Optics and Photonics Journal
Vol.3 No.6A(2013), Article ID:38551,5 pages DOI:10.4236/opj.2013.36A006
Synthesis of a Novel Bluish-Green Emitting Oxynitride Ca3Al8Si4O17N4:Eu2+ Phosphor in a CaAl4−xSixO7−xNx Solid Solution System
Institute of Multidisciplinary Research for Advanced Materials, Tohoku University, Sendai, Japan
Email: *kakihana@tagen.tohoku.ac.jp
Copyright © 2013 Jihae Kim et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received August 8, 2013; revised September 11, 2013; accepted September 27, 2013
Keywords: Oxynitride Phosphor; Eu2+ Activator; Ca3Al8Si4O17N4:Eu2+; Solid Solution
ABSTRACT
Synthesis of oxynitride solid solutions CaAl4−xSixO7−xNx:Eu2+ (x = 0 - 4) was attempted by the solid state reaction (SSR) methods using Si3N4 and AlN as nitrogen sources. The Ca3Al8Si4O17N4 (x = 4/3) sample with the high phase purity was obtained when AlN was used as a nitrogen source whereas the sample synthesized using Si3N4 as another nitrogen source contained a Ca2Al2SiO7 impurity. Thus, it was revealed that AlN was a preferable nitrogen source for the synthesis of Ca3Al8Si4O17N4 by the SSR method. The solid solutions around x = 4/3 activated with Eu2+ exhibited bluish-green luminescence with emission maxima at 480 nm by the excitation at 250 - 450 nm. Thus, the CaAl4-xSixO7-xNx: Eu2+ solid solutions especially for Ca3Al8Si4O17N4:Eu2+ (x = 4/3) were developed as novel Eu2+-activated oxynitride phosphors capable of the excitation by the near ultraviolet (NUV) LEDs.
1. Introduction
White light-emitting diodes (LEDs) have been developing rapidly over the past decade, since its advanced properties such as long life time, high efficiency, and environmentally friendliness without use of mercury. The application of white LEDs is expanding into extensive fields such as residential lighting, medical lighting, mobile, back lights, traffic lights, emotional lighting and so on. General white LEDs are composed of a blue LED chip and yellow phosphor such as Y3Al5O12:Ce3+ [1]. Such combination certainly achieves generation of artificial white light; however, it is not preferable in respect of the high color temperature and the low color rendering index value (CRI < 75) owing to the lack of red and green components. In case of residential lighting, CRI value should satisfy over 80 [2]. In order to realize such a high CRI value for the white LED, highly efficient bluegreen (470 - 510 nm) and red (650 nm) emitting phosphors capable of excitation by blue or NUV LEDs are demanded.
Eu2+ ions are widely used as activators in phosphors since the emission from Eu2+ attributed to the electron transition 4f65d1 → 4f7 is strongly affected by its surrounding environment, i.e. symmetry, covalence, bond length, crystal-field strength. In other words, the emission wavelengths from Eu2+ ions are able to be tuned from blue to red region with the selection of suitable materials as the hosts. Oxynitrides are regarded as suitable hosts since excitation and emission bands at longer wavelength are expected from the nephelauxetic effect owing to the larger covalence nature for M-N (M: metal) bonds than that for M-O [3,4]. It results in the extensive research for the Eu2+-doped oxynitrides particularly silicon-contained oxynitrides such as β-SiAlON:Eu2+ [5], MSi2O2N2:Eu2+ (M = Ca, Sr, and Ba) [6-8] and Ba3Si6O12N2:Eu2+ [9]. Development of new oxynitride phosphors is an important research topic to enrich the phosphor library with various excitation and emission properties. Sun et al. have reported the synthesis of solid solutions between CaAl4O7 and Ca3Al8Si4O17N4 [10]. Their research has attracted the authors’ interest in investigation of photoluminescence properties of Eu2+-activated CaAl4−xSixO7−xNx solid solutions.
On the other hand, homogeneous distribution of the Eu2+ activators in given host materials is one of the important factors in order to achieve high luminescence efficiency. Solution-based processes are potential methods to achieve homogeneous distribution of the activators [11-18]. Our research group recently has succeeded in improvements of emission intensities for oxynitride phosphors, Na1–xMxAlSiO4–xNx:Eu2+ (M = Mg2+, Ca2+, Sr2+) [13] and Ba3Si6O12N2:Eu2+ [14], by the combined synthesis methods composed of the preparation of oxide precursors by solution-based method and its subsequent nitridation under ammonium atmosphere.
Based on the background described above, the synthesis of oxynitride solid solutions CaAl4−xSixO7−xNx (x = 0 - 4) by the SSR method and investigation of their photoluminescence properties with the Eu2+ activation were examined in the present study. In addition, the combined method involving the solution-based process and the subsequent nitridation of oxide precursor was applied for the synthesis of Ca3Al8Si4O17N4:Eu2+.
2. Experimental
Two series of synthesis were examined for the CaAl4−xSixO7−xNx:Eu2+ solid solutions by a conventional solid state reaction (SSR) method as summarized in Table 1. Raw materials of CaCO3, Eu2O3, α-Al2O3, SiO2, AlN and Si3N4 were weighed and mixed thoroughly in an agate mortal with a pestle according to the ratios listed in Table 1. 2 mol% of europium was substituted for calcium. The mixed powder was heat-treated at 1673 K for 4 h in a H2(4%)-Ar stream. For the x = 4/3 sample corresponding to Ca3Al8Si4O17N4, the combined synthesis method involving the preparation of an oxide precursor by an amorphous metal complex (AMC) method, one of solution-based methods, and its subsequent nitridation under ammonia atmosphere was also examined [13,14]. The oxide precursor having a composition of (Ca, Eu):Al:Si = 3:8:4 was prepared by the AMC method. After dissolving CaCO3 in an aqueous citric acid solution, aqueous solutions of 1 M Eu(NO3)3 and 1 M Al(NO3)3∙9H2O were added. Then, an aqueous solution of propylene glycol-modified silane (PGMS) was added.
PGMS was obtained by an alkoxy group exchange reaction for tetraethoxysilane with propylene glycol at 353 K in the presence of hydrochloric acid as a catalyst [11,15]. The mixed solutions were heated on a heating plate operated at 393 K with stirring to promote polymerization. The obtained polymer gel was pyrolyzed at 723 K for 3 h, and subsequently at 823 K for 10 h to remove organic compounds gradually. The obtained oxide precursor, finally, was heat-treated at 1673 K for 4 h under an ammonia stream (50 ml/min).
The X-ray diffraction (XRD; Bruker AXS: D2 Phaser) was used for the phase identification. Photoluminescence spectra were measured using a fluorescence spectrometer (Hitachi: F-4500) at room temperature. Internal quantum efficiency was measured using another fluorescence spectrometer (Jasco: FP-6500) equipped with an integrating sphere.
3. Results and Discussion
Figure 1 shows XRD patterns of the samples in the sersies A using Si3N4 as a nitrogen source as listed in Table 1. CaAl4O7 (x = 0) was successfully obtained without any impurities. The x = 0.5 sample contained CaAl4O7 and Ca2Al2SiO7 whereas the x = 1 sample was the mixture of Ca2Al2SiO7, Ca3Al8Si4O17N4, Al2O3, and CaAl4O7. The x = 4/3 sample whose nominal composition was equal to Ca3Al8Si4O17N4 of the known Ca-Al-Si-O-N compound was also crystallized in the multiphase of Ca3Al8Si4O17N4, Ca2Al2SiO7, and Al2O3 although there is a report on the synthesis of Ca3Al8Si4O17N4 in a single phase [10]. The x = 2 sample showed a diffraction pattern similar to that for the x =3/4 sample. The x = 3 sample was composed of Ca2Al2SiO7 and unknown phase while the x = 4 sample was mixture of Ca3Si3O9, Si2N2O, and Si3N4. Thus, no known Ca-Al-Si-O-N phases were formed in the samples of x = 3 and 4. The results in the synthesis of the samples in the series A implied the difficulties in the synthesis of the CaAl4–xSixO7–xNx samples using Si3N4 as the nitrogen source even for the known
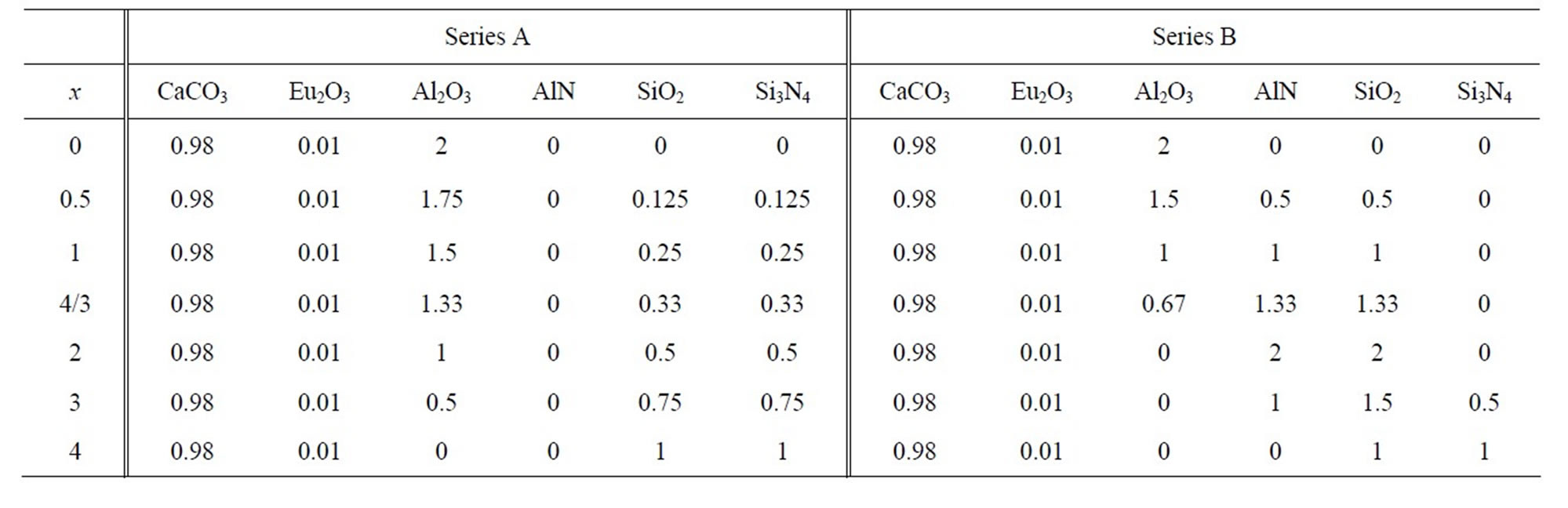
Table 1. Compositions for CaAl4−xSixO7−xNx:Eu2+ (x = 0 - 4) solid solutions.
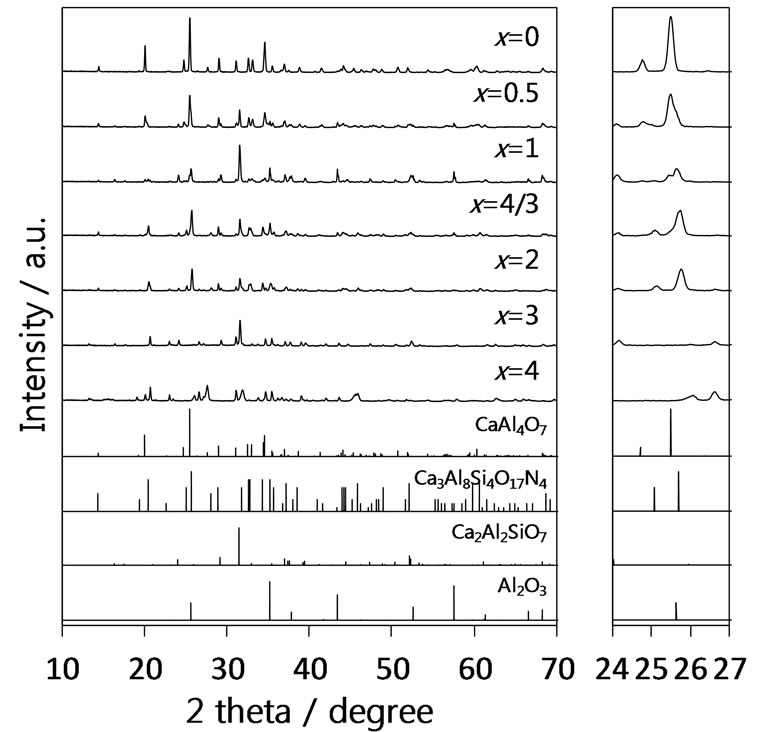
Figure 1. XRD patterns of CaAl4−xSixO7−xNx:Eu2+ samples (series A) synthesized by the SSR method using Si3N4 as a nitrogen source.
compound, Ca3Al8Si4O17N4.
Figure 2 shows XRD patterns of the samples in the series B using AlN as a nitrogen source except for the x = 4 sample where the samples of x = 0 and 4 were the same samples shown in Figure 1. The samples of x = 0.5, 1, and 2 showed the diffraction patterns similar to that of Ca3Al8Si4O17N4, although some oxide impurities such as Ca2Al2SiO7 and Al2O3 were also contained. The x = 4/3 sample was almost the pure phase of Ca3Al8Si4O17N4 although the trace amount of AlN still remained. This result indicates that AlN is a preferable nitrogen source rather than Si3N4 for the synthesis of Ca3Al8Si4O17N4. The diffraction pattern of the x = 3 sample was almost the same as the corresponding sample in the series A. It was interesting that the continuous shifts in the diffraction peaks attributed to the Ca3Al8Si4O17N4 phase were observed for the samples of x = 1 - 2 in the series B. Such shifts indicated formation of the CaAl4–xSixO7–xNx solid solutions. In contrast to the series B, the shifts in the diffraction peaks were not clear in the series A as shown in Figure 1. Thus, it was found that AlN was proper nitrogen source for the formation of the CaAl4−xSixO7–xNx solid solutions than Si3N4. It was assumed that the replacement of Al-O bonds in Al2O3 with Al-N bonds was a quite tough reaction in comparison with the replacement of Si-O bonds in SiO2 with Si-N bonds.
The authors examined photoluminescence properties for the samples of x = 1 - 2 in the series B, which contained the CaAl4–xSixO7–xNx solid solutions as mentioned above. Figure 3 represents their photoluminescence spectra. All samples exhibited bluish-green luminescence showing broad emission bands attributed to the electron transition 4f65d1 → 4f7 in Eu2+ with the corresponding excitation ranging from 250 to 450 nm. The emission maxima were observed around 480 nm for all the sam-
Figure 2. XRD patterns of CaAl4−xSixO7−xNx:Eu2+ samples (series B) synthesized by the SSR method using AlN mainly as a nitrogen source.
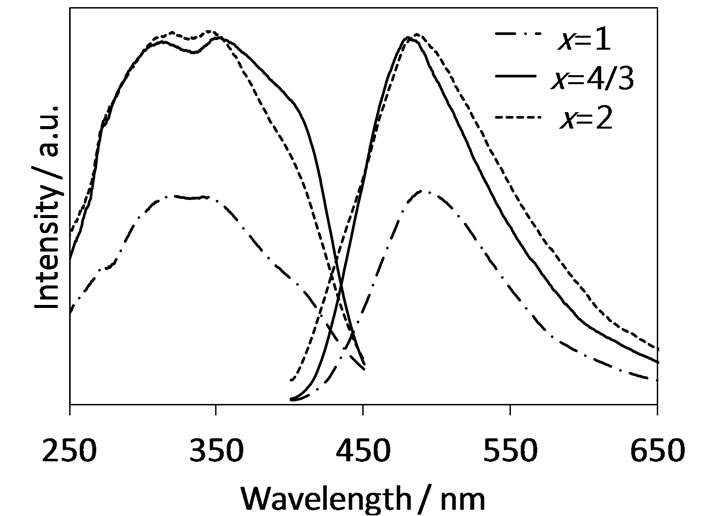
Figure 3. Photoluminescence spectra of the solid solutions CaAl4−xSixO7−xNx:Eu2+ (x = 1 - 2) in the series B.
ples. The influence of photoluminescence from Ca2Al2SiO7:Eu2+ contained as impurity phase in the samples of x = 1 and 2 can be excluded because the emission intensity of Ca2Al2SiO7:Eu2+, which was synthesized in a single phase by the SSR method, was much lower (<10%) than those of the present samples. In contrast to these oxynitride samples, the oxide CaAl4O7:Eu2+ (x = 0) showed no emission. Thus, it has been found that the Eu2+-activated CaAl4–xSixO7–xNx solid solutions around x = 4/3 (Ca3Al8Si4O17N4) are novel oxynitride phosphors in the Ca-Al-Si-O-N system. Their characteristics especially for the strong excitation intensity at 400 nm indicate that these phosphors can be excited by the NUVLED. From the results of the synthesis with the high phase purity and the strong emission, the x = 4/3 sample corresponding to Ca3Al8Si4O17N4 was regarded as the most interesting one among them.
As described above, synthesis of the Eu2+-activated Ca3Al8Si4O17N4 has been achieved by the SSR method using AlN as a nitrogen source. However, the SSR method is not a proper synthesis method for phosphors in respect of the distribution of activators in the host material. Solution-based synthesis methods are regarded as promising synthesis method for phosphors because they allow the homogeneous distribution of activators. The authors have reported that oxynitride phosphors synthesized by nitridation of oxide precursors prepared by solution-based methods exhibit stronger emission than those synthesized by the SSR methods [13,14]. In the present study, the authors attempted the synthesis of Ca3Al8Si4O17N4:Eu2+ by the combined synthesis method that was designed with the synthesis of an oxide precursor using the AMC method of a solution-based method followed by its nitridation under ammonia atmosphere (this combined synthesis method was represented as the AMC-NH3 method hereafter). Figure 4 shows XRD patterns of Ca3Al8Si4O17N4:Eu2+ synthesized by the AMC-NH3 and SSR methods. It was confirmed that the Ca3Al8Si4O17N4 phase was formed as the main phase in the sample synthesized by the AMC-NH3 method although parasitic impurities of Ca2Al2SiO7 and Al2O3 were present. Figure 5 shows the excitation and emission spectra of Ca3Al8Si4O17N4:Eu2+ phosphor synthesized by the AMC-NH3 and SSR methods. In spite of the presence of the impurity phases the AMC-NH3 sample exhibited photoluminescence intensity comparable to the SSR sample. It would be due to homogeneous distribution of the Eu2+ activators brought by the step of the solutionbased process in the present combined synthesis method. Finally, the performance of the novel Ca3Al8Si4O17N4: Eu2+ phosphor was evaluated from the point of view of qualitative analysis. The AMC-NH3 sample showed 48% of an internal quantum efficiency with 76% absorption of the excitation beam at 400 nm.
4. Conclusion
The oxynitride solid solutions CaAl4–xSixO7–xNx around x = 4/3 were synthesized by the SSR method. It has been found that AlN is a preferable nitrogen source rather than Si3N4 for the synthesis of Ca3Al8Si4O17N4. The Eu2+-activated Ca3Al8Si4O17N4:Eu2+ exhibited bluish-green lu-
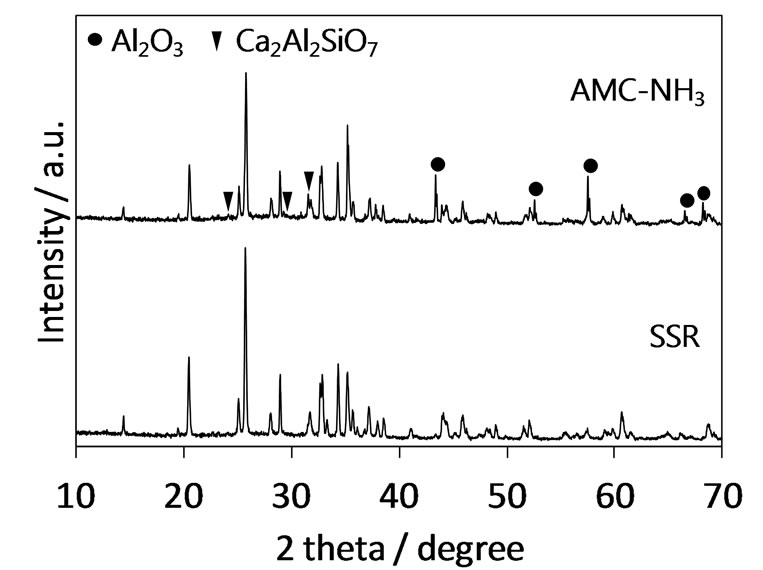
Figure 4. XRD patterns of Ca3Al8Si4O17N4:Eu2+ synthesized by AMC-NH3 and SSR methods.
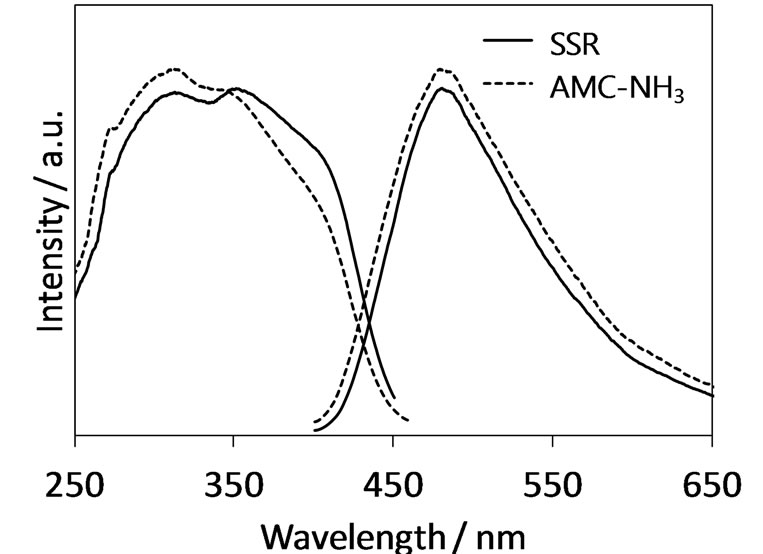
Figure 5. Photoluminescence spectra of Ca3Al8Si4O17N4: Eu2+ synthesized by AMC-NH3 and SSR methods.
menescence at 480 nm by the excitation at 250 - 450 nm. Thus, Ca3Al8Si4O17N4:Eu2+ and its solid solutions have been developed as novel Eu2+-activated oxynitride phosphors excitable by the NUV LEDs in the present study. The Ca3Al8Si4O17N4:Eu2+ phosphor was also synthesized by the combined synthesis method (AMC-NH3) involving the solution-based process. The Ca3Al8Si4O17N4: Eu2+ phosphor synthesized by the AMC-NH3 contained Ca2Al2SiO7 and Al2O3 impurities, resulted in the low phase purity of Ca3Al8Si4O17N4. However, the AMCNH3 sample showed the emission intensity comparable to that synthesized by the SSR method. The homogeneous distribution of Eu2+ activators in the AMC-NH3 sample would contribute to the relative strong intensity of luminescence.
5. Acknowledgements
This work was supported by a Grant-in-Aid for Scientific Research (A) (No. 22246081) from Japan Society for the Promotion of Science, Japan.
REFERENCES
- S. Ye, F. Xiao, Y. Pan, Y. Ma and Q. Zhang, “Phosphors in Phosphor-Converted White Light-Emitting Diodes: Recent Advances in Materials, Techniques And Properties,” Materials Science and Engineering: Reports, Vol. 71, No. 1, 2010, pp. 1-34. http://dx.doi.org/10.1016/j.mser.2010.07.001
- X. Li, J. Budai, F. Liu, J. Howe, J. Zhang, X. Wang, Z. Gu, C. Sun, R. Meltzer and Z. Pan, “New Yellow Ba0.93Eu0.07Al2O4 Phosphor for Warm-White Light-Emitting Diodes through Single-Emitting-Center Conversion,” Light: Science & Applications, Vol. 2, 2013, p. e50. http://dx.doi.org/10.1038/lsa.2013.6
- R. Xie and N. Hirosaki, “Silicon-Based Oxynitride and Nitride Phosphors for White LEDs—A Review,” Science and Technology of Advanced Materials, Vol. 8, No. 7-8, 2007, pp. 588-600. http://dx.doi.org/10.1016/j.stam.2007.08.005
- R. Xie, N. Hirosaki, Y. Li and T. Takeda, “Rare-Earth Activated Nitride Phosphors: Synthesis, Luminescence and Applications,” Materials, Vol. 3, No. 6, 2010, pp. 3777-3793. http://dx.doi.org/10.3390/ma3063777
- S. Yamada, H. Emoto, M. Ibukiyama and N. Hirosaki, “Properties of SiAlON Powder Phosphors for White LEDs,” Journal of the European Ceramic Society, Vol. 32, No. 7, 2012, pp. 1355.-1358. http://dx.doi.org/10.1016/j.jeurceramsoc.2011.05.050
- X. Song, R. Fu, S. Agathopoulos, H. He, X. Zhao and S. Zhang, “Photoluminescence properties of Eu2+-activated CaSi2O2N2: Redshift and concentration quenching,” Journal of Applied Physics, Vol. 106, No. 3, 2009, Article ID. 033103. http://dx.doi.org/10.1063/1.3190522
- R. Liu, Y. Liu, N. Bagkar, and S. Hu, “Enhanced Luminescence of SrSi2O2N2:Eu2+ Phosphors by Codoping with Ce3+, Mn2+, and Dy3+ Ions,” Journal of Applied Physics, Vol. 91, No. 6, 2007, Article ID. 061119. http://dx.doi.org/10.1063/1.2768916
- B. Yun, T. Horikawa, H. Hanzawa and K. Machida, “Preparation and Luminescence Properties of Single-Phase BaSi2O2N2:Eu2+, a Bluish-Green Phosphor for White Light-Emitting Diodes” Journal of The Electrochemical Society, Vol. 157, No. 10, 2010, pp. J364-J370. http://dx.doi.org/10.1149/1.3479763
- K. Komeya, Y. Cheng, J. Tatami and M. Mitomo, “New Green Phosphor Ba3Si6O12N2:Eu for White LED: Crystal Structure and Optical Properties,” Key Engineering Materials, Vol. 403, 2009, pp. 11-14. http://dx.doi.org/10.4028/www.scientific.net/KEM.403.11
- W. Y. Sun and T. S. Yen, “Subsolidus Phase Relationships in Part of the System Si, AI, Ca/N, O,” Ceramics International, Vol. 14, No. 4, 1988, pp. 199-205. http://dx.doi.org/10.1016/0272-8842(88)90022-3
- K. Yoshizawa, H. Kato, M. Kakihana, “Synthesis of Zn2SiO4:Mn2+ by Homogeneous Precipitation Using Propylene Glycol-Modified Silane,” Journal of Materials Chemistry, Vol. 22, No. 33, 2012, pp.17272-17277. http://dx.doi.org/10.1039/c2jm33056c
- M. Kim, M. Kobayashi, H. Kato and M. Kakihana, “Enhancement of Luminescence Properties of a KSrPO4: Eu2+ Phosphor Prepared Using a Solution Method with a Water-Soluble Phosphate Oligomer,” Journal of Materials Chemistry C, Vol. 1, No. 25, 2013, pp. 5741-5746. http://dx.doi.org/10.1039/c3tc31121j
- J. Kim, H. Kato and M. Kakihana, “Control of NaAlSiO4:Eu2+ Photoluminescence Properties by Charge-Compensated Aliovalent Element Substitutions,” Journal of Information Display, Vol. 13, No. 3, 2012, pp. 97-100. http://dx.doi.org/10.1080/15980316.2012.691079
- C. Yasushita, H. Kato and M. Kakihana, “Synthesis of an Oxynitride-Based Green Phosphor Ba3Si6O12N2:Eu2+ via an Aqueous Solution Process Using Propylene GlycolModified Silane,” Journal of Information Display, Vol. 13, No. 3, 2012, pp. 107-111. http://dx.doi.org/10.1080/15980316.2012.692725
- Y. Suzuki and M. Kakihana, “Preparation of Water Soluble Silicon Compound and its Application for Synthesis of (Y, Ce, Gd)2SiO5 Blue Emission Phosphor,” Journal of the Ceramic Society of Japan, Vol. 117, No. 3, 2009, pp. 330-334. http://dx.doi.org/10.2109/jcersj2.117.330
- M. Kakihana, “Synthesis of High-Performance Ceramics Based on Polymerizable Complex Method,” Journal of the Ceramic Society of Japan, Vol. 117, No. 8, 2009, pp. 857-862. http://dx.doi.org/10.2109/jcersj2.117.857
- N. Naruse, K. Tomita, M. Iwaoka and M. Kakihana, “Synthesis and Morphology Control of YBO3:Tb3+ Green Phosphor by Precipitation from Homogeneous solution” Journal of the Ceramic Society of Japan, Vol.121, No. 6, 2013, pp. 502-505. http://dx.doi.org/10.2109/jcersj2.121.502
- Y. Luo, D. S. Jo, K. Senthil, S. Tezuka, M. Kakihana, K. Toda, T. Masaki and D. H. Yoon, “Synthesis of High Efficient Ca2SiO4:Eu2+ Green Emitting Phosphor by a Liquid Phase Precursor Method,” Journal of Solid State Chemistry, Vol. 189, 2012, pp. 68-74. http://dx.doi.org/10.1016/j.jssc.2011.11.046
NOTES
*Corresponding author.

