Open Journal of Genetics
Vol.4 No.2(2014), Article ID:44685,11 pages DOI:10.4236/ojgen.2014.42015
Expression of Heterosis and Heritability in Vegetative Traits of Gongronema latifolia
Christian Ugwu Agbo1*, Jaime A. Teixeira da Silva2
1Department of Crop Science, University of Nigeria, Nsukka, Nigeria
2Miki Cho Post Office, Ikenobe, Japan
Email: *Christian.agbo@unn.edu.ng, c_agbogenetics@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/


Received 16 January 2014; revised 15 February 2014; accepted 14 March 2014
ABSTRACT
The existence of hybrid vigour with high heritability in vegetative traits of Gongronema latifolia will increase its productivity. This study was therefore undertaken to estimate heterosis and heritability in the vegetative traits of G. latifolia. Stem cuttings of five heterozygous parents of different geographical origin, and seeds of 10 hybrids resulting from their crosses were laid out in a randomized complete block design with three replications in a field. Data on the vegetative traits were obtained at the maximum growth period in each year of study and was used to estimate heterosis and heritability over the years. Better parent heterosis (BPH) ranged from −72% to 78% over three years. All the vegetative traits—with the exception of cordate base size—showed positive heterosis in a minimum of four cross combinations in two years. The cross AKS-33-EKPENE EDIENE X ANS-53-AWKA gave a higher BPH ranging from 5% to 6% in leaf area in the three years. The crosses ENS-48-MBU X IMS-50-NJIABA and ENS-48-MBU X ANS-53-AWKA gave higher BPH for leaf length and breadth for the first two years. All crosses showed positive and negative BPH for number of vines/plant and cordate base size, respectively. The hybrids ENS-48-MBU X IMS-50- NJIABA and EBS-49-ISHIAGU X IMS-50-NJIABA, with highly heritable BPH wang##Bracket# 60 cm2 leaf area, will be selected in favour of other hybrids and parents. The selection of these hybrids will improve and maintain productivity in the species as the leaves are the harvestable part and the species is vegetatively propagated.
Keywords:Hybrid Vigour, Hybrids, Leaves, Productivity, Selection

1. Introduction
Gongronema latifolia Benth. is a leafy vegetable that was, until recently, commonly harvested from virgin forests in West Africa; now, propagation methods for field establishment have been developed [1] [2] . The leaf is the main harvestable part of the plant for human food consumption or for medicine in Nigeria and other subSaharan African countries. The crop has been reported to be nutritionally high in protein, amino acids and vitamins [3] -[5] . G. latifolia traditionally plays a very vital role and in recent times has served as a herbal medicine useful in the management of diabetes mellitus, high blood pressure, and in the treatment of loss of appetite, dysentery, typhoid, worm infections, malaria and stomach ache; moreover, the aqueous and ethanolic extracts of the leaves have anti-inflammatory properties [3] [6] -[8] .
The expression of heterosis is the average superiority of an F1 hybrid over its parents. Heterosis plays a vital role in improving crop productivity and quality in order to feed ever-increasing human populations, especially in developing countries like Nigeria. Hybrid maize and rice technology have had a tremendous impact on food security, production efficiency and generation of employment [9] . The exploitation of heterosis has been largely responsible for the tremendous increase in maize yield in global agriculture [10] . Burton and Brownie [11] evaluated the F1 generation of two combinations derived from crosses between then-recent soybean cultivars and obtained an average yield of +16% from one cross and +5% better parent heterosis (BPH) from the other cross combination. Hakim et al. [9] reported a 48% increase in heterotic value of seed weight in sunflower (Helianthus annuus L.) hybrids.
Heritability, a measure of the phenotypic variance attributable to genetic causes provides an estimate of the genetic advance of a population under selection in a breeding programme [9] . Higher heritability estimates suggests simpler selection procedures. Hence, heritability estimates of traits are vital for decisions at the level of selection pressure at early stages of crop breeding programmes.
There is a paucity of information on the existence of heterosis in vegetative traits of G. latifolia in the literature. Previous reports have been on expression of heterosis in hybrids of other lines of G. latifolia for phytochemicals [12] and some vitamins and lycopene [5] . The presence of hybrid vigour in the vegetative traits will increase crop productivity to satisfy diverse needs from a given area of production. The objectives of the study were to access the level of heterosis in the vegetative traits of G. latifolia and to estimate heritability of those traits.
2. Materials and Methods
The plant materials used were five heterozygous parents of different geographical origin (different States in Nigeria) including: AKS-33-EKPENE-EDIENE, ENS-48-MBU, EBS-49-ISHIAGU, IMS-50-NJIABA and ANS- 53-AWKA and 10 hybrids resulting from their crosses. The States represented are: AKS = Akwaibom; ENS = Enugu; EBS = Ebonyi; IMS = Imo and ANS = Anambra.
Stem cuttings of the parent plants and seeds of the hybrids were raised in a nursery according to the procedures developed by Agbo and Omaliko [2] and Agbo and Obi [1] . The experiment was laid out in a randomized complete block design (RCBD) with three replications in 2008. The soil texture of the field was sandy loam, and the field was fertilized with well-decomposed poultry droppings at 10 tons/ha. The field was manually kept free of weeds. Data was sampled over three years (2008, 2009 and 2010) from five randomly selected plants at the maximum period of growth in each plot for all vegetative traits assessed: leaf area/plant (LAP), leaf breadth (LB), leaf length (LL), size of the cordate base (SCB), length of the longest vine (LLV), number of leaves/plant (NLP) and number of vines/plant (NVP). Leaf area was measured using a sensitive leaf area meter (Burle, Delta T. Devices Ltd., Serial No. 9884). Leaf length, broadest lamina width and cordate base size were measured with a metric ruler.
Statistical Analyses
Data on the vegetative traits were analyzed according to a randomized complete block design (RCBD) procedure using GENSTAT 3.0 Discovery edition [13] . A mixed model analysis of variance (ANOVA) was performed separately for each year, and combined analysis of variance for the three years was also conducted separately on each of the traits. Differences between treatment means were compared for significance by Duncan’s New Multiple Range Test in accordance with the procedure outlined by Gomez and Gomez [14] . The magnitude of heterosis for all traits was estimated separately in each year. Hybrid performance relative to the parents was measured as better parent heterosis (BPH) according to Uguru [15] .
BPH was determined as:
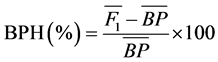
where 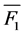 = mean performance of the hybrid and
= mean performance of the hybrid and 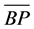 = mean performance of the better parent. A student’s t-test was used to test the level of significance of the heterosis values at the 5% level of probability.
= mean performance of the better parent. A student’s t-test was used to test the level of significance of the heterosis values at the 5% level of probability.
3. Results
There were significant differences (P = 0.05) between genotypes, years and genotype (G) × year (Y) interaction for all the traits with the exception of LLP and SCB in which year did not have a significant effect (Table 1). The pooled broad sense heritability (BSH) estimate was high for LAP, LB, LL, and SCB and ranged from 98.65 to 99.95%. BSH was low for LLV, NLP and NVP, ranging from 57.86% to 72.06%. The hybrid ENS-49- NSUKKA x IMS-50-NJIABA had significantly (P = 0.05) higher LAP, LB and LL and significantly (P = 0.05) lower SCB than all other parents and hybrid for the three years (Table 2 and Table 3). The parent ANS-53- AWKA, on the other hand, had a significantly (P = 0.05) lower LAP, LB and LL than the parents and other hybrids. The hybrid ENS-48-MBU x IMS-50-NJIABA had highest LLV (329.70 cm) in the three years. The hybrid AKS-33-EKPENE EDIENE x IMS-50-NJIABA had significantly (P = 0.05) higher NLP and NVP (237.39 and 8.67, respectively) than other parents and hybrids for the three years. The parent AKS-33-EKPENE EDIENE had significantly lower LLV, NLP and NVP and significantly (P = 0.05) higher SCB (161.47 cm, 165.92, 5.0 and 1.17 cm, respectively) than other parents and hybrids for the three years. Significantly (P = 0.05) higher LAP, LB, LLV and NLP was recorded in the second year. The third year of harvest, however, resulted in higher NVP and lower SCB.
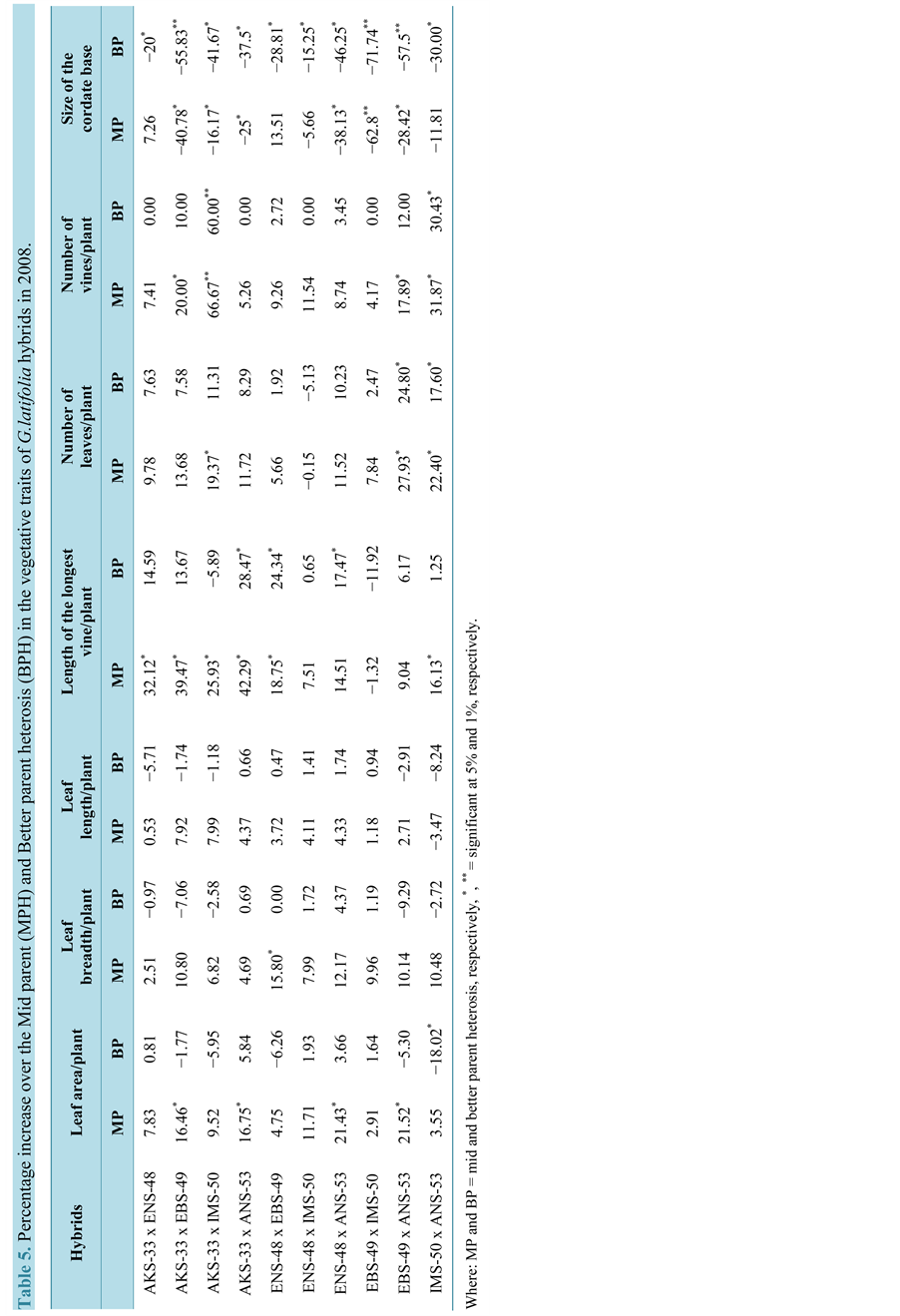
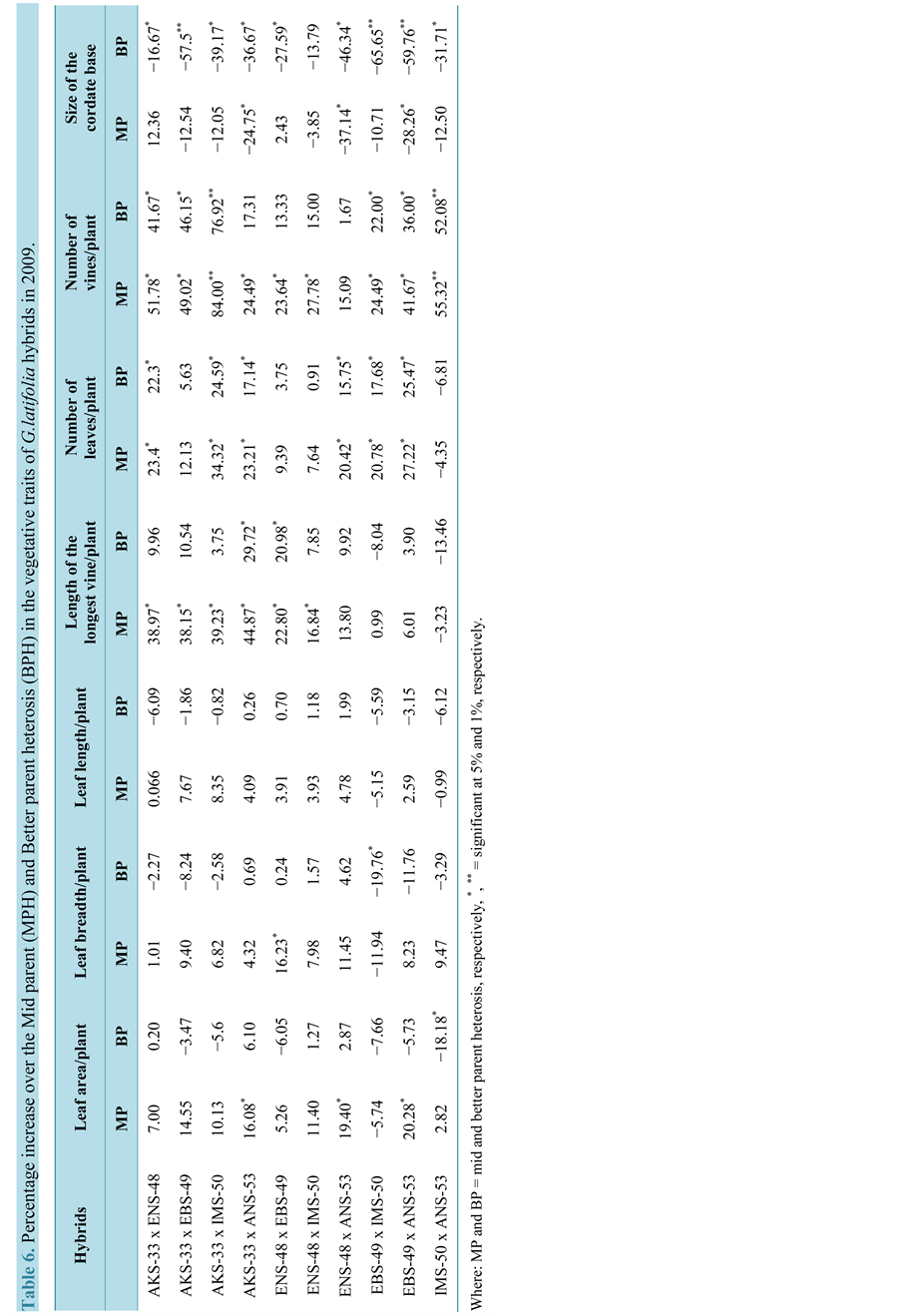
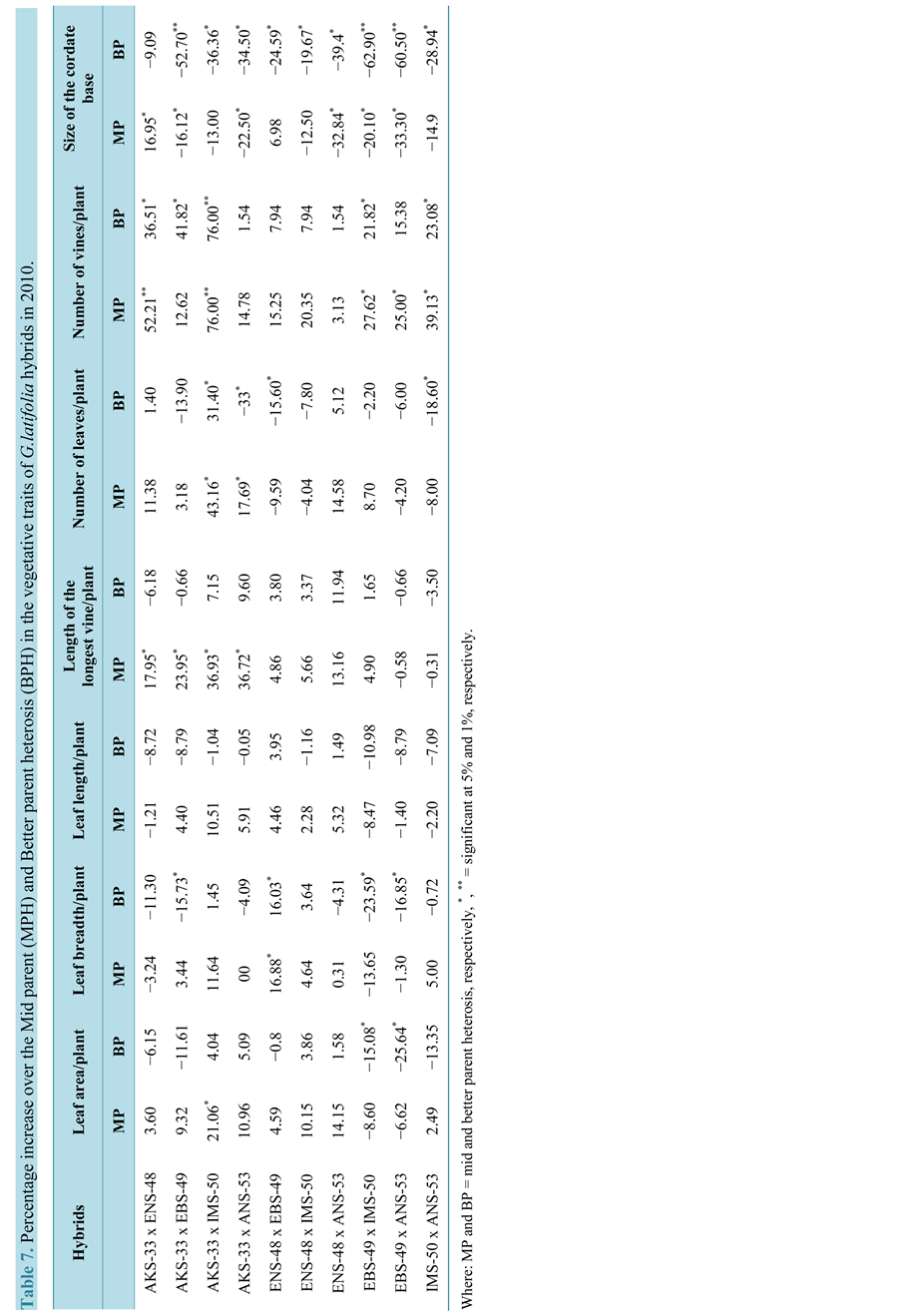
The hybrids expressed some levels of BPH for the vegetative traits in the three years (Tables 3-5). The hybrid AKS-33-EKPENE EDIENE x ANS-53-AWKA showed 5.8%, 6.1% and 5% increases in LAP in 2008, 2009 and 2010, respectively. The cross ENS-48-MBU x ANS-53-AWKA had higher level of BPH of 3.66% and 2.90% in LB in 2008 and 2009, respectively (Table 5 and Table 6). The cross however, had a BPH of −4.30% in 2010 which is considerably reduced compared to the original parental values (Table 7). The cross also showed higher BPH in LL in the three years. Significant (P = 0.05) BPH in LLV was shown by the crosses AKS-33-EKPENE EDIENE x ANS-53-AWKA and ENS-48-MBU x EBS-49-ISHIAGU in 2008 and 2009 over the parents. In 2010, the cross ENS-48-MBU x ANS-53-AWKA had a higher and significant BPH in NLP. The cross EBS-49-ISHIAGU x ANS-53-AWKA showed significantly (P = 0.05) higher BPH of 21% in NLP in 2008 and 2009. The cross however, had a negative BPH of −6.62% in NLP in 2010. Significantly (P = 0.05) higher level of BPH was shown for NVP than other traits in the cross AKS-33-EKPENE EDIENE x IMS-50-NJIABA in the three years (60%, 77% and 76%, respectively). SCB showed a significant BPH of −72%, −66% and −63%, respectively, for 2008, 2009 and 2010 in the cross EBS-49-ISHIAGU x IMS-50-NJIABA.
The magnitude of BPH varied among the vegetative traits and within the years. Positive BPH was exhibited by about 50% of the hybrids in leaf-related traits (area, breadth and length) for the three years. Almost 90% of the hybrids showed positive BPH that was significant (P = 0.05) in some cases, specifically LLV and NLP. All crosses showed positive and negative BPH in NVP and SCB, respectively for the three years. Higher levels of BPH were shown in the second year (2009) by the crosses with higher BPH in all vegetative traits.
Table 1. Mean squares and pooled heritability (broad sense) estimates for the vegetative traits of parents and F1 hybrids for three years.
** = Significant at P = 0.01, * = Significant at P = 0.05.
Table 2. Performance of the vegetative traits of the parents and hybrids of G. latifolia in 2008.
Where: LAP = Leaf area/plant, LBB = Leaf breadth, LLP = leaf length/plant, LLV = Length of the longest vine, NLP = Number of leaves/plant and SCB = Size of cordate base.
Table 3. Performance of the vegetative traits of the parents and hybrids of G. latifolia in 2009.
Where: LAP = Leaf area/plant, LBB = Leaf breadth, LLP = leaf length/plant, LLV = Length of the longest vine, NLP = Number of leaves/plant and SCB = Size of cordate base.
Table 4. Performance of the vegetative traits of the parents and hybrids of the G. latifolia in 2010.
Where: LAP = Leaf area/plant, LBB = Leaf breadth, LLP = leaf length/plant, LLV = Length of the longest vine, NLP = Number of leaves/plant and SCB = Size of cordate base.
4. Discussion
Significant differences among the hybrids indicate a high level of genetic recombination among the crosses. F1 hybrids resulting from crosses of vegetatively propagated plants have been reported to show a high level of heterozygosity [16] . The leaf traits (LAP, LB and LL) of the hybrids that showed high broad sense heritability (BSH) estimates of more than 99% each indicated that selections based on such traits would be effective in improving the productivity of the crop. The result is in agreement with the report of Hakim et al. [9] on effective selection of sunflower genotypes for specific traits based on high level of BSH. Selection of hybrids for improved productivity based on leaf traits will increase production because leaves are the main harvested parts of this plant species.
The BPH estimates were variable across years and parent combinations, hence, the observed G × Y interactions in the expression of the traits. A higher LAP, LB and LL in some cross combinations over the parents indicated the expression of heterosis [5] [17] . The hybrid EBS-49-ISHIAGU x IMS-50-NJIABA with hybrid vigour in LAP, LB and LL could be selected to improve productivity as NLP from the cross will give higher weight with an equal number of leaves for either parent. This provides a wide scope for selecting higher leafyielding genotypes. The leaf traits (area, breadth and length) that seem to be equally inherited have been shown to be positively and significantly correlated [18] . This latter report further indicated that the magnitude of leaf breadth and length have a direct effect on leaf area in this species. Even though the crosses AKS-33-EKPENE EDIENE x ANS-53-AWKA and ENS-48-MBU x ANS-53-AWKA showed higher BPH over the respective parents, the hybrid ENS-49-NSUKKA x ANS-50-ENUOGBU with lower BPH but higher LAP will be selected taking leaf size into consideration. This shows that the magnitude of BPH is relative to values of the cross combinations.
The significantly (P = 0.05) higher values for leaf traits (area, breadth and length), LLV and NLP in the second year have important agronomic implications. The reduced leaf sizes in the third year could be attributed to profuse flowering that occurred in some of the sampled plants of the hybrids by the third year. This species has
been observed to flower 16 months [2] after establishment and full profuse flowering starts in February of a year until June of the same year [2] . It has also been observed that plants established from seeds in this species fall into two categories: one category that flowers as described above and a second category that does not flower at all over 10 years of establishment (maximum period of monitoring). Hence, in the hybrids established from seedlings from the crosses, some of them flowered while others did not. Flowering thus limited growth of vines as some vines acted like determinate vines and leaf sizes and numbers were reduced even as the same level of organic manure was applied each year. For example, about 60% of the sampled plants in the hybrid ENS-48-MBU x ANS-53-AWKA with higher levels of BPH in 2008 and 2009 flowered profusely by the third year (2010). It thus had a negative BPH of −4.3% implying that the profuse flowering contributed to the observed reduction in leaf sizes. Further research is required to understand the mechanics of sexes and their effects on leaf and vine growth in G. latifolia species. The positive BPH in all the crosses for NVP indicated that the hybrids expressed vigour over their parents. It has been observed that G. latifolia established from seeds for over 10 years flowers more profusely and develops more secondary vines than plants established from stem cuttings even though the former starts to flower earlier (Agbo, personal observation). The higher number of secondary vines in seedlings could be attributed to shorter internodes that are available on a seedling compared to stem cuttings that have long internode lengths (data not shown). The nodes are points of development of secondary vines, hence the recorded BPH in all the crosses over their parents established from stem cuttings. There was a reduction in SCB in all the crosses when compared to the parents, hence the negative BPH for this trait. Lower SCB and high LBB have been reported to increase leaf area in G. latifolia [18] .
Profuse flowering in the third year could have also caused the reduction in NLP in the hybrids thus resulting in negative BPH in the cross EBS-49-ISHIAGU x ANS-53-AWKA. The cross had earlier shown a significant (P = 0.05) BPH of 21% in 2008 and 2009. The determinate nature of the flowering in some crosses caused cessation of growth on every vine that flowered, hence the reduction in vine length and leaf number. Determinate growth pattern in crop species had been shown to limit leaf yield [19] [20] . The reduced SCB in all hybrids is advantageous as reduced SCB coupled with broad LB has been shown to increase leaf area [18] .
The varied magnitude of BPH for all the vegetative traits in the crosses has also been reported for the chemical components of this species [5] [12] . Reports of high heterozygosity have been reported in vegetatively propagated crops such as plantain [21] . Most of the vegetative traits measured in this study exhibited a lower magnitude of BPH compared to values of chemical components of other G. latifolia hybrids. Jayalakim et al. [22] reported a wide range of heterosis for seed yield in sunflower plants. Burton and Brownie [11] evaluated the F1 generation of two combinations derived from crosses between soybean cultivars. The average yield of one cross showed +16% BPH while the other cross showed +5% BPH. These results suggest that significant yield increases are possible for some cross combinations in some traits and crop species.
In G. latifolia, heterosis is not yet well understood and a few explanations have been proposed by Agbo and Odo [12] and Agbo et al. [5] : 1) genetic distance, and 2) over dominance occurring in one of the parents. The suggested explanations are trait-specific and are likely not a general rule. In the present study, the hybrids AKS- 33-EKPENE EDIENE x ANS-53-AWKA and IMS-50-NJIABA x EBS-53-ISHIAGU had parental distance average leaf area of 42.8 and 34.8 cm2 for AKS-33-EKPENE EDIENE and ANS-53-AWKA, respectively, and 59.65 and 34.8 cm2 for IMS-50-NJIABA and ANS-53-AWKA, respectively. However, the first hybrid showed positive BPH of +6 while the second hybrid showed a negative BPH of −18. On the other hand, all hybrids showed varying levels of negative BPH for SCB for the three years. Hence, the suggestion of any common measurement for a hybrid, such as genetic distance between parents and over dominance will be insufficient to explain heterosis for all traits and cross combinations. This finding agrees with the results of quantitative trait loci analyses which revealed that the genetic basis of heterosis for specific traits is multigenic [23] -[25] and results that found the loci underlying variation in heterosis to often be trait-specific [26] [27] . The general principle of heterosis from a genetic and molecular stand-point suggests that heterosis is the result of many loci that have small effects that interact through a variety of molecular mechanisms [28] .
In the current study, a limited number of cross combinations were evaluated and the results provided information on the complexities and difficulties of heterosis evaluation in this species, as not being different from other crop species. However, the possibility of obtaining a G. latifolia hybrid expressing heterosis in the vegetative traits has been established. Selection of three hybrids including AKS-33-EKPENE-EDIENE x EBS-49- ISHIAGU, ENS-48-MBU x IMS-50-NJIABA and EBS-49-ISHIAGU x IMS-50-NJIABA with high heritable BPH in LAP will improve productivity of the species. As G. latifolia is vegetatively propagated, coupled with its high level of heritability of leaf traits, increased productivity will be sustained in the selected hybrids for a long time under good agronomic practice.
References
- Agbo, C.U. and Obi, I.U. (2008) Germination Potentials of Gongronema latifolia Benth. Seeds at Different Stages of Maturity and Storage. Seed Science and Technology, 36, 114-121.
- Agbo, C.U. and Obi, I.U. (2009) Patterns of Vegetative Propagation of Stem Cuttings of Three Physiological Ages of G. latifolia Benth. over Two Seasons in Nsukka. Agro-Science, 8, 290-293.
- Okafor, J.C. (1997) Conservation and Use of Traditional Vegetable from Woody Forest Species in Southeastern Nigeria. In Promoting the Conservation and Use of Underutilized and Neglected Crops. Proceedings of the IPGRI International Workshop on Genetic Resources of Traditional Vegetables in Africa. Conservation and Use, Nairobi, 29-31 August 1995.
- Agbo, C.U., Umeh, P.C. and Obi, I.U. (2009) Genetic Variability in Protein and Amino Acid Composition of Some Clones of G. latifolia Leaves. Food, 3, 98-106.
- Agbo, C.U., Teixeira da Silva, J.A. and Aleke, F.I. (2011) Selection of Gongronema latifolia Hybrids Exhibiting Heterosis for Certain Vitamins and Lycopene. Journal of Crop Improvement, 25, 742-755, http://dx.doi.org/10.1080/15427528.2011.606522
- Ugochukwu, N.H. and Babady, N.E. (2002) Antioxidant Effects of Gongronema latifolia in Hepatocytes of Rat Models of Non-Insulin Dependent Diabetes Mellitus. Fitoterapia, 73, 612-628. http://dx.doi.org/10.1016/S0367-326X(02)00218-6
- Morbise, O., Fatus, M.A., Makinde, J.M., Olajide, O.A. and Awe, E.O. (2002) Anti-Inflammatory Properties of Leaves of G. latifolia. Phytother Resource, 16, 75-77. http://dx.doi.org/10.1002/ptr.784
- Agbo, C.U., Baiyeri, K.P. and Obi, I.U. (2005) Indigenous Knowledge and Utilization of G. latifolia Benth: A Case Study of Women in University of Nigeria, Nsukka. Bio-Research Journal, 3, 66-69.
- Hakim, K., Hidayat-ur-Rahman, H., Ahmed, H., Ali, I. and Alam, M. (2008) Magnitude of Heterosis and Heritability in Sunflower over Environments. Pakistan Journal of Botany, 40, 301-308.
- Duvick, D.N. (2001) Biotechnology in the 1930s: The Development of Hybrid Maize. Nature Reviews Genetics, 2, 69-74. http://dx.doi.org/10.1038/35047587
- Burton, J.W. and Brownie, C. (2006) Heterosis and Inbreeding Depression in Two Soybean Single Crosses. Crop Science, 46, 2643-2648. http://dx.doi.org/10.2135/cropsci2006.03.0156
- Agbo, C.U. and Odo, G.C. (2008) Hybrid Vigour in Some Phytochemical and Mineral Compositions of Fresh and Air-Dried Leaves of G. latifolia Benth. Journal of Genetics and Breeding, 62, 1-6.
- GENSTAT (2007) Genstat Discovery Edition 3.0. Lawes Agricultural Trust Rothamsted Experimental Station, Herefordshire.
- Gomez, K.A. and Gomez, A.A. (1984) Statistical Procedure for Agricultural Research. 2nd Edition, John Wiley & Sons Publishers, New York, 680p.
- Uguru, M.I. (2005) Crop Genetics and Breeding. Comic Printers, Onitsha, 30.
- Anonymous (2001) Potato Breeding, Engineering Plant Roots. Swiss Federal Institute of Technology, Zurich.
- Perez, P.T., Cianzio, S.R. and Palmer, R.G. (2009) Evaluation of Soybean (Glycine max. (L.) Merr.) Hybrids. Journal of Crop Improvement, 23, 1-18. http://dx.doi.org/10.1080/15427520802417832
- Agbo, C.U. and Obi, I.U. (2005) Analysis of the Relationships between Gongronema latifolia Leaf Traits and Its Leaf Area Using Path Coefficients. Proceedings of Genetics Society of Nigeria (GSN) Conference, 5-8 September 2005, University of Nigeria, Nsukka.
- McWilliams, D.A., Berglund, D.R. and Endres, G.J. (2004) Soybean Growth and Management. North Dakota State University Extension Service, A1174, 8.
- Emmam, Y., Shekoofa, A., Salehi, F. and Jalali, A.H. (2010) Water Stress Effect on Two Common Bean Cultivars with Contrasting Growth Habits. American-Eurasian Journal of Agriculture and Environmental Science, 9, 495-499.
- Vuglsteke, D., Ortiz, R., Ferris, R.S.B. and Crouch, J.H. (1997) Plantain Improvement. Plant Breeding Revolution, 14, 267-320.
- Jayalakshimi, V., Narendra, B., Sridhar, V. and Devi, K.R. (2000) Heterosis in Sunflower (Helianthus annus L.). Agriculture Science Digest, 20, 114-115.
- Stuber, C.W., Lincoln, S.E., Wolf, D.W., Helentjaris, T. and Lander, E.S. (1992) Identification of Genetic Factors Contributing to Heterosis in a Hybrid from Two Elite Maize Inbred Lines Using Molecular Markers. Genetics, 132, 823-839.
- Semel, Y., Nissenbaum, J., Menda, N., Zinder, M. and Krieger, U. (2006) Overdominant Quantitative Trait Loci for Yield and Fitness in Tomato. Proceedings of the National Academic of Sciences of the United States of America, 103, 12981-12986. http://dx.doi.org/10.1073/pnas.0604635103
- Garcia, A.A., Wang, S., Melchinger, A.E. and Zeng, Z.B. (2008) Quantitative Trait Loci Mapping and the Genetic Basis of Heterosis in Maize and Rice. Genetics, 180, 1707-1724. http://dx.doi.org/10.1534/genetics.107.082867
- Frascaroli, E., Cane, M.A., Landi, P., Pea, G. and Gianfranceschi, L. (2007) Classical Genetic and Quantitative Trait Loci Analyses of Heterosis in a Maize Hybrid between Two Elite Inbred Lines. Genetics, 176, 625-644. http://dx.doi.org/10.1534/genetics.106.064493
- Li, L., Lu, K., Chen, Z., Mu, T., Hu, Z. and Li, X. (2008) Dominance, Overdominance and Epistasis Condition of Heterosis in Two Heterotic Rice Hybrids. Genetics, 180, 1725-1742. http://dx.doi.org/10.1534/genetics.108.091942
NOTES

*Corresponding author.