Advances in Biological Chemistry
Vol.3 No.2(2013), Article ID:30777,10 pages DOI:10.4236/abc.2013.32025
Enzymatic activities and kinetic properties of β-glucosidase from selected white rot fungi
![]()
1Department of Biological & Environmental Sciences, Alabama Agricultural and Mechanical University, Normal, USA
2Department of Agronomy, Kansas State University, Manhattan, USA
3Department of Natural Resources & Environmental Design, North Carolina A & T State University, Greensboro, USA
Email: *zachary.senwo@aamu.edu
Copyright © 2013 Priscilla M. Mfombep et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 15 January 2013; revised 8 March 2013; accepted 15 April 2013
Keywords: White Rot Fungi; β-Glucosidase; EC 3.2.1.21; Enzymatic Activities; Kinetic Properties; Plant Biomass
ABSTRACT
Beta-glucosidase is among the suite of enzymes produced by white rot fungi (WRF) to biodegrade plant biomass. This study investigated the enzymatic activities and kinetic properties of β-glucosidase from seventeen WRF comprised of the following species from various geographical locations: Pleurotus ostreatus, Auricularia auricular, Polyporus squamosus, Trametes versicolor, Lentinula edodes, and Grifola frondosa. All the WRF studied showed β-glucosidase activities. Significant variations in protein and carbohydrate contents were also recorded. Beta-glucosidase activities after 30 min of incubation ranged from 6.4 µg (T. versicolor) to 225 µg (G. frondosa). The calculated kinetic constant (Km) ranged from 0.47 µM (A. auricular-1120) to 719 µM (L. edodes-7). The Vmax depending on the kinetic transformation model ranged from 0.21 µg·min−1 (T. versicolor) to 9.70 µg·min−1 (G. frondosa-28). Beta-glucosidase activities also exhibited pH optima between 3.5 and 5.0 while temperature optima were between 60˚C and 70˚C with some media exhibiting a secondary temperature peak at 90˚C attributable to the presence of thermostable isoenzyme. WRF if appropriately screened and purified can be harnessed to potentially improve the bioconversion of cellulose to glucose and also facilitate efficient plant biomass biodegradation and production of useful plant bio-products.
1. INTRODUCTION
White rot fungi (WRF) are microorganisms of great interest that secrete complex suites of nonspecific extracellular ligninolytic enzymes, i.e., lignin peroxidase (EC 1.11.1.14), manganese peroxidase (EC 1.11.1.13), and laccase (EC 1.10.3.2) to biodegrade lignin [1] or digest substrates required for their proliferation and growth. The importance of white rot fungi has been noted in medicine [2], biodegradation of environmental wastes [3,4], pollutants [5,6], and in providing protein-rich foods [7]. Although cellulose and hemicellulose are biodegraded by several microbes, WRF which predominantly degrade lignin have been widely studied [8-11]. WRF have been screened for cellulase production potentials [12, 13] , but despite the vast amount of knowledge available on their enzymes, much is still to be understood about their specific enzymatic potential activities and kinetic properties. Enzymes involved with the biodegradation of lignocellulosic plant biomass are those of the “cellulase system”, of which β-glucosidase is a constituent [14]. This is because the complete hydrolysis of cellulose to glucose requires this system of enzymes (cellulases) comprised of endoglucanases, exoglucanases (cellobiohydrolases) and β-glucosidase.
The β-glucosidase family (EC 3.2.1.21) is a widespread group of enzymes that catalyze the hydrolysis of a broad variety of glycosides [15]. While some organisms secrete either endoglucanase or β-glucosidase, in other organisms, β-glucosidase is either lacking or produced in insufficient quantities [16]. When β-glucosidase secretion is low, cellobiose accumulates instead of glucose [17]. Cellobiose accumulation acts as a feedback-inhibitor of cellulose depolymerization by endoand exoglucanases [18,19], which is a critical factor in the industrial scale conversion of cellulose to glucose [20]. This situation can be alleviated during industrial scale conversion of cellulosic biomass by exogenous incorporation of β-glucosidase enzyme. Other enzymatic parameters that influence cellulosic bioconversion include pH, temperature, adsorption and presence of inhibitors [16]. The importance of β-glucosidase in industrial and environmental processes has been summarized [21,22]. The versatile nature of β-glucosidase relative to substrate specificity has increased its application in several environmentally-friendly biotechnological processes although individual WRF differ in their ability to produce this enzyme.
This study focused on investigating β-glucosidase activities and kinetic properties of selected WRF in submerged fermentation and the effects of different genera and geographical origins on the activities. The aim was also to enhance our understanding of the variabilities in biochemical characteristics and catalytic properties of β-glucosidase among selected fungal genera. White rot fungi showing best activities can be used individually or in combination with other organisms for plant biomass conversion to fermentable sugars and other bioproducts.
2. MATERIALS AND METHODS
2.1. Reagents
1) Coomassie Plus—The Better BradfordTM Assay Kit (Pierce Rockford, IL); 2) concentrated sulfuric acid (H2SO4); 3) phenol (5%) prepared by diluting 55.6 mL 90% w/w phenol in about 800 mL distilled deionized H2O, then adjusted to a final volume of 1 L with distilled deionized H2O and stored at 4˚C; 4) acetate buffer (50 mM, pH 5.0) prepared by dissolving 6.8 g sodium acetate trihydrate crystals in about 700 mL distilled deionized H2O then titrated to pH 5.0 with 99% glacial acetic acid and adjusted to a final volume (1 L) with distilled deionized H2O; 5) sodium carbonate (0.2 M) prepared by dissolving 21.2 g in distilled deionized H2O. Glucose standard solution prepared by dissolving 1.0 g anhydrous glucose (Sigma Co.) in distilled deionized water; 6) p-Nitrophenyl β-D-glucopyranoside (β-PNPGLU) (5 mM) prepared by dissolving 1.51 g (β-PNPGLU) (Sigma Chemical Co., St. Louis, MO) in about 800 mL sodium acetate buffer (50 mM, pH 5.0) and adjusted to the final volume (1 L) with the same buffer and stored at 4˚C; and 7) p-Nitrophenol standard solution prepared by dissolving 1.0 g p-nitrophenol (PNP) in distilled deionized H2O.
2.2. Organisms
White rot fungi isolates from the order Agaricales, Auriculariales and Aphylophorales (Table 1) used in this study are from the Mushroom Biology and Fungal Biotechnology Laboratory (MBFBL) at North Carolina A & T State University, Greensboro, NC.
2.3. Fungal Culture Conditions
Actively growing mycelia of each isolate were carefully scraped off the surface of potato dextrose agar plates and used to inoculate 100 mL sterile liquid cultivation medium in 250 mL Erlenmeyer flask under sterile conditions. The medium was composed of 5 g soluble starch, 0.1 g yeast extract, and 10 mL sawdust extract and made-up to 1 L with distilled deionized water (ddH2O). Inoculated flasks were incubated at 23˚C ± 2˚C for 14 days after which the mycelia mat was carefully removed and the remaining liquid from each of the triplicates per strain was pooled, filtered through a 0.22 µm acetate filter, then transferred into sterile bottles and kept at 4˚C until used.
2.4. Determination of Carbohydrate Contents
Carbohydrate content of the filtrate was measured using the phenol-sulfuric acid method for total carbohydrate determination [23]. Briefly, 1 mL of each sample was placed in labeled 50 mL test tubes, to which 1 mL of 5% aqueous phenol solution was added. The mixture was then placed on ice and 2 mL of concentrated sulfuric acid was added, heated in a water bath at 80˚C for 30 min and brought to room temperature by placing it in a water bath for 15 min. All samples including the controls were treated in triplicates and the absorbance measured at 490 nm against water blank using a spectrophotometer (GENESYS 10 spectrophotometer, Model 335902P-000, Thermo Electron Corp. Madison WI). The standard curve prepared contained 0, 20, 40, 60, 80, 100 µg·mL−1 glucose and was also subjected to the same treatment conditions as the extracts.
2.5. Determination of Protein Contents
Protein concentration was determined as described by Bradford [24] using a standard test tube protocol with a working range of 100 to 1500 µg·mL−1 protein concentration. Fifty µL of each WRF extract including controls in triplicates was placed in test tubes to which was added 1.5 µL of the Bradford assay reagent (containing G-250 dye, methanol, and phosphoric acid and solubilizing agents in water). Each tube was thoroughly mixed by swirling for a few seconds and the mixtures incubated at room temperature for 10 min. The absorbance of the blue color developed for the triplicates per sample was measured at 595 nm against water blank. A standard curve was prepared using bovine serum albumin (BSA) containing 0, 25, 125, 250, 500, 750, 1000, 1500, and 2000 µg·mL−1 BSA, that was subjected to the same experimental treatment conditions as the extracts.
2.6. Determination of β-Glucosidase Activities
The β-glucosidase activity in extracts was assayed using p-Nitrophenyl-β-D-glucopyranoside (β-PNPGLU) as substrate. Two mL of 5 mM β-PNPGLU (prepared in 50 mM
Table 1. Classification and sources of white rot fungi (source of mushroom extracts).
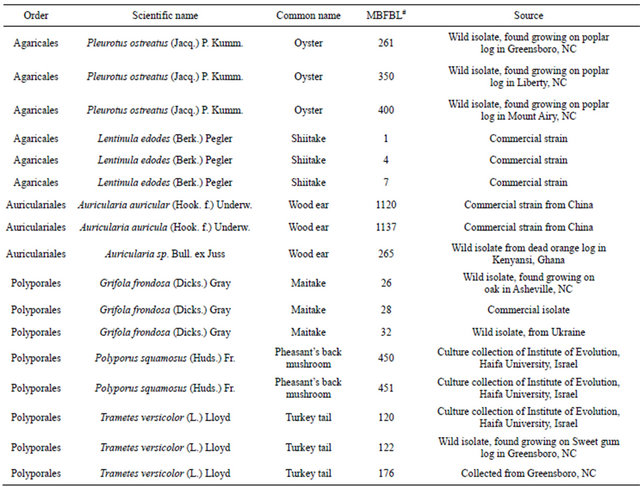 #MBFBL, Mushroom biology and fungal biotechnology laboratory at North Carolina A & T State University, Greensboro, NC.
#MBFBL, Mushroom biology and fungal biotechnology laboratory at North Carolina A & T State University, Greensboro, NC.
sodium acetate buffer, pH 5.0) was placed in a 15-mL centrifuge triplicate tubes to which was added 0.5 mL WRF extract and incubated at 37˚C for 30 min. The reaction was stopped using 1.5 mL 0.5 M Na2CO3 and the absorbance measured against a blank (sodium acetate) at 400 nm after 30 min. Each experiment had a control consisting of substrate without enzyme that was also subjected to the same experimental conditions. A PNP standard was prepared containing 0, 20, 40, 60, 80 and 100 µg of PNP.
2.7. Determination of Kinetic Parameters
The kinetic parameters were assessed by estimating β- glucosidase activities at various β-PNPGLU concentrations (0.02, 0.05, 0.1, 0.5, 1.0, 1.5 mM). The MichaelisMenten constant (Km) and maximum reaction rate (Vmax) were calculated using both linear and non-linear transformations of the Michaelis-Menten equation. The measurements to determine the temperature effects and temperature coefficients (Q10) for β-glucosidase activities were at 10˚C intervals (between 10˚C and 100˚C) while the activation energy (Ea), was determined using the Arrhenius equation. Two milliliters of 5 mM β-PNPGLU (prepared in 50 mM sodium acetate buffers at pH ranging from 3 to 7 in 0.5 increments) was used to assess the effects of pH on the β-glucosidase activities.
3. RESULTS AND DISCUSSION
3.1. Protein and Carbohydrate Contents
Growth media after the static cultivation of 17 WRF isolates spanning six fungal genera [Pleurotus, Auricularia, Polyporus, Trametes, Lentinula, and Grifola] (Table 1) were evaluated for their β-glucosidase activities. Mycelial growth resulted in the accumulation of secreted proteins in the culture media. The analyses showed significant differences at p ≤ 0.05 in the total protein secreted (Table 2) and varied widely ranging from 68 µg·mL−1 (P. ostreatus-261, wild isolate from North Carolina) to 228 µg·mL−1 (Auricularia sp.-265, wild strain from Ghana).
Table 2. Total protein and carbohydrate contents in media of various white rot fungi.
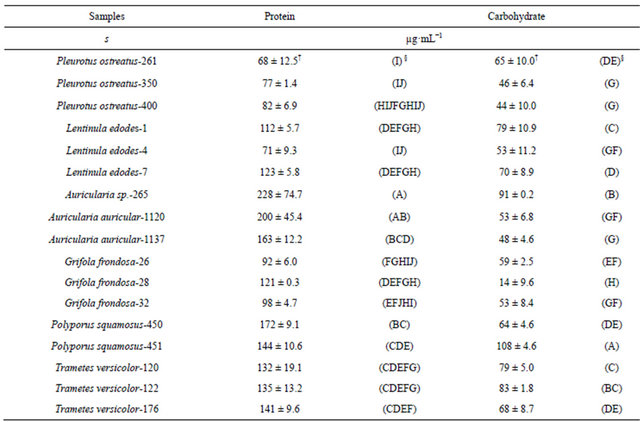 †Standard deviation (n = 3). §ANOVA (Means with the same letters within the same columns are not significantly different).
†Standard deviation (n = 3). §ANOVA (Means with the same letters within the same columns are not significantly different).
Protein content in WRF enzyme production media ranging between 2.8 and 24.0 µg·mL−1 have been reported [25], which are lower compared to this study, probably because of differences in the organisms used and the composition of the growth media. The media also had significant differences at p ≤ 0.05 in the carbohydrate content (Table 2) which ranged from 44 µg·mL−1 (P. ostreatus-400) to 108 µg·mL−1 (P. squamosus-451). However, there were differences in the carbohydrate contents both within and between the various genera in this study, indicating that the abundance of carbohydrate in the media is species and origin specific. The results also indicate that the carbohydrate contents of the liquid media were more variable than the protein contents.
3.2. Beta-Glucosidase Activities
Beta-glucosidase activities, estimated as PNP released after 30 min of incubation with β-PNPGLU at 37˚C indicated that G. frondosa-28 (commercial isolate) exhibited the highest activities. Trametes versicolor-122 (wild isolate from NC) showed the least total β-glucosidase activities; however, there were wide ranges in β-glucosidase activities within each genus, except in the Pleurotus strains. The range of β-glucosidase activities measured within genera after 30 min of incubation were; Pleurotus (10.3 - 12.5 µg), Grifola (39.1 - 225 µg), Lentinula (7.2 - 22.6 µg), Auricularia (16.1 - 24 ug), Polyporus (15 - 21 µg), and Trametes (6.4 - 41.6 µg). There were also differences in β-glucosidase activities between genera ranging from 6.4 (T. versicolor-122) to 225 µg (G. frondosa-28). Morais et al. [26], noted marked differences in β-glucosidase activities of L. edodes strains, which resulted from changes in enzyme production as the duration of fermentation increased. In this study the cultivation conditions were similar for all the genera; however, differences in activities could have only resulted from the differences in the amount of β-glucosedase enzyme protein secreted. Also, variations could be at the molecular level of gene organization which influences the enzyme response to such variables as: pH, temperature, ionic strength, and substrate-enzyme interactions in the reaction media.
Beta-glucosidase activities were not correlated with the protein contents possibly because protein in the crude extracts could include other proteins not associated with β-glucosidase activities. The variations in crude secretions could be explained by the fact that the protein contents of the extracts included other enzymes and amino acids not related to the enzyme activities under investigation. It has been reported that when enzymes are assayed from crude extracts, the results may not suggest a complete degradation or hydrolysis of the substrate [27]. In this study, any substrate degradation which was not the result of enzymatic activities was eliminated using controls. Beta-glucosidase activities showed significant negative correlations with carbohydrate contents which may be explained by the fact that less β-glucosidase is produced when higher available carbon is present in the medium. Also the fungi might have been taking up and assimilating the simple sugars released due to the enzymatic activities.
3.3. Kinetic Parameters
All the fungal β-glucosidase activities assayed fitted the Michaelis-Menten kinetic model (plots not shown) which has been reported to account for the kinetic properties of several enzymes. The Michaelis-Menten constant Km (indicative of enzyme-substrate affinity) and Vmax (maximum velocity at enzyme saturation) for β-glucosidase activities in the media were calculated using both the linear and non-linear regression fit of the Michaelis-Menten equation. The average Km value using the three linear transformations ranged from 0.47 (A. auricula-1120) to 719 µM (L. edodes-7), while Vmax ranged from 0.21 µg·min−1 (T. versicolor-122) to 9.63 µg·min−1 (G. frondosa-28). The lower Km values usually indicate higher enzyme-substrate affinity [28]. The three transformations (Table 3) showed considerable variations, because each transformation gives different weight to errors in the variables [29]. A non-linear regression fit was also used, which gave Km values ranging from 0.51 µM (A. auricular-1120) to 660 µM, (L. edodes-7), and Vmax values ranging from 0.21 µg·min−1 (T. versicolor-122) to 9.70 µg·min−1 (G. frondosa-28) (Table 3). Purified β-glucosidase from straw mushroom has shown Km values for β-PNPGLU between 90 and 500 µM [30], while Sun et al. [31], reported apparent Km between 347 and 70 µM from purified fruiting bodies of L. edodes. Therefore β-glucosidase from A. auricula-1120 had an affinity almost 1300 times greater for PNPGLU than L. edodes-7. The maximum velocity at enzyme saturation was about 45 times higher for Grifola-28 than T. versicolor-122. These ranges in Km and Vmax indicated great disparity with which enzymes from various WRF function to biodegrade substrate.
Table 3. Kinetic parameters of β-glucosidase activities of white rot fungi calculated from different linear and non-linear transformations of the Michaelis-Menten equation.
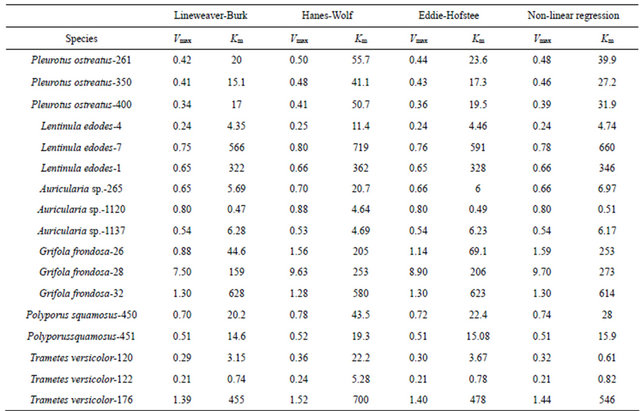 Vmax (µg·min−1); Km (µM). Numbers next to each species are assigned identification by Mushroom Biology and Fungal Biotechnology Laboratory (MBFBL).
Vmax (µg·min−1); Km (µM). Numbers next to each species are assigned identification by Mushroom Biology and Fungal Biotechnology Laboratory (MBFBL).
3.4. Effects of pH and Temperature
The pH profile of each fungal cultivation media for β-glucosidase activities showed that the extracts exhibited pH optima between 3.5 and 5.0 (Figure 1). Almost all displayed relative stability around their pH optima with the exception of P. ostreatus-400 and P. squamosus-451. Similar pH ranges have been reported for other fungal β-glucosidase activities. Previous studies have reported pH optima ranging between 3.8 and 5.0 for Thermoascus aurantiacus β-glucosidase activities [32] and between 4.0 and 6.0 for Aspergillus oryzae [22]. Enzymes, like all proteins are affected by changes in temperature which are used to characterize and evaluate their usefulness in certain processes. Under the experimental conditions in this study, the activities exhibited temperature optima between 60˚C and 70˚C (Figure 2). Most media showed a secondary minor temperature peak around 90˚C, which could be attributed to the possible presence of thermostable isoenzymes in the media. The Auricularia spp. showed more stability around their temperature optima (60˚C - 70˚C), while the other genera were generally more sensitive around their temperature optima. The optimum temperature is within the range of those reported for some other fungal β-glucosidase enzymes (80˚C for Thermoascus aurantiacus and 60˚C for Aspergillus oryzae) [22].
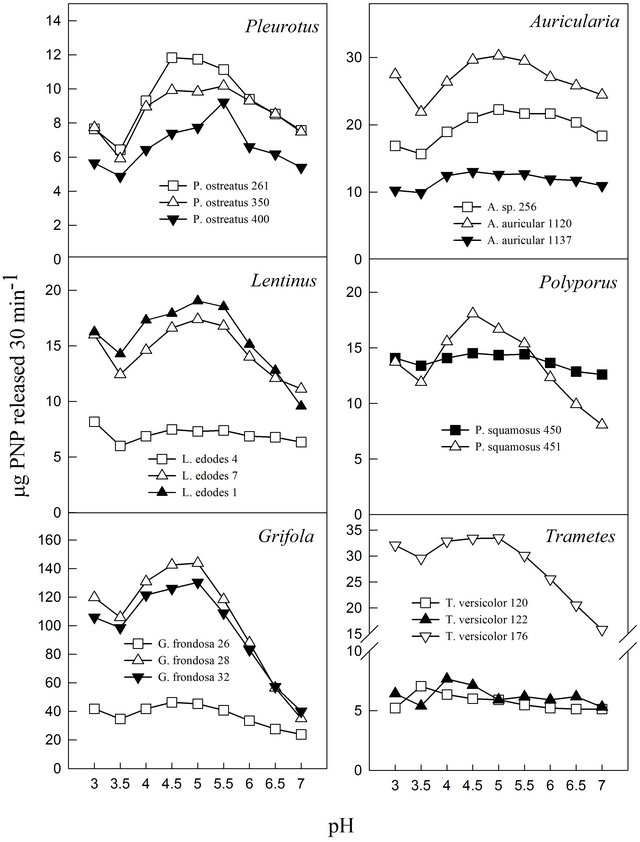
Figure 1. Effect of pH on β-glucosidase activities of various white rot fungi.
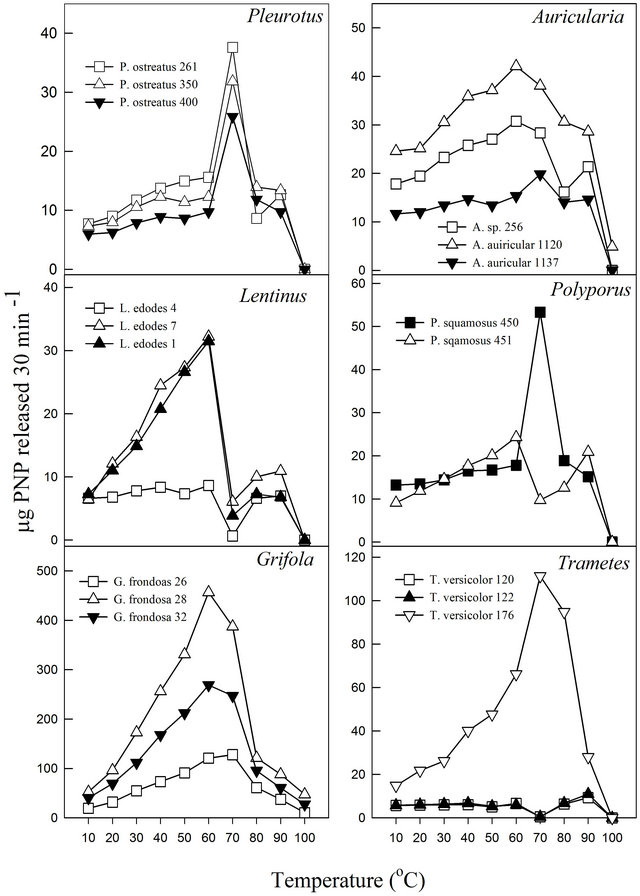
Figure 2. Effect of temperature on β-glucosidase activities of various white rot fungi.
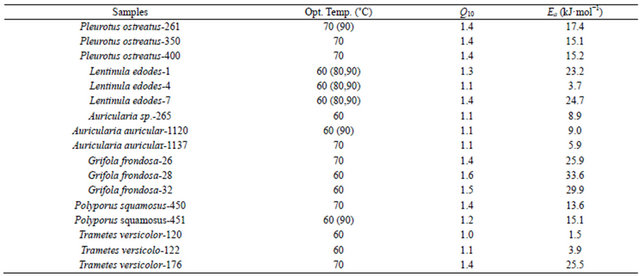
In this study, it was noted that the increase in product formed was relatively gradual with increasing temperature until the optimum temperature was reached, after which the enzyme denatured rapidly and consistently with the temperature profiles of other studies [33]. The activation energy (Ea) or energy barrier that must be overcome for a reaction to proceed was estimated from the logarithmic transformed Arrhenius equation (logK = (−Ea/2.303RT) + logA). The values for Pleurotus, Auricularia and Grifola strains tested were generally similar, while those for Polyporus, Lentinula and Trametes showed significant differences (Figure 3). The calculated Ea ranged from as low as 1.5 kJ·mol−1 (T. versicolor-120) to as high as 33.6 kJ·mol−1 (G. frondosa-28) with an average value of 16.0 kJ·mol−1 (Table 4). Generally, there is a doubling in reaction rate with every 10˚C increase in temperature for chemical reactions known as the temperature coefficient or temperature quotient (Q10). However, this is not always the case with biological systems such as enzyme-catalyzed reactions where the Q10 is generally in an order of magnitude < 2 [34]. The estimated Q10 values for the β-glucosidase activities ranged from 1.1 (Aricularia spp.) to 1.6 (G. frondosa-28) and were generally below 2.0 (Table 4).
Table 4. Temperature coefficients (Q10) and activation energies (Ea) of β-glucosidase activities of white rot fungi.
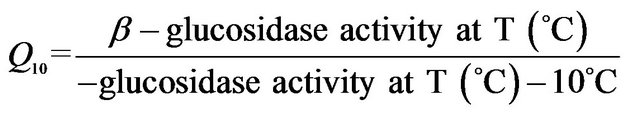
 .
.
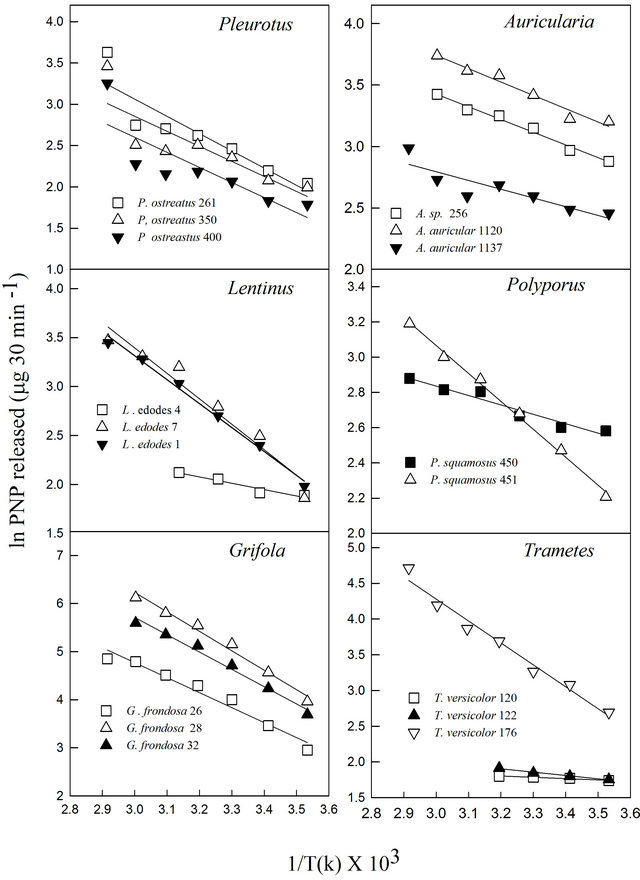
Figure 3. Arrhenius equation plot of β-glucosidase activities of various white rot fungi.
Jabbar et al. [34] reported Q10 of a purified cellulosehydrolyzing endoglucanase at 1.01. This indicates that the reaction rates less than doubled for every 10˚C increase in incubation temperature.
4. CONCLUSION
Significant quantitative and qualitative differences exist among the β-glucosidase activities of WRF. The enzymes also exhibited different response patterns to pH and incubation temperature conditions. Thus screening the WRF responsible for the biodegradation of most plant biomass for their β-glucosidase biocatalytic potentials offers an attractive tool for plant biomass transformation. If appropriately screened and purified, they can be harnessed to potentially improve the bioconversion of cellulose to glucose and also facilitate efficient plant biomass biodegradation to useful bio-products.
5. ACKNOWLEDGEMENTS
This work is a contribution of the Winfred Thomas Agricultural Research Station, Alabama A&M University, Normal, AL. Trade or manufacturers’ names mentioned are for information only and do not constitute endorsement, recommendation, or exclusion by Alabama A&M University and collaborating universities. This research was supported in part by USDA, Evans-Allen Grant # ALAX 011.
REFERENCES
- Barr, D.P. and Aust, S.D. (1994) Mechanisms white rot fungi use to degrade pollutants. Environmental Science and Technology, 28, 78A-87A.
- Wasser, S.P. (2002) Medicinal mushrooms as a source of antitumor and immunomodulating polysaccharides. Applied Microbiology and Biotechnology, 60, 258-274. doi:10.1007/s00253-002-1076-7
- Hadar, Y., Kerem, Z. and Gorodecki, B. (1993) Biodegradation of lignocellulosic agricultural wastes by Pleurotus ostreatus. Journal of Biotechnology, 30, 133-139. doi:10.1016/0168-1656(93)90034-K
- Isikhuemhen, O.S., Anoliefo, G.O. and Oghale, O.I. (2003) Bioremediation of crude oil polluted soil by the white rot fungus, Pleurotus tuberregium (Fr.) Sing. Environmental Science and Pollution Research, 10, 108-112. doi:10.1065/espr2002.04.114
- Bezalel, L., Hadar, Y. and Cerniglia, C.E. (1996) Mineralization of polycyclic aromatic hydrocarbons by the white rot fungus Pleurotus ostreatus. Applied and Environmental Microbiology, 62, 292-295.
- D’Annibale, A., Ricci, M., Leonardi, V., Quaratino, D., Mincione, E. and Petruccioli, M. (2005) Degradation of aromatic hydrocarbons by white rot fungi in a historically contaminated soil. Biotechnology and Bioengineering, 90, 723-731. doi:10.1002/bit.20461
- Mukherjee, R. and Nandi, B. (2004) Improvement of in vitro digestibility through biological treatment of water hyacinth biomass by two Pleurotus species. International Biodeterioration and Biodegradation, 53, 7-12. doi:10.1016/S0964-8305(03)00112-4
- D’Souza, T.M., Merrit, C.S. and Reddy, C.A. (1999) Lignin-modifying enzymes of the white rot basidiomycete Ganoderma lucidum. Applied and Environmental Microbiology, 65, 5307-5313.
- Heinzkill, M., Bech, L., Halkier, T., Schneider, P. and Anke, T. (1998) Characterization of laccases and peroxidases from wood-rotting fungi (family Coprinaceae). Applied and Environmental Microbiology, 64, 1601-1606.
- Isikhuemhen, O.S. and Mikiashvili, N.A. (2009) Lignocellulolytic enzymes activity, substrate utilization and mushroom yield by Pleurotus ostreatus cultivated on substrate containing anaerobic digester solids. Journal of Industrial Microbiology and Biotechnology, 36, 1353- 1362. doi:10.1007/s10295-009-0620-1
- Isikhuemhen, O.S., Mikiashvili, N.A., Adenipekun, C.O., Ohimain, E. I. and Shahbazi, G. (2012) The tropical white rot fungus, Lentinus squarrosulus Mont.: Lignocellulolytic enzymes activities and sugar release from cornstalks under solid state fermentation. World Journal of Microbiology and Biotechnology, 28, 1961-1966. doi:10.1007/s11274-011-0998-6
- Das, M., Royer, T.V. and Leff, L.G. (2007) Diversity of fungi, bacteria, and actinomycetes on leaves decomposing in a stream. Applied and Environmental Microbiology, 73, 756-767. doi:10.1128/AEM.01170-06
- Yu, H., Zeng, G., Huang, H., Xi, X., Wang, R., Huang, D., Huang, G. and Li, J. (2007) Microbial community succession and lignocellulose degradation during agricultural waste composting. Biodegradation, 18, 793-802. doi:10.1007/s10532-007-9108-8
- Platt, M.W., Hadar, Y. and Chet, I. (1984) Fungal activities involved in lignocellulose degradation by pleurotus. Applied Microbiology and Biotechnology, 20, 150-154. doi:10.1007/BF00252594
- Berrin, J.G., Czjzek, M., Kroon, P.A., McLauchlan, W.R., Puigserver, A., Williamson, G. and Juge, N. (2003) Substrate (aglycone) specificity of human cytosolic betaglucosidase. Biochemical Journal, 373, 41-48. doi:10.1042/BJ20021876
- Kumar, R., Singh, S. and Singh, O.V. (2008) Bioconversion of lignocellulosic biomass: Biochemical and molecular perspectives. Journal of Industrial Microbiology and Biotechnology, 35, 377-391. doi:10.1007/s10295-008-0327-8
- Stockton, B.C., Mitchell, D.J., Grohmann, K. and Himmel, M.E. (1991) Optimum beta-d-glucosidase supplementation of cellulase for efficient conversion of cellulose to glucose. Biotechnology Letters, 13, 57-62. doi:10.1007/BF01033518
- Howell, J.A. and Stuck, J.D. (1975) Kinetics of solka floc cellulose hydrolysis by Trichoderma viride cellulase. Biotechnology and Bioengineering, 17, 873-893. doi:10.1002/bit.260170608
- Morais, H., Ramos, C., Forgacs, E., Jakab, A. and Cserhati, T. (2004) Enzyme production of Pleurotus ostreatus evaluated by the three-way principal component analysis. Engineering in Life Sciences, 4, 165-170. doi:10.1002/elsc.200420017
- Cai, Z., Xing, G., Yan, X., Xu, H., Tsuruta, H., Yagi, K. and Minami, K. (1997) Methane and nitrous oxide emissions from rice paddy fields as affected by nitrogen fertilisers and water management. Plant and Soil, 196, 7-14. doi:10.1023/A:1004263405020
- Czjzek, M., Cicek, M., Zamboni, V., Burmeister, W.P., Bevan, D.R., Henrissat, B. and Esen, A. (2001) Crystal structure of a monocotyledon (maize ZMGlu1) beta-glucosidase and a model of its complex with p-nitrophenyl beta-D-thioglucoside. Biochemical Journal, 354, 37-46. doi:10.1042/0264-6021:3540037
- Langston, J., Sheehy, N. and Xu, F. (2006) Substrate specificity of Aspergillus oryzae family 3 beta-glucosidase. Biochimica Et Biophysica Acta-Proteins and Proteomics, 1764, 972-978. doi:10.1016/j.bbapap.2006.03.009
- Saha, A.K. and Brewer, C.F. (1994) Determination of the concentrations of oligosaccharides, complex type carbonyhdrates, and glycoproteins using tthe phenol-sulfuric acid method. Carbohydrate Research, 254, 157-167. doi:10.1016/0008-6215(94)84249-3
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantitites of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248-254. doi:10.1016/0003-2697(76)90527-3
- Szklarz, G.D., Antibus, R.K., Sinsabaugh, R.L. and Linkins, A.E. (1989) Production of phenol oxidases and peroxidases by wood-rotting fungi. Mycologia, 81, 234- 240. doi:10.2307/3759705
- Morais, H., Ramos, C., Forgacs, E., Cserhati, T., Oliviera, J. and Illes, T. (2001) Three-dimensional principal component analysis employed for the study of the beta-glucosidase production of Lentinus edodes strains. Chemometrics and Intelligent Laboratory Systems, 57, 57-64. doi:10.1016/S0169-7439(01)00121-6
- Ng, T.K. and Zeikus, J.G. (1981) Comparison of extracellular cellulase activities of clostridium-thermocellum-lqri and trichoderma-reesei-QM9414. Applied and Environmental Microbiology, 42, 231-240.
- Dick, W.A. and Tabatabai, M.A. (1984) Kinetic-parameters of phosphatases in soils and organic waste materials. Soil Science, 137, 7-15. doi:10.1097/00010694-198401000-00002
- Dowd, J.E. and Riggs, D.S. (1965) A comparison of estimates of Michaelis-Menten kinetic constants from various linear transformations. Journal Biological Chemistry, 240, 863-869.
- Cai, Y.J., Buswell, J.A. and Chang, S.T. (1998) Betaglucosidase components of the cellulolytic system of the edible straw mushroom, Volvariella volvacea. Enzyme and Microbial Technology, 22, 122-129. doi:10.1016/S0141-0229(97)00151-8
- Sun, Y.M., Zhang, Y.Z., Liu, N., Li, D.M. and Li, M.Z. (2010) Hydrolysis of genistin and daidzin by a beta-glucosidase purified from Lentinula edodes. Journal of Medicinal Plants Research, 4, 2684-2690.
- Parry, N.J., Beever, D.E., Owen, E., Vandenberghe, I., Van Beeumen, J. and Bhat, M.K. (2001) Biochemical characterization and mechanism of action of a thermostatble beta-glucosidase purified from Thermoascus aurantiacus. Biochemical Journal, 353, 117-127. doi:10.1042/0264-6021:3530117
- Gueguen, Y., Chemardin, P., Labrot, P., Arnaud, A. and Galzy, P. (1997) Purification and characterization of an intracellular beta-glucosidase from a new strain of Leuconostoc mesenteroides isolated from cassava. Journal of Applied Microbiology, 82, 469-476. doi:10.1046/j.1365-2672.1997.00136.x
- Jabbar, A., Rashid, M.H., Javed, M.R., Perveen, R. and Malana, M.A. (2008) Kinetics and thermodynamics of a novel endoglucanase (cmcase) from Gymnoascella citrina produced under solid-state condition. Journal of Industrial Microbiology and Biotechnology, 35, 515-524. doi:10.1007/s10295-008-0310-4
NOTES
*Corresponding author.

