Open Journal of Pediatrics
Vol.3 No.3(2013), Article ID:35874,6 pages DOI:10.4236/ojped.2013.33034
Hepatitis C virus antibodies among transfused children with sickle cell anaemia at University of Ilorin Teaching Hospital
![]()
1Department of Paediatrics and Child Health, Federal Medical Centre, Keffi, Nigeria
2Department of Paediatrics and Child Health, University of Ilorin Teaching Hospital, Ilorin, Nigeria
Email: kolade.ernest@yahoo.com
Copyright © 2013 C. E. Onuchukwu et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received 6 April 2013; revised 8 May 2013; accepted 16 May 2013
Keywords: Hepatitis C Virus; Sickle Cell Anaemia; Blood Transfusion
ABSTRACT
Background: Hepatitis C is an infectious disease of the liver caused by the Hepatitis C virus (HCV) resulting to a chronic Hepatitis. Chronic HCV infection constitutes a serious health challenge in places where prevalence is substantial. In Nigeria, there is a high risk because donor blood is not routinely screened for HCV. Patients with sickle cell anaemia (SCA) are considered a subset of the population at higher risk of acquiring the virus, due to their frequent needs for transfusion of blood and its products. However, the magnitude of HCV infection has not been adequately measured in our general population and specific data on HCV in SCA patients are scanty, hence a prospective case controlled study to determine the prevalence of HCV antibodies in transfused SCA patients attending the sickle cell anaemia clinic in the University of Ilorin Teaching Hospital (UITH), Ilorin was taken. Objective: To determine the prevalence of Hepatitis C virus antibodies among transfused children with SCA in Ilorin. Subjects and Method: Eighty two transfused SCA children aged 6 months to 14 years were recruited consecutively from February 2008 to January 2009 while eighty four non transfused SCA children of the same age range recruited over the same period served as controls. Hepatitis C virus antibody screening was done using a second generation ELISA method. Results: The overall prevalence of HCV antibody was 3.0%, while it was 3.7% and 2.4% in the transfused and non transfused SCA patients respectively (χ2 = 0.23, p = 0.68). The patients were also comparable across the social class when subcategorized into high and low social class (χ2 = 0.37, p = 1.00 (subjects), χ2 = 0.42, p = 1.00 (controls). Conclusion: The prevalence of Hepatitis C virus anti- bodies in transfused SCA patients is low. The difference in prevalence between transfused and nontransfused SCA patient was not statistically significant. This was cautiously interpreted due to the hospital based premise of the work. Therefore, Hepatitis C virus antibody acquisition might be from sources other than transfusion of unscreened blood.
1. INTRODUCTION
Viral hepatitis is a major cause of morbidity and mortality in Sub-Saharan Africa [1]. The Hepatitis C virus (HCV) has become an important cause of chronic liver disease and liver cancer worldwide [2] Hepatitis C virus infection is highly prevalent in Africa, however the epidemiology of this infection is yet to be well defined [1]. Hepatitis C virus infection may lead to chronicity in 70% - 85% of cases [3], which is similar to Hepatitis B virus infection (HBV) that may become chronic in about 75% of individuals infected [4]. It was estimated that about 170 million people worldwide are chronically infected with HCV and majority are in developing countries [5]. About 30% of those infected with HCV will progress to liver cirrhosis and ultimately to end stage liver failure and hepatic carcinoma [3,4,6]. It is one of the agents transmissible by blood transfusion and is now known to be the major cause of non-A, non-B post transfusion hepatitis (NANBH) [7]. The risk factors that are associated with transmission of both HCV and HBV infections are transfusion of blood and blood products, tattooing, scarification with re-usable instruments, body piercing, injection/illicit drug use, perinatal transmission and multiple sex partners [5,8]. Since HCV was first identified[9,10] one of the best known and most extensively studied routes of its transmission has been blood and blood derivative transfusion [11,12], therefore patients at risk for HCV infection include transfusion dependent Haemophiliacs, homozygous β-Thalassaemia and sickle cell anaemia (SCA) patients [13]. Though donor blood was generally screened for evidence of HBV, only recently was screening for HCV started.
The commonest genetic condition in Nigeria for which patients receive repeated blood transfusion is SCA [14], which has a prevalence of 1.6% - 3% [15] in Nigeria.
Few studies have been done on HCV in SCA patients and these have shown a relatively high prevalence of HCV infection. Hasan et al. [16] reported an overall prevalence of 10.1% in adult patients with sickle cell disease in New York. De Vault et al. [17] in Philadelphia, reported prevalence of 20.7% in adults, with HCV infection commoner in SCA patients with multiple transfusions. Torres et al. [18] in Brazil found a prevalence of 14.1% in patients with SCA compared to 3% in the general population. Mutimer et al. [1] studying a mixture of adults and children also reported a prevalence of 20% in SCA patients and 14% in blood donors in Benin Nigeria. Ejele et al. [19] also reported an overall prevalence of 3% in the Niger Delta area of Nigeria with higher values in females, uneducated and unmarried subjects [19]. This study was carried out among adult population. Lesi and Kehinde [2] reported an overall 5% prevalence rate in transfused SCA patients in Lagos though most of the subjects were adults. Adewuyi [14] in Ilorin studying a group of adult and children found an overall prevalence of 5% in blood donors and SCA patients with multiple transfusions in 1996 and concluded that there was no significant difference between transfused and non transfused patients. Some of these studies suggested that the prevalence of HCV was directly related to the frequency of exposure to blood and blood products transfusion, [16,17] while others [12,14] suggested otherwise. Most of these studies were done in a mixed population of adults and children having sickle cell disease and none done specifically in children. None of them evaluated impact of geography, culture and local hospital policy which might have affected the prevalence reported.
The risk of transfusion acquired HCV infection and its potentially devastating consequences are clearly greater in this environment where routine pre-donor HCV screening is not standard practice [2]. This study therefore aims to determine the prevalence of HCV antibodies among transfused children with SCA and the results will contribute to developing a policy for pre-donor screening of blood for anti-HCV.
2. SUBJECTS AND METHODS
The study was carried out at the University of Ilorin Teaching Hospital (UITH) Ilorin, Nigeria. The University of Ilorin Teaching Hospital (U.I.T.H.) is a tertiary health facility that serves as a referral centre for Kwara, Kogi, Niger, Osun and Ekiti States of Nigeria and also offers secondary health services to the public. It runs a well-established Sickle Cell Disease Clinic for patients below the age of 14 years. An average of 30 mainly old and few new patients are seen in the clinic every Monday. On attaining the age of 14 years such individuals are transferred to the adult Sickle Cell Disease Clinic of the same hospital. The hospital provides a blood transfusion service and laboratory that screens blood for antibodies to HCV.
Eighty-two transfused SCA children aged 6 months to 168 months were recruited consecutively from February 2008 to January 2009 while 84 non transfused SCA children of the same age range recruited over the same period served as controls. Pre-transfusion anti-HCV status was not documented. Antibodies to HCV screening were done using a second generation ELISA method. The age of the subjects in this study were taken as their completed months at recruitment. The subjects were transfused at least 6 months before presentation to be eligible for the study. The reasons for transfusion in these cohorts were severe anaemia with heart failure or rapidly falling heamatocrit.
Haemoglobin (Hb) electrophoresis was carried out using electrophoretic tank (Volkman SAE 2761) with cellulose acetate paper at pH 8.4 to confirm their status in the Haematology department of U.I.T.H Ilorin. A pretested questionnaire was used to obtain information on age, sex, history of blood transfusion, parents’ educational level as well as occupation to obtain their social class.
Ethical clearance was obtained from the Ethical Review Committee of the hospital and official permission obtained from the head of the Haematology Department. After a clear explanation of the project to them, informed consent was also obtained from either or both parents/ guardian and the children before subject enrolment.
Five milliliters of venous blood was collected from each subject after a verbal consent and then transported to the laboratory where the serum was separated and assayed for antibodies to HCV immediately using a 2nd generation HCV one step Hepatitis C virus test strip manufactured by Acumen diagnostic incorporated, USA which is a rapid chromatographic immunoassay for the qualitative detection of antibody to HCV in serum was used for HCV analysis. The sensitivity and specificity of the test strip were 99.6% and 99.5% respectively.
Statistical analysis was done using SPSS statistical package. The chi-square test was used to assess the significance of the difference amongst the groups and a p-value of <0.05 was considered significant.
3. RESULTS
A total of 82 transfused sickle cell anaemia patients and 84 non-transfused sickle cell anaemia patients were recruited into the study. The male to female ratio was 1:0.6 in the subjects and 1:0.9 in the controls. The mean age of the subjects and the controls were 95.8 ± 48.7 months and 93.5 ± 54.0 months respectively and are comparable (p = 0.77), Table 1. Table 2 shows that five of the children had detectable antibodies to HCV, constituting 3% of the total population studied, giving an overall hospital-based prevalence of Hepatitis C infection in sickle cell anaemia (SCA) to be 3%. The prevalence of antiHCV in both the subjects and the controls were comparable (p = 0.68), as shown in Table 2 below.
Table 3 shows that the subjects and controls were comparable in terms of social stratification in Classes I, IV and V, but more controls were in social Classes II and more subjects in social Class III in the subjects (p = 0.003 and 0.002 for Classes II and III respectively). There is no significant difference in the seroprevalence of HCV infection in the low and high social classes, (Table 4) both in the subjects and controls.
Table 5 shows that the rate of blood transfusion in the subjects is comparable in both sexes and across the different age groups. More males received transfusions than females in all the age groups and were not statistically significant except in the 5 - 10 yr age group (p = 0.002).
Two males and one female of the subjects were posi-
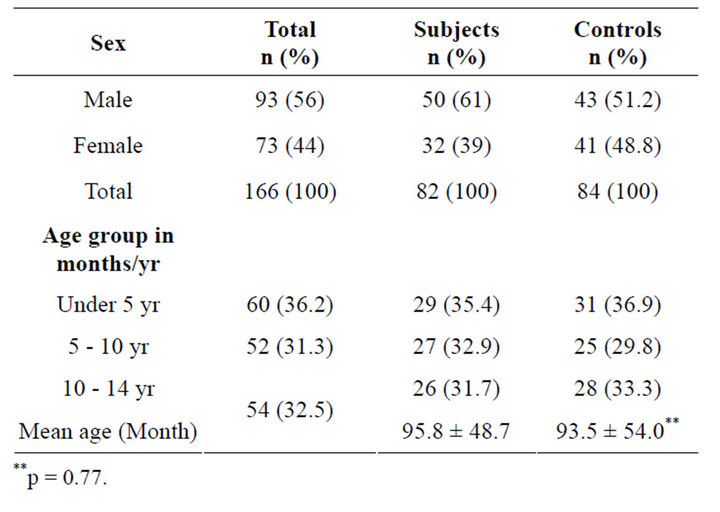
Table 1. Sex and age distribution of the total population studied.
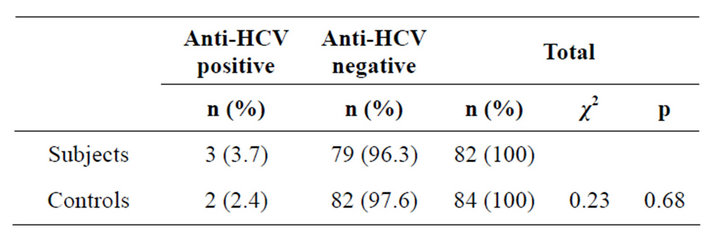
Table 2. The anti-HCV seropositivity in the subjects and controls.
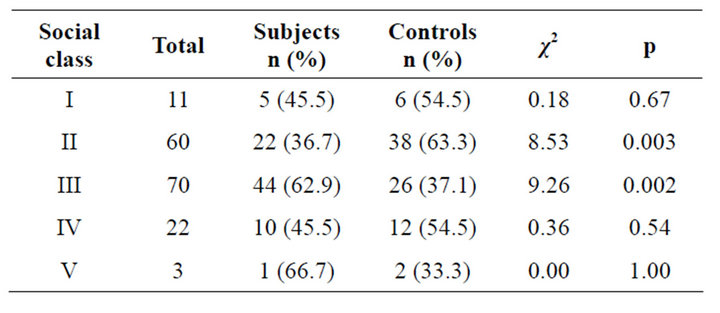
Table 3. The socioeconomic classification of the subjects and controls.
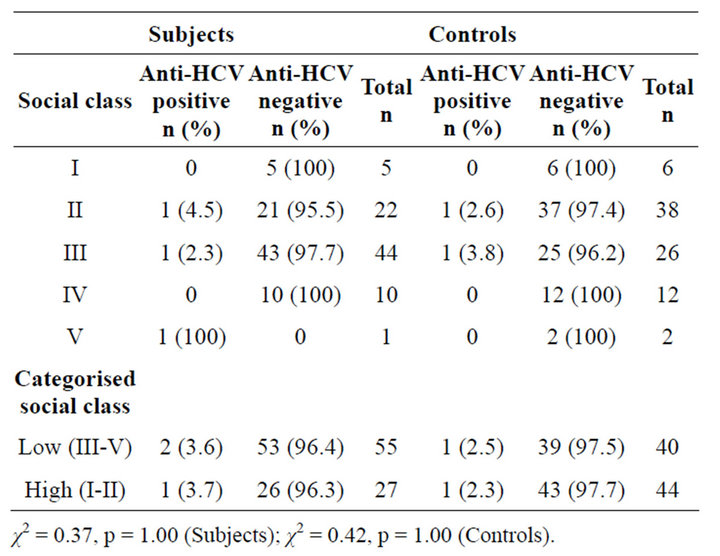
Table 4. Anti-HCV positivity in subjects and controls according to social class.
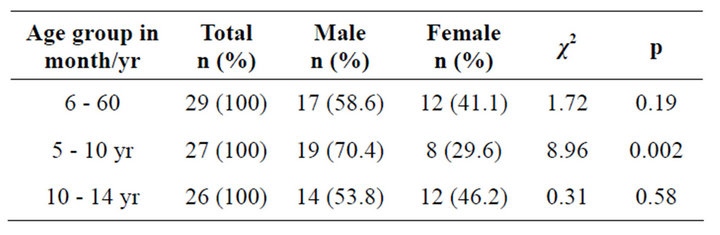
Table 5. Age and sex distribution of subjects that received blood transfusion.
tive, while a male and female each were positive amongst the control group and this difference is not statistically significant (p = 0.69 and 0.74 for subjects and controls respectively, Table 6).
Table 7 shows that among those that are positive for anti HCV the distribution was similar in the different age groups in the subjects and controls. None of the patients in the 6 to 60 months age group was positive in the controls. The subjects and controls were comparable in terms of positivity across the age groups (p = 0.48). Table 8 shows that among the subjects, anti-HCV seropositivity increased with the number of units of blood transfused. This increase was such that 2.2% of recipients of 1 - 2 units and 6.9% of recipients of 3 - 4 units were antiHCV positive.
All the subjects received blood transfusion at one time
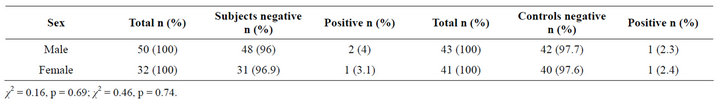
Table 6. The anti-HCV status of the subjects and controls according to sex.
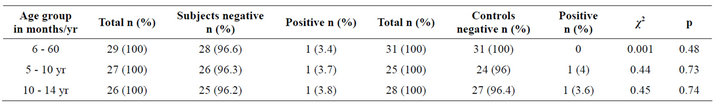
Table 7. Age distribution of anti-HCV positive patients in the subjects and controls.
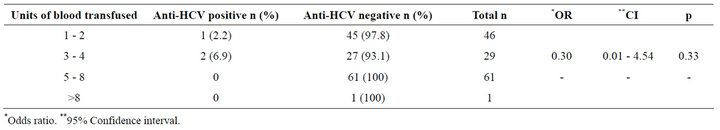
Table 8. Correlation between anti-HCV seropositivity and the units of blood transfused.
or the other with the number of transfusions ranging from 1 to 13 units with a mean of 2.63 ± 1.67 units per subject. A patient in the age group 121 to 168 months received greater than eight units of blood (Figure 1).
4. DISCUSSION
In this study, the seroprevalence rate of anti-HCV is 3.7% in the transfused sickle cell anaemia (SCA) patients and 2.4% in the controls that were not transfused, a finding that is similar to previous reports [2,4].
An overall prevalence of 3% was detected in sickle cell anaemia patients generally, with a higher prevalence of 3.7% in transfused patients, and 2.4% in non-transfused sickle cell anaemia patients. These values parallels the frequency of HCV infection in the world population in general estimated at 3% [4], while the frequency among SCA patients subjected to transfusion of blood or blood derivatives reported in the literature ranges from 2% - 30%. [2,5-9,14,16-18]. In the present study, under 5 years old group have the highest percentage of seropositivity Table 1. This may indicate that this age group are more vulnerable due to the fact that many sociocultural and biologic activities occur during this age group.
The prevalence rate reported in this study is lower than previous studies [2,14,16-19] which involved mostly adults in high-risk groups, sickle cell anaemia patients, patients with icteric hepatitis, HIV infected patients and hepatocellular carcinoma. These studies were carried out in a culturally different environment which could have accounted for the differences. Anti-HCV seroprevalence rate found in this study is similar to that reported by other workers in Ilorin, [14,20] Lagos [2] and in the Niger Delta [19]. This similarity might be explained by the fact that these areas are within the same country and share some practices. A preliminary finding of a prevalence of 3.7% suggests that HCV infection may be common and may not be fully accounted for by blood transfusion alone.
Sickle cell anaemia patients who received multiple blood transfusions did not appear to be at a greater risk of acquiring Hepatitis C virus infection since the prevalence is comparable in the transfused cases and non transfused controls suggesting that blood transfusion may not be the only or major route of transmission in this environment and needs further evaluation.
There was also no correlation in prevalence between multi-transfused and non-transfused sickle cell anaemia patients, a finding that is similar to earlier reports [2,14] but at variance with others [1,16-18,21], that reported a higher prevalence with multi-transfused SCA patients. Again, climate, culture, age of patients, social status and local hospital policy may play significant roles. The patients were evenly distributed in the five social classes and the subjects and controls were comparable except in social classes two and three where the differences in the subjects and controls were statistically significant. Over two-thirds of those that are anti-HCV positive were in the low socioeconomic class, a finding that is similar to
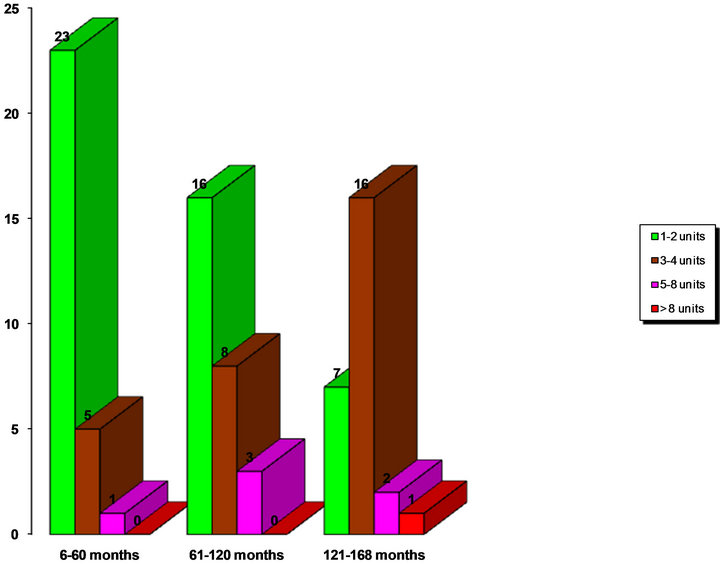
Figure 1. Bar chart showing the units of blood transfused in the different age groups amongst the subjects.
other reports from the Niger Delta [19] Area of Nigeria and in the USA [21]. This can be attributed to the poverty and resultant ignorance and poor health seeking behaviour of this group of people.
The sexual route may also not be important since the result of this study compared to others in adults [16,17, 19] did not show significant difference.
Gender did not correlate with seropositivity as the prevalence rate is comparable in both sexes. Many previous studies did not mention any gender difference in the prevalence of anti-HCV except some workers [19,22] who found a higher anti-HCV prevalence in females and was statistically insignificant. This may suggest a chance finding and larger studies are however required to confirm this finding.
There was a linear correlation between the prevalence of anti-HCV and increasing number of units of blood transfused and this is similar to the trend in other studies. [1,16-18] The highest prevalence was in those who had more than 3 units of blood. This is comparatively higher than those that received more than 10 units of blood in both US studies with seroprevalence of 23% and 30% [16,17].
The higher magnitude of the risk of positivity with each transfusion in the Nigerian studies may suggest that more contaminated blood is being transfused. This is not surprising considering that blood is not usually screened for HCV antibodies, it is therefore pertinent that blood be given only when necessary and blood for transfusion should also be screened for Hepatitis C virus antibodies.
Whatever the source of infection, screening of blood and blood products alone may not prevent the transmission of HCV infection effectively in this environment. Increasing awareness of the disease and its route of transmission, adequate enlightenment of the general population as well as health care workers on safe medical practices could make a significant impact on the spread of HCV infection. Furthermore, premarital antenatal counseling and screening for sickle cell disease will contribute to a decrease in prevalence through a corresponding decrease in the sickle cell gene frequency.
4. CONCLUSION
We conclude that in this study blood transfusion has not significantly contributed to increased risk of HCV seropositivity in SCA patient and suggested a higher sample size and further search for other driving factors in our population. However, it will be good practice to maintain policy and practice that has been proven to mitigate increased prevalence of HCV among the general population.
REFERENCES
- Mutimer, D.J., Olomu, A., Skidmore, S., et al. (1994) Viral hepatitis in Nigeria sickle cell disease and comercial blood donors. QJM, 87, 407-411.
- Lesi, O.A. and Kehinde, M.O. (2003) Hepatitis C virus infection in patients with sickle cell anaemia at Lagos University Hospital. Nigerian Postgraduate Medical Journal, 10, 79-83.
- Hoognagle, J.H. and Hepatitis, C. (1997) The clinical spectrum of disease. Hepatology, 26, 5S-20S.
- Esteban, J.I., Lopez-Televera, J.C., Genesca, J., et al. (1999) High rate of infectivity and liver disease in blood donors with antibodies to hepatitis C virus. Annals of Internal Medicine, 15, 443-449.
- WHO (1997) Hepatitis C WHO fact sheet No 164. http://www.who.int/inffs/en/fact 164.html
- Coursaget, P., Bourdil, C., Kastally, R., et al. (1990) Prevalence of hepatitis C virus infection in Africa; anti HCV antibodies in the general population and in patients suffering from cirrhosis or primary liver cancer. Research in Virology, 141, 449-454. doi:10.1016/0923-2516(90)90045-K
- Choo, Q.L., Weiner, A.J., Overby, L.R., et al. (1990) Hepatitis C virus: The major causative agent of viral non-A, non-B hepatitis. British Medical Bulletin, 46, 423-441.
- Finleyson, M.D., Hayes, P.C. and Simpson, K.J. (1999) Diseases of the liver and biliary system. In: Christopher, H., Ed., Davidson’s Principle and Practice of Medicine, Churchill Livingstone (Publishers) Ltd, Edinburgh, 683- 736.
- Choo, Q.L., Kuo, G., Weiner, A.J., et al. (1989) Isolation of A cDNA clone derived from a blood borne non-A, non-B viral hepatitis genome. Science, 244, 359-362. doi:10.1126/science.2523562
- Kuo, G., Choo, Q.L., Alter, H.J., et al. (1989) An essay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science, 244, 362-364. doi:10.1126/science.2496467
- Donahue, J.G., Munoz, A., Ness, P.M., et al. (1992) The declining risk of post-transfusion hepatitis C virus infection. The New England Journal of Medicine, 327, 369- 373. doi:10.1056/NEJM199208063270601
- Schreiber, G.B., Busch, M.P., Kleinman, S.H., et al. (1996) The risk of transfusion—transmitted viral infections. The New England Journal of Medicine, 334, 1685- 1690. doi:10.1056/NEJM199606273342601
- Bhattacharya, D.K., Bhattacharjee, S., De, M. and Lahiri, P. (1991) Prevalence of hepatitis C in transfusion dependent thalassaemics and haemophiliacs. Indian Journal of Medical Research (B), 94, 430-432.
- Adewuyi, J.O. (1996) Prevalence of antibodies to hepatictis C virus among normal blood donors and multi-transfused sickle-cell anaemia patients in Nigeria. Tropical Doctor, 26, 29-30.
- Akinyanju, O.A. (1989) A profile of sickle cell disease in Nigeria. Annals of the New York Academy of Sciences, 565, 126-136. doi:10.1111/j.1749-6632.1989.tb24159.x
- Hasan, M.F., Marsh, F., Posner, G., et al. (1996) Chronic hepatitis C in patients with sickle cell disease. The American Journal of Gastroenterology, 91, 1204-1206.
- De Vault, K.R., Friedman, L.S., Westerberg, S., et al. (1994) Hepatitis C in sickle cell anaemia. Journal of Clinical Gastroenterology, 18, 206-209. doi:10.1097/00004836-199404000-00006
- Torres, M.C.M.R., Pereira, L.M.M.B., Ximenes, R.A.A., et al. (2003) Hepatitis C virus infection in a Brazilian population with sickle-cell anaemia. Brazilian Journal of Medical and Biological Research, 36, 323-329. doi:10.1590/S0100-879X2003000300006
- Ejele, O.A., Nwauche, C.A. and Erhabor, O. (2006) Seroprevalence of hepatitis C virus in the Niger Delta of Nigeria. Nigerian Postgraduate Medical Journal, 13, 103- 106.
- Agbede, O.O., Iseniyi, J.O., Ojuawo, A., et al. (2006) Risk factors and seroprevalence of hepatitis C antibody in mothers and their pre-school age children in Ilorin. African Journal of Clinical and Experimental Microbiology, 7, 153-157.
- Thomas, D.C., Zerilman, J.M. and Alter, H.J. (1995) Sexual transmission of hepatitis C among patients attending Baltimore sexually transmitted diseases clinic: An analysis of 309 sexual partnerships. The Journal of Infectious Diseases, 171, 768-775.
- Jose, L., Sanchez, M.H. and Sjorgren, J.D. (2000) Hepatitis C in Peru: Risk factors for infection, potential iatrogenic transmission and genotype distribution. The American Journal of Tropical Medicine and Hygiene, 63, 242-248.

