Journal of Tuberculosis Research
Vol.04 No.04(2016), Article ID:72760,22 pages
10.4236/jtr.2016.44022
Effects of the Cytotoxic T-Cells on the Dynamics of Co-Infection of HIV-1 and Mycobacterium tuberculosis
Chipo Mufudza, Senelani D. Hove-Musekwa, Edward T. Chiyaka
Department of Applied Mathematics, Modelling Biomedical Systems Research Group, National University of Science and Technology, Bulawayo, Zimbabwe

Copyright © 2016 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 28, 2016; Accepted: December 12, 2016; Published: December 15, 2016
ABSTRACT
Enhancement of the Human Immunodeficiency Virus (HIV) specific cytotoxic T- cells mechanisms in an HIV-1 and Mycobacterium tuberculosis (Mtb) co-infected individual seems to improve the clinical picture of an individual by reducing Acquired Immuno Deficiency Syndrome (AIDS) state progression rate. In this paper, we develop a system of deterministic differential equations representing the immune cells involved in an HIV-1 and Mtb co-infected individual. Results show that although the non-lytic arm of the HIV-1 cytotoxic T-cells affects the co-infection dynamics more than the lytic factors, a combination of both factors results in a more positive reduced progression to the AIDS state. This is due to the increased protection of the CD4+ T-cells by the CTL mechanisms by further reducing infections and replications by the HIV. Thus, HIV-1 specific CTLs mechanisms’ involvement is here recommended to be part of a solution to the HIV and Mtb co-infection problems.
Keywords:
Mycobacterium tuberculosis, HIV, Co-Infection, Cytotoxic T-Cells, Lytic and Non-Lytic Factors

1. Introduction
1.1. HIV and Mycobacterium tuberculosis (Mtb) Co-Infection
Since the resurgence of HIV, Mtb and HIV has been closely linked, the HIV-1, Mtb co-infection causes a two-infection disease endangering human immune response which may also differ depending on the genetic background [1] . Approximately one- third of the world’s population is latently infected with Mtb [2] . The rate of progression from infection to full blown disease varies greatly with at least 10% of Mtb infected individuals developing clinical disease and about half of them developing disease more than two years after infection, commonly named reactivation or post-primary TB [3] . Thus, the lifetime risk of developing active TB in immunocompetent adults is estimated to be 5% - 10%, but in HIV-positive individuals this risk is increased to 5% - 15% annually [4] . The depletion of CD4+ T-cells, which is a main feature of AIDS, is certainly an important contributor to the increased risk of reactivation of latent TB and susceptibility to new Mtb infection. HIV also manipulates macrophage bactericidal pathways [5] , deregulates chemotaxis [6] , and tips Th1/Th2 balance [7] and may impair Tumor Necrosis Factor (TNF)-mediated macrophage apoptotic response to Mtb and thus facilitates bacterial survival [8] . Specifically, TB patients with AIDS present a dominant granulocytic infiltrate and necrosis without the typical necrosis seen in non- HIV-infected TB granulomas due to the killing of CD4+ T-cells in the granuloma by the HIV [9] .
HIV produces a progressive decline in the cell mediated immunity by virtue of the pathogen targeting the CD4+ T-cells eventually disabling them and making them dysfunctional. The decline impacts negatively on the control of the Mtb by the immune system since the CD4+ T-cells are also needed for the cell immune mediated response for the Mtb infection to keep TB in check. HIV also alters the pathogenesis of TB, greatly increasing the risk of developing active disease in a co-infected individual leading to more extra pulmonary involvement and radiographic manifestations.
The function of many immune cells, including macrophages and Dendritic cells (DCs), is modulated by both HIV and Mtb, with the presence of Mtb increasing replication of the virus in a co-infected individual [10] . Mtb has been reported to up-regu- late HIV-1 replication in chronically or acutely infected T-cells or macrophages [11] [12] . The primary target for Mtb, the alveolar macrophage, can also be infected with HIV exacerbating HIV replication in macrophages and lung cells obtained by bronchoalveolar lavage from co-infected individuals [10] [12] [13] . In-vitro Mtb infection can up-regulate both HIV infection and replication within monocyte-derived macrophages (MDMs), increase the efficiency of virus transmission from infected MDMs to T-cells, and favor replication of X4 HIV variants by upregulation of CXCR4 [14] . It is therefore evident that the presence of each of these diseases has a profound effect on the other due to the interactions with the immune system. This is because the frequency at which HIV and Mtb occurs together is determined by the epidemiology of each of the disease in a given population. Thus, co-infection has also become one of the main complications worldwide with TB being the cause of death for one out of every three people with AIDS.
1.2. Why Cytotoxic Cells?
The challenges that the world is facing in HIV and Mtb co-infection can possibly be solved through a combined vaccine. However, design of candidate vaccines is a parti- cularly difficult task since laboratory correlates of protection have not been defined for Mtb and HIV infections. Vaccination efforts have shown that Mtb infection diminish HIV-specific Immunoglobulin A (IgA) responses at mucosal surfaces which help prevent HIV infection or reduce the viral load [15] . In addition, vaccine-induced immune responses need to be tipped towards protection, avoiding those that may result in immunopathology, this requires meticulous study of appropriate adjuvants, antigens, and vaccination regimens for the novel vaccines [16] . Even with treatment, it has been noted with serious concern regarding current recommendations for treatment of HIV- Mtb co-infection since data suggest that at least 8 months duration of rifapentine (RPT), or rifampin (RIF) therapy, initial daily dosing, and concurrent antiretroviral therapy might be associated with better outcomes, but adequately powered randomized trials are urgently needed to confirm [17] . The criteria for deciding between strategies to treat a co-infected individual still remains a challenge although a more robust quan- titative measure could help by incorporating rate of change of CD4+ count as a measure of Integrated Risk Information System (IRIS) risk, as well as viral and bacterial load, drug toxicity, and improved measures of drug interaction [18] . The presence of the CTLs cannot be sidelined in an effort to come up with solutions to HIV-Mtb co- infections. Studies have shown that the presence of Mtb affect the C38 expression on the CD8+ cells responsible for CTL differentiation. This negatively impacts the HIV progression which may also depend on the ethnic group (genes) [19] . There have been several hypotheses set regarding the relationship between HIV specific CTLs and viral load. The findings strongly support the involvement of CTLs in the control of HIV infection [20] but does it have the same effect in the case of co-infection? The CD8+ cellular responses like CTLs are potential mediators of protection against HIV [21] .
Understanding the vital effects of Cytotoxic T-cells as an immune system component might be of paramount importance for vaccine development. The tropism of the co-infection between HIV-1, Mtb and the immune system studied by Kirshner [22] can be bridged by including the role of the Cytotoxic T-cells (CTLs: a special type of the CD8+ T-cells) in the immune system. CTLs play an important role in controlling both HIV and Mtb. This is made possible by incorporating the lytic and non-lytic responses of the CTLs. The lytic response involves the destruction of infected T-cells by the effector cells. The CTLs are antigen specific as they possess receptor molecules on their surface that can recognise the antigen epitope (portion of antigen which which specifically interacts with the immune system). These receptors are designed in such a way that they recognise antigen only when they are bound to a cell surface molecule called the major histocompatibility complex (MHC). The MHC presents peptides to CTLs which then destroy the infected cell if it can bind the peptide-MHC class1 complex [23] . In a co-infected individual, the CTLs are responsible for killing infected T-cells by both HIV and Mtb. This is made possible when naive CD8+ T-cells are converted into CTLs which are either HIV specific CTLs or Mtb specific CTLs through the B-cells. The CTLs are essential in many ways in the control of Mtb infection through their different effector mechanisms including activation of macrophages when they induce the release of the Interferon gamma (INF-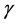 ) thereby by activating infected macrophages to induce reactive nitrogen intermediates [24] . They also mediate lysis of infected macrophages through the Fas-FasL pathway predominantly achieved by the CD4+ T-cells and through the granule exocytosis pathway [24] . CTLs also induce target cell apoptosis and kill the bacteria directly by the granulysin which result in the alteration of bacterial cell membrane.
) thereby by activating infected macrophages to induce reactive nitrogen intermediates [24] . They also mediate lysis of infected macrophages through the Fas-FasL pathway predominantly achieved by the CD4+ T-cells and through the granule exocytosis pathway [24] . CTLs also induce target cell apoptosis and kill the bacteria directly by the granulysin which result in the alteration of bacterial cell membrane.
In this paper, we focus on the HIV specific CTLs only and how they help to reduce the two infection disease progression. Non-lytic response of the CTLs involves the prevention of infection of new cells and inhibition of replication by soluble mediators secreted by the immune system. The CTLs then caters for the non-lytic response by producing chemokines like the 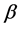 -chemokine that helps to reduce viral load at the early stages of the HIV infection (the asymptomatic stage) as they block the entry of virions into the CD4+ T-cells [25] . They also produce cytokines. The HIV specific CTLs principally produce the INF-
-chemokine that helps to reduce viral load at the early stages of the HIV infection (the asymptomatic stage) as they block the entry of virions into the CD4+ T-cells [25] . They also produce cytokines. The HIV specific CTLs principally produce the INF-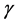 , Interleukin-6 (IL-6) and Interleukin-10 (IL-10) which suppress the rate of viral production [26] . The INF-
, Interleukin-6 (IL-6) and Interleukin-10 (IL-10) which suppress the rate of viral production [26] . The INF-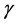 cytokines produced by the CTLs are also responsible for the activation of the macrophages so that they clear the Mtb bacteria and the HIV virus. Although we are not going to incorporate the Mtb specific CTLs, it should be noted that they also produce the INF-
cytokines produced by the CTLs are also responsible for the activation of the macrophages so that they clear the Mtb bacteria and the HIV virus. Although we are not going to incorporate the Mtb specific CTLs, it should be noted that they also produce the INF-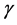 cytokine and the TNF which again are responsible for the inhibition of new infection by the Mtb. We will also consider the dynamics looked at by Kirshner [22] and incorporate the HIV specific CTLs mechanisms in the immune system and propose a mathematical model describ- ing the dynamics of uninfected T-cells, virally infected CD4+ T-cells, macrophages, HIV pathogen, Mtb pathogen and the HIV specific CTLs with the immune system.
cytokine and the TNF which again are responsible for the inhibition of new infection by the Mtb. We will also consider the dynamics looked at by Kirshner [22] and incorporate the HIV specific CTLs mechanisms in the immune system and propose a mathematical model describ- ing the dynamics of uninfected T-cells, virally infected CD4+ T-cells, macrophages, HIV pathogen, Mtb pathogen and the HIV specific CTLs with the immune system.
2. Model Formulation
The model by Kirshner [22] is here introduced and used as our basic model. This model focuses on the study of the hypothesis that the presence of infection of HIV with Mtb in the body worsens the clinical picture of HIV and that the presence of HIV can activate the Mtb infection. This was done incorporating the interactions of the immune system's key players which include the lymphocytes or T-cells, macrophages, HIV and Mtb. Four population groups of cells and pathogens are here used namely: the armed CD4+ and CD8+ T-cell population at time 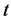 represented by
represented by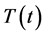 , the macrophage population at a given time represented by
, the macrophage population at a given time represented by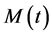 , the HIV population is represented by
, the HIV population is represented by 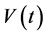 and the Mtb population at a time
and the Mtb population at a time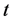 , represented by
, represented by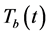 . The model is explained in detail in [22] . The following system of equations were proposed:
. The model is explained in detail in [22] . The following system of equations were proposed:
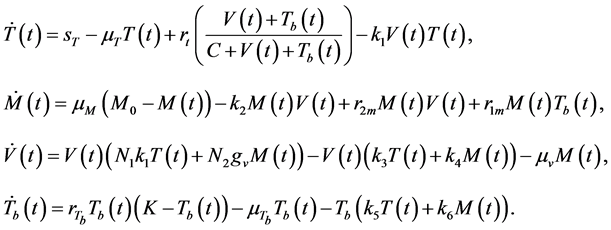 (1)
(1)
The interactions of both pathogens with the immune system are explored in [22] using system (1) and results show that the T-cells population is lower in the presence of both pathogens than in the case of HIV alone. The results also showed that viral load is higher in a co-infected patient than in a single infected patient and the same trend was observed for the Mtb. Thus, since progression to AIDS is based on the CD4+ T-cell count and the viral load, the presence of Mtb in an HIV infected individual worsens the clinical picture of the AIDS state.
Model with CTL Mechanisms
The lytic and non-lytic factors of the HIV specific CTLs are here incorporated to system (1), in order to analyse their effects to the dynamics of disease progression in a co-infected individual. In particular, HIV specific CTL T-cells response in HIV infection and its effects to the whole immune system dynamics. Thus CTLs are here modelled with their HIV specific function and not just as general T-cells as done by [27] and by monitoring the dynamics of six groups of population cell densities: the population density for the uninfected T-cells at a time 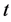 (a pool of the CD4+ T-cells, Mtb specific CTLs and naive CD8+ T-cells)
(a pool of the CD4+ T-cells, Mtb specific CTLs and naive CD8+ T-cells)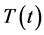 ; the virally infected CD4+ T-cells at a time,
; the virally infected CD4+ T-cells at a time,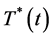 ; the free viral particles population at a time,
; the free viral particles population at a time,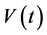 ; the density of the resting, activated and infected macrophages,
; the density of the resting, activated and infected macrophages,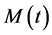 ; the population density of the Mtb pathogen at a time,
; the population density of the Mtb pathogen at a time, 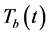 and the density of the HIV-1 specific CTLs population at a time
and the density of the HIV-1 specific CTLs population at a time

Uninfected CD4+ T-cells are produced from the thymus at a rate of 

HIV pathogens are modelled by the factors 

tively, where 







fection [27] . Infected CD4+ T-cells are also directly killed by the CTLs at a rate 



factor of

duction. Macrophage proliferation due to infection by the HIV and Mtb are modeled at the rates 











3. Initial Conditions and Reproductive Number, R0
3.1. Disease Free State (E0)
The disease free equilibrium is given by 


3.2. Reproductive Number, R0
The basic reproductive number (R0) measures the number of new secondary infections generated by a single co-infected individual cell in the presence of CTLs mechanisms. It is a dimensionless parameter which can be used to investigate the potency of the immune system. It can be used to control infections and disease progression so that they are kept at low levels. R0 is here given as the dominant eigenvalue of the next generation matrix of the Jacobian matrix by Watmough’s method [30] . There are several 
・ 
・ 
・ 

Diseases are not competing, therefore an individual can die of either HIV or Mtb or both and so they both contribute to overall 
4. Endemic States Analysis
The model system (2) has three endemic states: the virally infected state (where only the HIV pathogen is present), the Mtb state (which represents the presence of the Mtb pathogen only) and the co-infected state (where both pathogens are present). The stability analysis of these states is here discussed and analysed using the Jacobian method.
4.1. Virally Infected State
The population density of the uninfected CD4+ T-cells, infected CD4+ T-cells, Virus, Mtb, Macrophages and the HIV specific CTLs at the virally infected endemic state are respectively given by 

where






The abundance of the infected CD4+ T-cells at the virally infected state is given by

This is effectively reduced by increase in the inhibition of viral infection and direct killing of the virus by the CTLs. The population however, increases with increase in the viral population which inturn increases the reproductive ratio.
The viral load at this endemic steady state is given by the expression:

The equilibrium value of the viral load is dependent on the average number of the new viruses produced by the infected macrophages as well as infected CD4+ T-cells. Increase in the viral production attribute to an increase in the viral load. This viral population is however, reduced by the effectiveness of the CTLs to inhibit viral pro- duction and replication, a mechanism of the CTLs that reduces viral multiplication and protects the CD4+ T-cells. Thus, inclusion of these mechanisms in the new model helps to understand how the virus can be reduced. Positivity of the viral population is possible when
The macrophage population is given by

This population is sustained when 
Finally, the HIV specific CTLs population density is given by:

Increase in the viral population density increases the R0v which then stimulate more CTL to be released to either directly kill the virus via their phagocytic nature or inhibit replication and more infections although it also depends on the rate of production of
the CTLs from the source. This is possible when the condition 
4.2. Mtb Equilibrium State
The second endemic equilibrium state is the Mtb infected steady state where the viral load and the infected T-cell populations are all zeros and is given by:

where 





The uninfected CD4+ T-cells density exist only if
The macrophages are given by:

This value is dependent on the interactions of the macrophages with the Mtb patho- gen and the natural death rate for the macrophages. When Mtb approaches zero then the macrophage population goes to the uninfected steady state value of

The Mtb population at the Mtb endemic steady state is given by the following ex- pression:

The equilibrium value depends totally on the interaction of the Mtb with the macro- phages and the killing by the CD8+ T-cells together with the Mtb natural death rate and the Mtb carrying capacity. Mtb exists only if the following condition is satisfied

4.3. Co-Infected State
The last endemic state is the co-infected endemic state where both pathogens are present given by

where







where 

where 


5. Stability Analysis
5.1. Disease Free State,
Stability analysis of all the steady states are here done using the characteristic poly- nomial of the Jacobian matrix at each equilibrium state for all our equilibrium states. The stability analysis of the uninfected steady is analysed using 
where 


The other two eigenvalues are obtained from the quadratic equation




Stability of 


























5.2. Virally Infected State
Stability of 











5.3. Mtb Infectected State
At Mtb infected state stability is characterised by the characteristic polynomial of 
where 

The roots are negative if and only if 





Theorem 1 Let 






Thus, from the theorem we have the Mtb infected endemic state stable since the other roots of the Jacobian matrix have negative real parts since the routh determinants of the polynomial 
The Mtb infected steady state can only be stable also when the 

Therefore, stability of the Mtb infected steady state is stable when 












5.4. Co-Infected State
The co-infected endemic steady state can only be stable when the number of pathogens for both diseases are greater than zero and this corresponds to the condition when 



6. Numerical Simulations
Matlab 6.5 version was used for all our simulations for both models using ODE45 solver. Simulations on this model give us a portrait of the general behaviour of immune cells population in the presence of the HIV and Mtb pathogens. We are also concerned about the parameters which are of importance in stabilising the model and the ranges in which the system is stable and unstable. Table 1 shows all the parameter values used for all our simulations are here in used. The initial condition for systems (1) and (2) are given as





7. Effects of CTL Mechanisms
7.1. Effects of CTLS on R0
The presence of the lytic factor is almost insignificant in a co-infected state since it depicts the severe stage of HIV and Mtb. The different mechanisms affect the repro- ductive ratio differently. Figure 1(a) shows how 








Table 1. Numerical values for the simulations.
mediated killings is low relative to the rate of cell-mediated killings and 







Figure 1. Effects of different CTL mechanisms on the reproductive ratio and Uninfected CD4+ T-cells. (a) Graph of reproductive ratios, (b) Cell populations propagations without CTLs, (c) Cell populations propagations with CTLs mechanisms incorporated, (d) Effects of CTLs on viral load.
lytic arm is in operation. Here the 




7.2. Cell Population
There is also evidence of the uninfected CD4+ T-cell depletion due the presence of CTL mechanisms as shown by Figure 1(b) and Figure 1(c). The viral population can never reach out to higher levels above 



8. Conclusions
The HIV specific CTLs have a very big role to play in limiting viral spread and con- trolling infection by the virus in the immune system, which means that they have profound effects to the dynamics of the co-infection of Mtb and HIV in general. In the early stages of infection when the HIV specific CTLs are still in small amounts, we have noticed that the lytic factors are more important than the non-lytic factors and reaching the AIDS state is accelerated when the lytic factors are limited in the immune system, a weakness of our basic model by Kirshner [22] since it had no mechanisms of the CTL effects. CTLs are believed to be in abundance at the chronic stage of HIV infection and hence when
The study shows that both the lytic factors and the non-lytic factors are important in the controlling of HIV infection in a co-infected individual. The non-lytic mechanisms are more effective in controlling infection in both cases when rate of CTL mediated killings is low relative to the rate of cell-mediated killings. The lytic factors are more important when rate of virus induced CD4+ T-cells killings is very high relative to the rate of CTL mediated killing regardless of the CTL levels. However, the lytic arm is more important when it comes to control infection than replication since it is believed that CTLs will remain high and non-lytic arms are more important in controlling replication of the virus as they are believed to be more effective at the chronic stage of the infection [27] as also confirmed by Figure 1(a). The CTLs’ ability to hinder viral replication protects the clinical picture of a co-infected individual hence protecting the individual from fast AIDS stage as witnessed by Figure 1(a), Figure 1(b). Effectively the presence of CTLs reduces the viral load levels and protects the CD4+ T cell levels. Thus, we can conclude that the effector mechanisms of the HIV specific CTLs are relevant in controlling infection by the immune system although there is need to deter- mine which on-lytic factors are more important at the chronic phase. The increase in the CTLs mechanisms reduces viral multiplication, which results on the reduction of viral load, with the non-lytic factors being more effective in viral load reduction. Reduction of the viral load protects the macrophages too, thereby slowing down the rate of progression to AIDS. Thus, if drugs can be put in place to enhance these CTL mechanisms either as a vaccine or treatment, then rate of depletion of CD4 count is reduced and macrophages are protected. Ultimately, the progression to AIDS state is reduced thereby increasing the life span of individual co-infected by HIV and Mtb.
Acknowledgements
This research was made possible due to the support and insights from Dr G. Magom- bedze. Thank you very much.
Cite this paper
Mufudza, C., Hove-Musekwa, S.D. and Chiyaka, E.T. (2016) Effects of the Cytotoxic T-Cells on the Dynamics of Co-Infection of HIV-1 and Mycobacterium tuberculosis. Journal of Tuberculosis Research, 4, 191-212. http://dx.doi.org/10.4236/jtr.2016.44022
References
- 1. Shankarkumar, U. and Shankarkumar, A. (2011) Role of HLA-A, HLA-B, HLA-DRB1 and HLADQB1 Alleles in HIV-1 Patients with Pulmonary Tuberculosis Co-Infection from Western India. World Journal of AIDS, 1, 136-138.
https://doi.org/10.4236/wja.2011.14019 - 2. Dye, C., Scheele, S., Dolin, P., Pathania, V. and Raviglione, M.C. (1999) Consensus Statement. Global Burden of Tuberculosis: Estimated Incidence, Prevalence, and Mortality by Country. WHO Global Surveillance and Monitoring Project. JAMA, 282, 677-686.
https://doi.org/10.1001/jama.282.7.677 - 3. Selwyn, P.A., Hartel, D., Lewis, V.A., Schoenbaum, E.E., Vermund, S.H., et al. (1989) A Prospective Study of the Risk of Tuberculosis among Intravenous Drug Users with Human Immunodeficiency Virus Infection. New England Journal of Medicine, 320, 545-550.
https://doi.org/10.1056/NEJM198903023200901 - 4. Aaron, L., Saadoun, D., Calatroni, I., Launay O., Memain N., et al. (2004) Tuberculosis in HIV-Infected Patients: A Comprehensive Review. Clinical Microbiology and Infection, 10, 388-398.
https://doi.org/10.1111/j.1469-0691.2004.00758.x - 5. Spear, G.T., Kessler, H.A., Rothberg, L., Phair, J. and Landay, A.L. (1990) Decreased Oxidative Burst Activity of Monocytes from Asymptomatic HIV-Infected Individuals. Clinical Immunology and Immunopathology, 54, 184-191.
https://doi.org/10.1016/0090-1229(90)90080-A - 6. Wahl, S.M., Allen, J.B., Gartner, S., Orenstein, J.M., Popovic M., et al. (1989) HIV-1 and Its Envelope Glycoprotein Down-Regulate Chemotactic Ligand Receptors and Chemotactic Function of Peripheral Blood Monocytes. Journal of Immunology, 142, 3553-3559.
- 7. Havlir, D.V. and Barnes, P.F. (1999) Tuberculosis in Patients with Human Immunodeficiency Virus Infection. New England Journal of Medicine, 340, 367-373.
https://doi.org/10.1056/NEJM199902043400507 - 8. Patel, N.R., Zhu, J., Tachado, S.D., Zhang, J. and Wan, Z. (2007) HIV Impairs TNF-Alpha Mediated Macrophage Apoptotic Response to Mycobacterium tuberculosis. Journal of Immunology, 179, 6973-6980.
https://doi.org/10.4049/jimmunol.179.10.6973 - 9. Diedrich, C.R. and Flynn, J.L. (2011) HIV-1/Mycobacterium tuberculosis Co-Infection Immunology: How Does HIV-1 Exacerbate Tuberculosis? Infection and Immunity, 79, 1407-1417.
https://doi.org/10.1128/IAI.01126-10 - 10. Nakata, K., Rom, W.N., Honda, Y., Condos, R., Kanegasaki, S., et al. (1997) Mycobacterium tuberculosis Enhances Human Immunodeficiency Virus-1 Replication in the Lung. American Journal of Respiratory and Critical Care Medicine, 155, 996-1003.
https://doi.org/10.1164/ajrccm.155.3.9117038 - 11. Shattock, R.J., Friedland, J.S. and Griffin, G.E. (1993) Modulation of HIV Transcription in and Release from Human Monocytic Cells Following Phagocytosis of Mycobacterium tuberculosis. Research in Virology, 144, 7-12.
https://doi.org/10.1016/S0923-2516(06)80005-1 - 12. Zhang, Y., Nakata, K., Weiden, M. and Rom, W.N. (1995) Mycobacterium Tuberculosis Enhances Human Immunodeficiency Virus-1 Replication by Transcriptional Activation at the Long Terminal Repeat. Journal of Clinical Investigation, 95, 2324-2331.
https://doi.org/10.1172/JCI117924 - 13. Orenstein, J.M., Fox, C. and Wahl, S.M. (1997) Macrophages as a Source of HIV during Opportunistic Infections. Science, 276, 1857-1861.
https://doi.org/10.1126/science.276.5320.1857 - 14. Mancino, G., Placido, R., Bach, S., Mariani, F., Montesano, C., et al. (1997) Infection of Human Monocytes with Mycobacterium Tuberculosis Enhances Human Immunodeficiency Virus Type 1 Replication and Transmission to T-Cells. Journal of Infectious Diseases, 175, 1531-1535.
https://doi.org/10.1086/516494 - 15. Ignatowicz, L., Mazurek J., Leepiyasakulchai, C., Skold, M., Hinkula, J., et al. (2012) Mycobacterium Tuberculosis Infection Interferes with HIV Vaccination in Mice. PLoS ONE, 7, e41205.
https://doi.org/10.1371/journal.pone.0041205 - 16. Pawlowski, A., Jansson, M., Skold, M, Rottenberg, M.E. and Kallenius, G. (2012) Tuberculosis and HIV Co-Infection. PLOS Pathogens, 8, e1002464.
https://doi.org/10.1371/journal.ppat.1002464 - 17. Khan, F.A., Minion, F., Pai, M., Royce, S., Burman, W., Harries, A.D. and Menzies, D. (2010) Treatment of Active Tuberculosis in HIV-Co-Infected Patients: A Systematic Review and Meta-Analysis. Clinical Infectious Diseases, 50, 1288-1299.
https://doi.org/10.1086/651686 - 18. Ramkissoon, S., Mwambi, H.G. and Matthews, A.P. (2012) Modelling HIV and MTB Co-Infection including Combined Treatment Strategies. PLoS ONE, 7, e49492.
https://doi.org/10.1371/journal.pone.0049492 - 19. Sharada, R.M., Rani, H.S., Pydi, S.S., Subbanna, J. and Vallur, V.L. (2012) CD38 Expression on CD8+ Cells—Its Influence on Development of Tuberculosis in HIV Positive Individuals. Open Journal of Immunology, 2, 65-71.
https://doi.org/10.4236/oji.2012.22008 - 20. Ogg, G.S., Dunbar, P.R., Rowland-Jones, S.L., Cerundolo, V. and McMichael, V.J. (1998) Quantitation of HIV-1-Specific Cytotoxic T Lymphocytes and Plasma Load of Viral RNA. Science, 279, 2103-2106.
https://doi.org/10.1126/science.279.5359.2103 - 21. Rowland-Jones, S.L., Dong, T., Fowke, K.R., Kimani, J., et al. (1998) Cytotoxic T Cell Responses to Multiple Conserved HIV Epitopes in HIV-Resistant Prostitutes in Nairobi. Journal of Clinical Investigation, 102, 1758-1765.
https://doi.org/10.1172/JCI4314 - 22. Kirshner, D. (1999) Dynamics of Co-Infection with M. Tuberculosis and HIV-1. Theoretical Population Biology, 55, 94-109.
https://doi.org/10.1006/tpbi.1998.1382 - 23. Ganusov, V.V. (2003) The Role of the CTL Response and Virus Cytopathogenecity in the Virus Decline during Antiviral Therapy. Proceedings of the Royal Society of London B, 270, 1513-1518.
https://doi.org/10.1098/rspb.2003.2401 - 24. Ernst, W.A., Thoma-Uszynski, S., Teitelbaum, R., Ko, C., Hanson, D.A., Clayberger C., Krensky, A.M., Leippe, M., Bloom, B.R., Ganz, T., et al. (2000) Granulysin, a T Cell Product, Kills Bacteria by Altering Membrane Permeability. Journal of Immunology, 165, 7102-7108.
https://doi.org/10.4049/jimmunol.165.12.7102 - 25. Klenerman, P., Wu, P. and Phillips, R. (2002) HIV: Current Opinion on Escapology. Current Opinion on Microbiology, 5, 28-36.
https://doi.org/10.1016/S1369-5274(02)00339-9 - 26. Magombedze, G., Garira, W. and Mwenje, W.E. (2006) Mathematical Models of Chemotherapy of Human Tuberculosis Infection. Biological Sciences, 14, 509-553.
- 27. Garira, W., Hove-Musekwa, S.D. and Shiri, T. (2005) Optimal Control of Combined Therapy in a Single Strain HIV-1 Model Electronic. Journal of Differential Equations, 52, 1-22.
- 28. Wodarz, D., LLyod, A.L., Jansen, V.A.A. and Nowak, M.A. (1999) Dynamics of Macrophages and T Cell Infection by HIV. Journal of Theoretical Biology, 196, 101-113.
https://doi.org/10.1006/jtbi.1998.0816 - 29. Wodarz, D. and Nowak, M.A. (2000) Immune Responses and Viral Phenotype:do Replication Rate and Cytopathogenecity Influence Virus load? Journal of Theoratical Medicine, 2, 113-117.
https://doi.org/10.1080/10273660008833041 - 30. Driessche, V.P. and Watmough, J. (2002) Reproduction Numbers and Sub-Threshold Endemic Equilibria for Compartmental Models of Disease Transmission. Mathematical Biosciences, 180, 29-48.
https://doi.org/10.1016/S0025-5564(02)00108-6 - 31. Mufudza C. (2008) Analaysing the Effects of Cytotoxic T-Cells in the HIV-1 and Myco-Bacterium Tuberculosis Co-Infection. Msc Thesis, University of Zimbabwe, Harare, 1-95.
Appendix
10.1. A1
10.2. A2
where
The eigenvalues are calculated by solving the characteristic polynomial of the Jacobian matrix 
where
10.3. A3
The Jacobian matrix evaluated at the virally infected endemic steady state is given by
where
10.4. A4
where
the characteristic polynomial of the 
where

























































