDD patients who have had sub-optimal response to antidepressant monotherapy (http://www.ema.europa.eu).
With regard to predictors of adjunctive therapy, females were more likely to receive adjunctive therapy compared with males, possibly relating to the greater incidence, duration, and chronicity of depression that occurs in women compared with men [21,22]. Females, compared with males, may have a more complex treatment trajectory that requires multifaceted interventions such as adjunctive therapy. Greater health-seeking behaviours in females for overall medical care, compared with males, may also be a contributory factor as men may have less opportunity to fully explore the treatment options when experiencing an inadequate response to antidepressant therapy [22-24]. Age was also predictive of adjunctive therapy, with the highest magnitude among those aged 60 years and older. Depression in the elderly can have a poorer prognosis with high rates of relapse and chronicity [25,26] as well as a slower response to antidepressants compared with younger patients [26]. Moreover, the elderly are at higher risk of adverse outcomes from their depression, such as suicide. Hence, adjunctive therapy among elderly patients may be necessary to ensure that they are adequately treated. Also, the elderly tend to have more somatic disorders than younger people and higher associated GP consultation rates [27], thus, providing more opportunity to seek treatment for MDD.
Illness severity was also predictive of adjunctive therapy. Not only was severity of depression associated with likelihood of adjunctive treatment, but a higher burden of general comorbidity and the presence of IBS were associated with higher incidence of adjunctive therapy compared with patients without these comorbidities. Similarly as observed with the elderly, more frequent consultations with GPs due to comorbidity may increase opportunity for the use of adjunctive treatment for MDD; furthermore, patients with comorbid conditions often fare worse in terms of both their depression and their comorbid condition [26,28,29].
Strengths and Limitations
The GPRD provided longitudinal data on a large sample of MDD patients and good statistical power for examining the trajectory of treatment for MDD in UK primary care. Patients on SSRIs who had a definitive diagnosis for MDD were specifically selected, since SSRIs can also be used to treat other conditions such as anxiety.
Several limitations should be noted:
1) GPs may not enter diagnoses according to DSM IV or ICD criteria, and MDD patients without a specific Read code for MDD (i.e., only a general code for depression) and no depression screening questionnaire score may have been missed in the database.
2) The GPRD contains information on drugs prescribed and does not verify whether the medication was dispensed or taken by the patient.
3) Data included only the comorbidities for which the patient had consulted and that the GP had recorded.
4) The predictors of adjunctive therapy may not be generalizable to the population of MDD patients outside of primary care.
5. CONCLUSION AND IMPLICATIONS
While this study demonstrated that certain groups such as females, older people, those with a higher comorbidity burden, and those initiating treatment in recent years were more likely to receive adjunctive therapy, the overall rates of adjunctive therapy in primary care were low relative to other treatment strategies such as SSRI dose increases and antidepressant switching. According to UK treatment guidelines, adjunctive therapy is likely to be initiated by mental health specialists. However this study shows little evidence of shared care maintenance treatment by GPs. Patients with partial and non-response may also not be referred for specialist care and hence may remain undertreated for their MDD. Given the high rates of inadequate response to antidepressant monotherapy, patients who initiate adjunctive therapy in specialist care may benefit from maintenance treatment from their GP under a shared-care arrangement [30].
6. ACKNOWLEDGEMENTS
Thanks are due to Dr. Andrew Copas for providing statistical advice.
REFERENCES
- NICE (2009) Depression. The treatment and management of depression in adults. NICE clinical guideline 90. National Institute for Health and Clinical Excellence, 2010.
- Rush, A.J., Trivedi, M.H., Wisniewski, S.R., Nierenberg, A.A., Stewart, J.W., Warden, D., Niederehe, G., Thase, M.E., Lavori, P.W., Lebowitz, B.D., McGrath, P.J., Rosenbaum, J.F., Sackeim, H.A., Kupfer, D.J., Luther, J. and Fava, M. (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. American Journal of Psychiatry, 163, 1905-1917. doi:10.1176/appi.ajp.163.11.1905
- Judd, L.L., Akiskal, H.S., Maser, J.D., Zeller, P.J., Endicott, J., Coryell, W., Paulus, M.P., Kunovac, J.L., Leon, A.C., Mueller, T.I., Rice, J.A. and Keller, M.B. (1998) Major depressive disorder: A prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. Journal of Affective Disorders, 50, 97-108. doi:10.1016/S0165-0327(98)00138-4
- Judd, L.L., Akiskal, H.S., Zeller, P.J., Paulus, M., Leon, A.C., Maser, J.D., Endicott, J., Coryell, W., Kunovac, J.L., Mueller, T.I., Rice, J.P. and Keller, M.B. (2000) Psychosocial disability during the long-term course of unipolar major depressive disorder. Archives of General Psychiatry, 57, 375-380. doi:10.1001/archpsyc.57.4.375
- Kennedy, S., McIntyre, R., Fallu, A. and Lam, R. (2002) Pharmacotherapy to sustain the fully remitted state. Journal of Psychiatry and Neuroscience, 27, 269-280.
- Thase, M.E., Simons, A.D., McGeary, J., Cahalane, J.F., Hughes, C., Harden, T. and Friedman, E. (1992) Relapse after cognitive behavior therapy of depression: Potential implications for longer courses of treatment. American Journal of Psychiatry, 149, 1046-1052.
- Keller, M.B. (2004) Remission versus response: The new gold standard of antidepressant care. Journal of Clinical Psychiatry, 65, 53-59.
- Chisholm, J. (1990) The read clinical classification. British Medical Journal, 300, 1092. doi:10.1136/bmj.300.6732.1092
- Dave, S., Petersen, I., Sherr, L. and Nazareth, I. (2010) Incidence of maternal and paternal depression in primary care: A cohort study using a primary care database. Archives of Pediatrics & Adolescent Medicine, 164, 1038-1044. doi:10.1001/archpediatrics.2010.184
- Joling, K.J., Van Marwijk, H.W., Piek, E., Van der Horst, H.E., Penninx, B.W., Verhaak, P. and Van Hout, H.P. (2011) Do GPs’ medical records demonstrate a good recognition of depression? A new perspective on case extraction. Journal of Affective Disorders, 133, 522-527. doi:10.1016/j.jad.2011.05.001
- Kroenke, K., Spitzer, R.L. and Williams, J.B. (2001) The PHQ-9: Validity of a brief depression severity measure. Journal of General Internal Medicine, 16, 606-613. doi:10.1046/j.1525-1497.2001.016009606.x
- Zigmond, A.S. and Snaith, R.P. (1983) The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica, 67, 361-370. doi:10.1111/j.1600-0447.1983.tb09716.x
- Beck, A.T., Steer, R.A. and Brown, G.K. (1996) Manual for the beck depression inventory-II. Psychological Corporation, San Antonio.
- Charlson, M.E., Pompei, P., Ales, K.L. and MacKenzie, C.R. (1987) A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. Journal of Chronic Diseases, 40, 373-383. doi:10.1016/0021-9681(87)90171-8
- Khan, N.F., Perera, R., Harper, S. and Rose, P.W. (2010) Adaptation and validation of the Charlson index for Read/OXMIS coded databases. BMC Family Practice, 11, 1. doi:10.1186/1471-2296-11-1
- Bauer, M., Monz, B.U., Montejo, A.L., Quail, D., Dantchev, N., Demyttenaere, K., Garcia-Cebrian, A., Grassi, L., Perahia, D.G., Reed, C. and Tylee, A. (2008) Prescribing patterns of antidepressants in Europe: Results from the Factors Influencing Depression Endpoints Research (FINDER) study. European Psychiatry, 23, 66-73. doi:10.1016/j.eurpsy.2007.11.001
- Martin-Lopez, L.M., Rojo, J.E., Gibert, K., Martin, J.C., Sperry, L., Duno, L., Bulbena, A. and Vallejo, J. (2011) The strategy of combining antidepressants in the treatment of major depression: Clinical experience in spanish outpatients. Depression Research and Treatment, 2011, 140194. doi:10.1155/2011/140194
- Gregor, K.J., Riley, J.A. and Downing, D.K. (1996) Concomitant use of anxiolytics and hypnotics with selective serotonin reuptake inhibitors. Clinical Therapeutics, 18, 521-527. doi:10.1016/S0149-2918(96)80034-8
- McManus, P., Mant, A., Mitchell, P., Birkett, D. and Dudley, J. (2001) Co-prescribing of SSRIs and TCAs in Australia: How often does it occur and who is doing it? British Journal of Clinical Pharmacology, 51, 93-98. doi:10.1046/j.1365-2125.2001.01319.x
- Classi, P., Ball, S. and Le, T.K. (2011) What happens next? Pharmacological Prescription patterns in patients with major depressive disorder who initiate selective serotonin reuptake inhibitor therapy. American Psychiatric Association Annual Conference, Honolulu.
- Weissman, M.M., Bland, R.C., Canino, G.J., Faravelli, C., Greenwald, S., Hwu, H.G., Joyce, P.R., Karam, E.G., Lee, C.K., Lellouch, J., Lepine, J.P., Newman, S.C., RubioStipec, M., Wells, J.E., Wickramaratne, P.J., Wittchen, H. and Yeh, E.K. (1996) Cross-national epidemiology of major depression and bipolar disorder. Journal of the American Medical Association, 276, 293-299. doi:10.1001/jama.1996.03540040037030
- Angst, J., Gamma, A., Gastpar, M., Lepine, J.P., Mendlewicz, J. and Tylee, A. (2002) Gender differences in depression. Epidemiological findings from the European DEPRES I and II studies. European Archives of Psychiatry and Clinical Neuroscience, 252, 201-209. doi:10.1007/s00406-002-0381-6
- Hall, R.H. (2003) Promoting men’s health. Australian Family Physician, 32, 401-407.
- Thorogood, M., Coulter, A., Jones, L., Yudkin, P., Muir, J. and Mant, D. (1993) Factors affecting response to an invitation to attend for a health check. Journal of Epidemiology & Community Health, 47, 224-228. doi:10.1136/jech.47.3.224
- Cole, M.G., Bellavance, F. and Mansour, A. (1999) Prognosis of depression in elderly community and primary care populations: A systematic review and metaanalysis. American Journal of Psychiatry, 156, 1182-1189.
- Sable, J.A., Dunn, L.B. and Zisook, S. (2002) Late-life depression. How to identify its symptoms and provide effective treatment. Geriatrics, 57, 18-19.
- NHS (2009) Trends in consultation rates in general practice 1995/1996 to 2008/2009: Analysis of the QResearch® database. The Health and Social Care Information Centre.
- Leo, R.J. (2005) Chronic pain and comorbid depression. Current Treatment Options in Neurology, 7, 403-412. doi:10.1007/s11940-005-0032-0
- Koike, A.K., Unutzer, J. and Wells, K.B. (2002) Improving the care for depression in patients with comorbid medical illness. American Journal of Psychiatry, 159, 1738-1745. doi:10.1176/appi.ajp.159.10.1738
- Agius, M., Murphy, C.L. and Zaman, R. (2010) Does shared care help in the treatment of depression? Psychiatria Danubina, 22, S18-S22.
NOTES
*Corresponding author.

