American Journal of Molecular Biology
Vol.3 No.1(2013), Article ID:27672,14 pages DOI:10.4236/ajmb.2013.31006
The molecular regulatory effect of intracerebroventricular thymulin on endotoxin-mediated NF-(B nuclear translocation and activation in vivo
![]()
Cellular and Molecular Physiology and Immunology Signaling Research Group, Biomedical Laboratory and Clinical Sciences Division, Department of Medical Laboratory Technology, Faculty of Health Sciences, Beirut Arab University, Beirut, Lebanon
Email: john.haddad@yahoo.co.uk
Received 30 November 2012; revised 27 December 2012; accepted 5 January 2013
Keywords: Anti-Inflammatory; Hippocampus; IkB; Inflammation; Intracerebroventricular; Intraperitoneal; Lipopolysaccharide; NF-kB; Thymulin
ABSTRACT
The nuclear factor-(B (NF-(B) is one member of a ubiquitously expressed family of Rel-related transcription factors that serve as critical regulators of proinflammatory genes. The immunomodulatory potential of thymulin and its effect on NF-(B in vivo, particularly in the central nervous system (CNS), is not well characterized. In this study, the role of endotoxin (ET) in regulating NF-(B was unraveled in various compartments of the CNS. Stereotaxic localization reverberated specific intracerebroventricular (ICV) injection of ET into the CNS, with or without pretreatment with ICV thymulin. Treatment with ET upregulated the expression and nuclear trans-localization of NF-(B1 (p50), NF-(B2 (p52), RelA (p65), RelB (p68) and c-Rel (p75) in the hippocampus (HC), an effect abrogated by ICV pretreatment with thymulin. Thymulin modulated the phosphorylation of I(B- ( in the HC by upregulating the cytosolic accumulation of I(B-( and downregulating its phosphorylation (pI(B-(). Further analysis of the DNA-binding activity revealed an upregulated activity in the HC relative to saline-constitutive expression of the RelA (p65) subunit. ET did not induce the DNA-binding activity of NF-(B in the diencephalon (DE) or substantia nigra (SN) at various time points, when compared with baseline levels of expression. Intraperitoneal (IP) injections of ET in vivo upregulated the expression of NF-(B subunits in the liver and reduced the cytosolic accumulation of I(B-( by inducing pI(B-(. Furthermore, IP pretreatment with thymulin followed by ICV injection of ET attenuated/reduced the DNAbinding activity of NF-(B in the HC. These results indicate that ICV injection of ET regulates the nu- clear translocation/activation of NF-(Bwithin specific compartments in the brain. Thymulin attenuated ETinduced response, with particular involvement of I(B-(. The in vivomolecular regulation of thymulin via the NF-(B pathway is critical to understanding the anti-inflammatory role of this nonapeptide and unraveling pathways associated with neuroimmune interactions mediating proinflammatory signals in the CNS.
1. INTRODUCTION
Burgeoning research over the past few decades and continuing apace has shown that the immune, nervous and endocrine systems, or the so called “trio”, are tightly linked via specialized communication pathways and mechanisms [1,2]. Interactions between the nervous and immune systems, specifically, provide a physiological (homeostatic) basis for understanding neuroimmuneassociated disorders and medical conditions emanating from them. In approximately 200 AD, the Greek author Galen wrote that “melancholic women were more susceptible to breast cancer than sanguine women”. Since then, a wealth of anecdotal evidence has convinced physicians, neuroscientists and researchers of the importance of psychological factors in the prognosis of disease. This belief is now bolstered by substantial evidence that the nervous system output can indeed modulate immune functions and mechanisms of action [1]. The nervous system, in fact, has a number of attributes that influence local immune responses, hence theentailing “bidirectional” concept [3].
Among biological modifiers that may contribute to this bidirectional interaction at the level of the CNS is thymulin. Thymulin, a nonapeptide hormone secreted by the thymusessentially for regulating T lymphocyte differentiation and function, is reported to havemajor immunomodulatory actions [4], thereby providing an interface between neuroendocrine-immune communication systems [1,5,6]. Originally known as “serum thymic factor” (Facteur Thymique Serique; FTS) [7], thymulin binds to a carrier protein and cationic zinc (Zn2+) to exert its modulatory properties [8,9]. The biological activity of thymulin is dependent on equimolar interaction with Zn2+, whose bioavailability affects cellular immune responses [10]. Moreover, it has been shown that thymulin is capable of modulating proinflammatory cytokines in vitro and in vivo [11-13], providing evidence for a novel anti-inflammatory potential [14].
Two distinct epithelial populations in the thymus produce thymulin, also known as thymic factor (TF) [4]. The hormone is believed to be involved in T-cell differentiation and enhancement of T and NK cell activities. Besides these rather paracrine or auto-organic effects on the thymus-dependent immune system, thymulin also seems to have neuroendocrine effects [3,15]. There exist bidirectional interactions between the thymic epithelium and the hypothalamus-pituitary axis (HPA); for example, thymulin follows a circadian rhythm, and physiologically elevated ACTH levels correlate positively with thymulin plasma levels and vice versa [3,16]. Recently, a new role for thymulin as an effector on proinflammatory mediators/ cytokineshas emerged [12,17,18]. For example, a peptide analog of thymulin (PAT) has been reported to have analgesic effects atsupraphysiologic concentrations and particularly neuroprotective anti-inflammatory effects in the CNS [15,18,19].
The underlying mechanisms of thymulin-mediated immuno-regulation are not fully understood [1,3,20]. It has been reported, for example, that the effects of thymulin in downregulating an inflammatory signal are mediated, at least in part, by modulating intracellular cyclic nucleotides [21-23]. In addition to the potent anti-inflammatory properties of thymulin, Zn2+ can synergistically down-regulate a pro-inflammatory signal by reducing the release of inflammatory mediators and by acting as an antioxidant [9]. Of particular importance, Zn2+ was shown to be required in mediating the antioxidant-dependent inhibition of the redox-responsive nuclear factorkB (NF-kB), a transcription factor essential to the expression of pro-inflammatory genes encoding cytokines and inflammatory mediators [3,14,24,25]. In addition, a redox-sensitive mechanism involving Zn2+ has been implicated in regulating the DNA-binding kinetics of NFkB in vitro [26]. The anti-inflammatory property assigned to thymulin in the brain, however, has yet to be compartmentalized and subsequently ascertained.
NF-κB was initially discovered through its interaction with an 11-base pair sequence in the immunoglobulin light-chain enhancer in B cells [24]. NF-κB is a collective subfamily of inducible dimeric transcription factors composed of members of the Rel family of DNA-binding proteins that recognize a common sequence (consensus) motif [25]. In its active DNA-binding form, NF-κB is a heterogeneous collection of dimers, composed of various combinations of members of the NF-κB/Rel family. At present, this family is composed of 5 members, termed p50, p52, p65, cRel and Rel B [24]. The homology between the members of the Rel family is essentially through the Rel homology domain (RHD), which is about 300 amino acids in size and constitutes the DNAbinding domain of these pervasive proteins.
The NF-κB family shares a unique RHD in their Nterminus. Moreover, a subfamily of NF-κB proteins, including RelA, RelB, and c-Rel, has a transactivation domain in their C-termini. In contrast, the NF-κB1 and NF- κB2 proteins are synthesized as large precursors, p105, and p100, which then undergo processing to generate the mature NF-κB subunits, p50 and p52, respectively [24, 25]. The processing of p105 and p100 is mediated by the ubiquitin/proteasome pathway and involves selective degradation of their C-terminal region containing ankyrin repeats (AR). Whereas the generation of p52 from p100 is a tightly-regulated process, p50 is produced via the constitutive processing of p105 [24,25].
NF-κB family members also share structural homology with the retroviral oncoprotein v-Rel, thereby resulting in their classification as NF-κB/Rel proteins [24]. The study of v-Rel has unequivocally demonstrated that Rel/NF-κB transcription factors can be oncogenic; therefore, it’s interesting to know how the ensuing mutations in v-Rel have altered its structure as compared to c-Rel. However, v-Rel has accumulated so many activating mutations that it may not be a precise model for the role of these transcription factors in human cancers, where a single mutation (or gene amplification event) has occurred. Thus, in some cases, it is not known whether the rearrangements, mutations, and amplifications in Rel/ NF-κB/IκB genes that have been repeatedly identified in several human cancers and the constitutive NF-κB signaling seen in certain human cancers or induced by oncogenic human viruses (e.g., Epstein-Barr virus [EBV] and Human Y-Lymphotropic virus [HTLV]-1) contribute to proliferation, abrogate growth suppression, influence the control of apoptosis, or affect all of these processes [24,25].
The nomenclature of the NF-κB family is complicated. Therefore, it is beyond the scope of this work to try and tackle the origin and nomenclature of NF-κB proteins; however, for illustrative purposes it is indicated that there are five proteins in the mammalian NF-κB family, sub-grouped into two classes: Class I and II [4,5]. Class I comprises of NF-κB1 and NF-κB2, whereas RelA (p65), RelB (p68) and c-Rel (p75) belong to Class II. There are excellent references out there that the reader would want to consult on the NF-κB family [24,25].
These observations, therefore, prompted the investigation of whether thymulin has an immunomodulatory, attenuating potential in the CNS and the relevant molecular pathways involved. It is particularly shown that stereotaxic intracerebroventricular injection of endotoxin (ET/LPS) differentially upregulates the expression of various NF-kB subunits, including RelA (p65), the major transactivating member of Rel family, and an effect particularly localized to the hippocampus (HC). Interestingly, thymulin ameliorated the ET-dependent nuclear localization of NF-kB1 (p50), RelA (p65), RelB (p68), c-Rel (p75) and, to a lesser extent, NF-kB2 (p52) in the HC.Unraveling the likely molecular pathway mediating the anti-inflammatory effect of thymulin, it is shown that this nonapeptide attenuates the phosphorylation of NFkB major cytosolic inhibitor, IkB-a, and induces its cytosolic accumulation. Furthermore, ICV ET enhanced, in a time-dependent manner, the NF-kB DNA-binding pattern in the HC, an effect abrogated by thymulin, in a dose-dependent manner. Despite the enhancementof ETmediated expression of NF-kB in the HC, no substantial upregulation was observed in the diencephalon (DE) or substantia nigra (SN), suggesting localized effect in the CNS. Moreover, intraperitoneal (IP) injections of ET in vivo upregulated the expression of NF-kB subunits in the liver and reduced the cytosolic accumulation of IkB-a by inducing pIkB-a. Furthermore, IP pretreatment with thymulin followed by ICV ET mildly reduced the DNAbinding activity of NF-kB in the HC, probably indicating systemic-central communication.
These results indicate that ICV injection of ET regulates the nuclear translocation and activation of NF-kB within specific compartments in the brain, an effect particularly localized to the hippocampus. Additionally, thymulin attenuates the ET-induced response, with particular involvement of the transduction pathway implicating IkB-a and its cytosolic phosphorylation.
2. MATERIALS AND METHODS
2.1. Chemicals and Reagents
Unless otherwise indicated, chemicals of the highest analytical grade were purchased from Sigma-Aldrich. Adult (200 - 250 g) male Sprague-Dawley rats were used in this study. The animals were housed under optimum conditions of light and temperature with food and water ad libitum and kept, in groups of 4 - 5, during the period of the experiment in clear plastic cages with solid floors covered with 3 - 6 cm of sawdust. All experimental procedures involving the use of live animals were reviewed and approved under the Animals (Scientific Procedures) Act, 1986 (UK). Different groups of rats received ET/ LPS prepared from Salmonella typhosa (strain 0901) (Difco, Detroit, MC, USA) in sterile, physiological saline. For ICV injections, the regarded ET concentration was stereotaxically administered in volumes of 5 - 10 mL. On the other hand, IP ET injections were administered in specified volumes of 50 mL.
2.2. Thymic Hormone Immunomodulatory Effect on ET-Induced NF-(B Expression
The carboxymethyl cellulose (1% CMC in 150 mM NaCl) was used as a carrier in which thymic hormones were administered [25]. Animals were stereotaxically monitored for accurate injections of ET intracerebroventricularly (ICV), with minimal stress under mild anesthesia. Various concentrations of thymulin were prepared in sterile, physiological saline using 1% CMC and injected in boluses of 5 - 10 mL, mimicking ET or saline control volumes (ICV/IP).Although the experiments run for this study used CMC as a carrier, subsequent studies in our laboratory have shown that the modulatory effects of thymulin were not affected with or without CMC. Hence, subsequent experiments introduced thymulin without CMC. In the therein reported experiments, thymulin was not conjugated with Zn2+, knowing that systemic circulating levels of Zn2+ may well contribute to the biological activity of thymulin [18]. However, in vitro experiments introduced thymulin and thymulin-Zn2+ conjugates where we have observed synergistic effect (Data not shown). Stereotaxically, animals were routinely situated and tested upon in our laboratory following exact coordinates as per CNS atlas for rats and mice (if experimented on). Animals were pretreated with thymulin (0.1 - 5 mg; ICV) for 30 minutes prior to stimulation with ET/LPS (1 mg) for 45 minutes and simultaneously monitored before sacrificing and tissue extracting, essentially as described elsewhere [27].
For time-dependent analysis, the aforementioned was undertaken and tissues were extracted at various time points, ranging from 0.5 h - 8 h. Naïve (untreated) and saline-injected (ICV) animals were handled exactly as would-be ET-treated animals and under the same conditions. In the series of IP experiments, ET injections were similar to ICV with IP pretreatment with thymulin. HC, DE and SN tissues were immediately removed and snap frozen for further analysis. Despite the anatomical limitations regarding the definitive stereotaxic isolation of HC, DE and SN tissues, extreme care and regimens were followed by expert individuals who were trained to perform the extraction experiments on sham animals prior to experiments, following exact coordinates and anatomical positioning under the direct supervision of Professor Nayef E. Saadé (American University of Beirut), who provided expertise and professional advice.
2.3. Cytosolic and Nuclear Protein Extraction for I(B and NF-(B Analysis
Cytosolic and nuclear extracts were prepared from tissues, essentially as reported elsewhere [14,25], with minor modifications. Samples were washed twice in 5 mL ice-cold, O2 pre-equilibrated phosphate buffered saline (PBS) and cells (»1 - 2 × 107) were collected and centrifuged at 420 g for 5 minutes at 4˚C. Nuclei were released by re-suspending the pellet in 250 mls buffer A containing (in mM): 10 Tris-HCl (pH7.8), 10 KCl, 2.5 NaH2PO4, 1.5 MgCl2, 1 Na3VO4, 0.5 dithiothreitol (DTT), 0.4 [4- (2-Aminoethyl)-benzene sulfonyl fluoride-HCl (AEBSF), and 2 mg/mL each of leupeptin, pepstatin A and aprotinin. The suspension was left in ice for 10 minutes followed by a 45-second homogenization at a moderate speed. Nuclei were collected by centrifuging the slurry at 4500 g for 5 minutes at 4˚C and re-suspending in 100 mL buffer B (Buffer A adjusted to (in mM): 20 Tris-HCl (pH7.8), 420 KCl, 20% (v/v) Glycerol). The supernatant formed is termed the cytosolic extract (used for IkB-a and pIkB-a analysis). The nuclei were then lysed at 4˚C for 30 minutes with gentle agitation, the debris cleared by centrifugation at 10,000 g for an additional 30 minutes at 4˚C and the supernatant frozen in liquid nitrogen and stored at -70˚C until used. In all cases, protein contents were determined by the Bradford method using BSA as a standard.
2.4. Western Analysis and Electrophoretic Mobility-Shift Assays
Cytosolic and nuclear proteins (20 - 25 mg) were resolved by SDS-PAGE using a 7.5% separating phase at room temperature at 150 V for 1 h. After electrophoretic transfer onto nitrocellulose, each membrane was washed in Tris-buffered saline (20 mM Tris-HCl (pH7.6); 500 mM NaCl) followed by blocking for 1 h at room temperature in TBS plus 0.1% (v/v) Tween-20 (TBS-T) with gentle agitation. After three washes in TBS-T, membranes were incubated with mouse monoclonal IgG1 anti-IkB-a (H-4), IgG2b anti-pIkB-a (B-9), rabbit polyclonal IgG anti-p50 (NF-kB1; NLS), anti-p52 (NF-kB1; K-27), anti-p65 (RelA; A), anti-p68 (RelB; C-19), and anti-p75 (c-Rel; N) antibodies for primary detection (Santa Cruz Biotechnology, CA., USA; 1:500) in TBS-T overnight at 4˚C. Primary conjugates were visualized on film using an anti-rabbit IgG-biotinylated antibody coupled with streptavidin-horseradish peroxidase enhanced chemiluminescence (ECL; Amersham Life Science). bActin standard was used as an internal reference for semi-quantitative loading in parallel lanes for each variable. Western blots were scanned by NIH MagiScanII and subsequently quantitated by UN-Scan-IT automated digitizing system (version 5.1; 32-bit), and the ratio of the density of the band to that of b-actin was subsequently performed. Nuclear extracts were analyzed for NF-kB DNA binding activity by electrophoretic mobility shift assay (EMSA), and supershift experiments with specific antibodies were performed as described previously (see below) [14,25]. Specific quantitation of the corresponding DNA gel shift bands was performed with phosphorimaging.
EMSA experiments were conducted using the following radiolabeled deoxy-oligonucleotide sequences purchased from Genosys: NF-kB (consensus sequence underlined) W-22: 5’-AGTTGAGGGGACTTTCCCAGGC-3’; (1 bp missense control, M-22: 5’-AGTTGAGGCGACTTTCCCAGGC-3’). After endlabeling with polynucleotide kinase (Boehringer Mannheim), purifying and annealing probes, identical amounts of radioactivity (2 × 104 counts∙min-1) were added to binding reactions containing 1 - 5 mg nuclear extracts in a final volume of 40 mL in DNA binding buffer (20 mM HEPES (pH7.9); 1 mM MgCl2; 4% Ficoll) containing 0.15 mg polydeoxyinosinic-deoxycytidylic acid [poly (dIdC)] (Boehringer Mannheim) as a non-specific competetor. Mixtures were incubated for 30 minutes at 25˚C before separating on native non-denaturing 4% polyacrylamide gels at room temperature by electrophoresis in Tris-Borate-EDTA buffer. Where indicated, non-labeled oligonucleotide competitor was added in 100-fold molar excess immediately prior to addition of a radiolabeled probe of the same sequence. For super-shift experiments, specific antibody for NF-kB (polyclonal IgG rabbit p65 (RelA) (Santa Cruz Biotechnology, CA) was used at 2 mg/reaction. The antibody was incubated with nuclear extracts prior to the addition of probe for 1 h at 4˚C, and then processed as indicated above. The distribution of 32P-label was visualized and quantitated on dried gels using a Canberra-Packard Instant Imager.
2.5. Statistical Analysis and Data Presentation
Data are presented as means ± SEM of at least 3 independent cell cultures. Statistical evaluation of the difference in mean separation was performed by one-way analysis of variance (ANOVA), followed by post hoc Tukey’s test, and the a priori level of significance at 95% confidence level was considered at P < 0.05.
3. RESULTS
3.1. Analysis of the Effect of Thymulin on ET-Induced NF-(B Subunit Translocation
In order to unravel the mechanism associated with the anti-inflammatory effect of thymulin in the CNS, varying concentrations of thymulin (0.1 - 5 mg; ICV) were introduced stereotaxically 30 minutes before ICV injection of ET (1 mg; 45 minutes). As shown in Figure 1, ET (Lane- 3) upregulated the nuclear translocation of NF-κB1 (p50), NF-κB2 (p52), RelA (p65), the major transactivating member of the Rel family [14], RelB (p68) and c-Rel (p75) in the hippocampus (HC). The protein expression of NF-κB1 (p50) and NF-κB2 (p52) was mild as compared with other NF-κBsubunits, with prominentexpression of p65. Thymulin (5 mg; ICV) alone (Lane-2) or control (Lane-1) that received no injections showed faint constitutive or no expression, respectively, as compared with ET. Despite the limiting factor of not having determined the systemic levels of thymulin in the therein reported studies, further experiments are essentially warranted formeasuring systemic and CSF levelsof circulating thymulin following injections of various concentrations of thymulin with or without endotoxin.
The inductive effect of ET is ameliorated and attenuated with thymulin, particularly at concentrations ³ 1 mg
(Lanes-4, 5 and 6), with prominent effects on p52, p65 and p75 subunits. To ensure semi-quantitative loading per lane, the housekeeping protein b-actin was assayed by Western analysis relative to expression of various NF- κB subunits, as shown in Figure 1. Therefore, b-actin standard was used as an internal reference for semiquantitative loading in parallel lanes for each variable.
3.2. Analysis of the Effect of Thymulin on ET-Induced I(B-( Phosphorylation and Cytosolic Accumulation
Further assessment of the underlying mechanism pertaining to the inhibitory effect of thymulin on ET-induced nuclear localization of NF-κB subunits, the major cytosolic inhibitor of NF-κB was put into light. Regulated by an upstream kinase, dubbed IKK [28], IκB-a is phosphorylated prior to the nuclear translocation of NF- κB. ET induced the phosphorylation of IκB-a, thereby tagging it for proteasome degradation, hence its cytosolic concentration was decreased (Lane-3) (Figure 2). Thymulin, in a dose-dependent manner, reduced the phos-

Figure 1. The expression of various NF-kB subunits in the hippocampus in response to ICV ET. Varying concentrations of thymulin (0.1 - 5 mg; ICV) were introduced stereotypically 30 minutes before ICV injection of ET (1 mg; 45 minutes). ET (Lane-3) upregulated the nuclear translocation of NF-kB1 (p50), NF-kB2 (p52), RelA (p65), RelB (p68) and c-Rel (p75) in the hippocampus (HC). The protein expression of NF-kB1 (p50) and NF-kB2 (p52) was mild as compared with other NF-kBsubunits, with prominent expression of p65. Thymulin (5 mg; ICV) alone (Lane-2) or control (Lane-1) that received no injections showed faint constitutive or no expression, respectively, as compared with ET. The inductive effect of ET is ameliorated and attenuated with thymulin, particularly at concentrations ³ 1 mg (Lanes-4, 5 and 6), with prominent effects on p52, p65 and p75 subunits. b-Actin standard was used as an internal reference for semi-quantitative loading in parallel lanes for each variable.
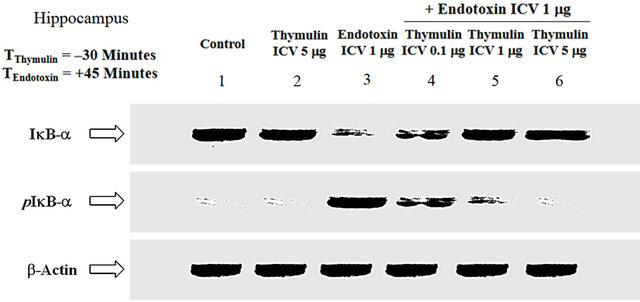
Figure 2. The effect of ET and thymulin pretreatment on IkB-a. Regulated by an upstream kinase (IKK), IkB-a is phosphorylated prior to the nuclear translocation of NF-kB. ET induced the phosphorylation of IkB-a, thereby tagging it for proteasome degradation, hence its cytosolic concentration was decreased (Lane-3). Thymulin, in a dose-dependent manner, reduced the phosphorylation of IkB-a, with maximal inhibitory effect at ICV concentration of 5 mg (Lanes-4, 5 and 6). Thymulin restored IkB-a cytosolic accumulation, in a dose-dependent manner, consistent with the downregulation of its phosphorylation (Lanes-4, 5 and 6; Figure 2). Neither the control (Lane-1) nor thymulin alone (Lane-2) showed any effect on the phosphorylation of IkB-a, but there is constitutive expression as compared with ET alone. b-Actin standard was used as an internal reference for semi-quantitative loading in parallel lanes for each variable.
phorylation of IκB-α, with maximal inhibitory effect at ICV concentration of 5 mg (Lanes-4, 5 and 6). Of note, thymulin restored IκB-α cytosolic accumulation, in a dose-dependent manner, consistent with the downregulation of its phosphorylation (Lanes-4, 5 and 6; Figure 2). Neither the control (Lane-1) nor thymulin alone (Lane-2) showed any effect on the phosphorylation of IκB-α, but there is constitutive expression as compared with ET alone. The b-actin distribution in Figure 2 depicts semiquantitative loading in parallel lanes.
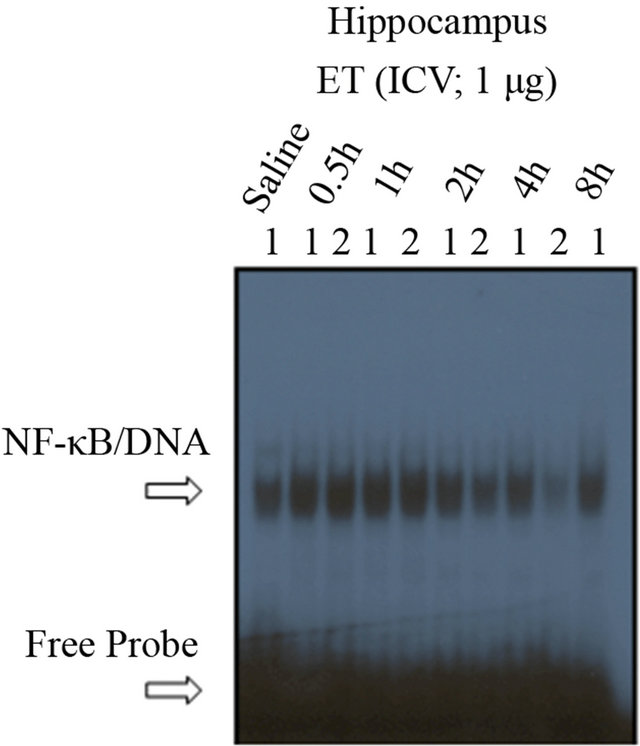
3.3. Analysis of the Effect of ICV ET on NF-(B DNA-Binding Activity—Time Dependency, and Assessment of the Specificity of ICV ET on NF-(B DNA-Binding Activity
To ensure that the nuclear translocation of NF-κB is associated with DNA-binding activity, electrophoretic mobility shift assaywas undertaken. As shown in Figure 3(a), ET induced, in a time-dependent manner, NF-κB activation in the HC, as compared with saline (Lane-1). The number relative to the lanes indicates assigned animals per lane at each time point. Densitometric analysis shown in the histogram (Figure 3(b)) indicates the timedependent induction of NF-κB activation mediated by ET, with maximal effectiveness at time points 0.5 h, 1 h and 2 h, declining thereafter. Hence, our choosing of the 45 minutes interval for subsequent experiments was accordingly designed.
We have previously shown that ET-mediated activation of NF-κB in vitro and ex vivo involves the p50-p65 complex [14,25]. In order to re-affirm and verify the constituency and the specificity of the NF-κB complex in the HC, supershift and mutation experiments were subsequently performed [25]. There was no shifted band in the control lane that contained no nuclear extracts (Figure 3(c)). Incubation of nuclear extracts treated with ET (1 mg; ICV) for 45 minutes with an oligonucleotide that has been mutated for 3 bp of the wild type κB moiety abrogated the binding of NF-κB complex to the specific DNA sequence (ET + M22). The supershifted band with an anti-RelA (p65) polyclonal antibody revealed the involvement of p65 subunit in ET-mediated activation of NF-κB (Figure 3(c)). The addition of cold competitor (ET + COMP) reduced, in a dose-dependent manner, the NF-κB/DNA-binding activity with complete obliteration of the specific band at 200×. The free probe (FP) denotes the faster migrating unbound radioisotope.
3.4. Analysis of the Effect of ICV ET on NF-κB DNA-Binding Activity and the Role of Thymulin
As shown in Figure 4(a), thymulin attenuated ETmediated NF-κB/DNA activation in the HC, as compared with saline (Lane-1) or thymulin alone (Lanes-2 and 3). The number relative to the lanes indicates assigned animals per lane at each time point. Densitometric analysis shown in the histogram (Figure 4(b)) indicates the dose-dependent inhibition of NF-κB activation mediated
 (a)
(a)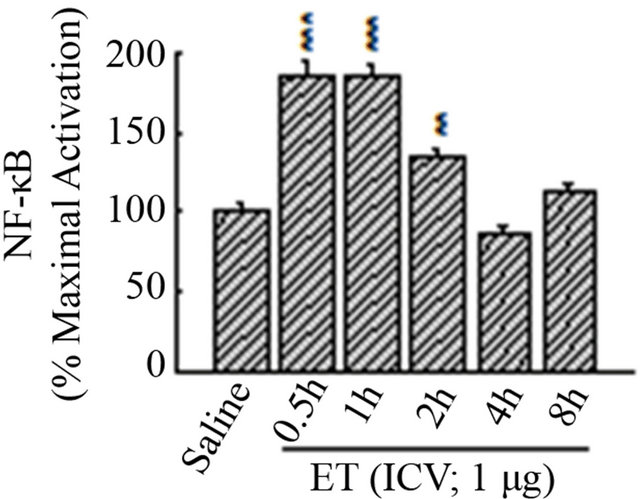 (b)
(b)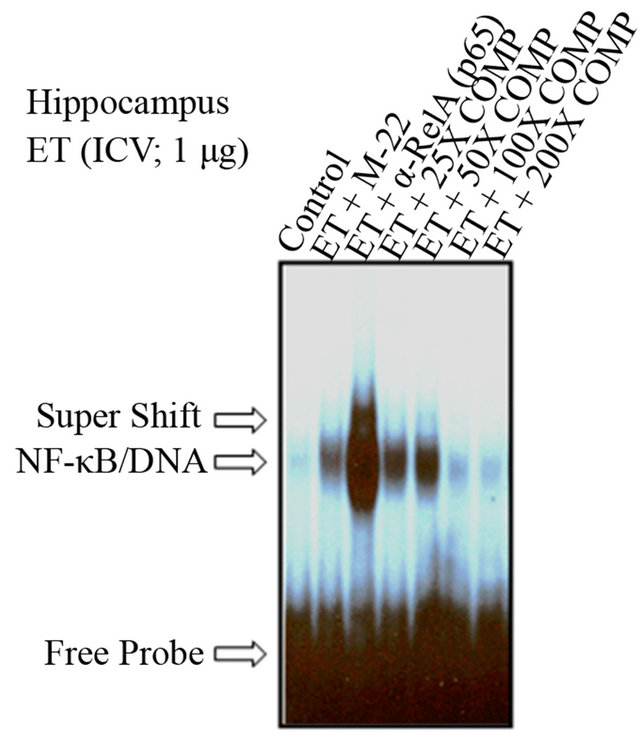 (c)
(c)
Figure 3. The time-response curve for ICV ET in the hippocampus. (a) ET induced, in a time-dependent manner, NF-kB activation in the HC, as compared with saline (Lane-1). The number relative to the lanes indicates assigned animals per lane at each time point. (b) Densitometric analysis shown in the histogram indicates the time-dependent induction of NF-kB activation mediated by ET, with maximal effectiveness at time points 0.5 h, 1 h and 2 h, declining thereafter. The free probe (FP) denotes the faster migrating unbound radioisotope. **P < 0.01, ***P < 0.001, as compared to saline. n = 2, which represents the number of independent experiments in duplicate. (c)The determination of the specificity of NF-kB activation in the hippocampus. There was no shifted band in the control lane that contained no nuclear extracts. Incubation of nuclear extracts treated with ET (1 mg; ICV) for 45 minutes with an oligonucleotide that has been mutated for 3 bp of the wild type kB moiety abrogated the binding of NF-kB complex to the specific DNA sequence (ET + M22). The supershifted band with an anti-RelA (p65) polyclonal antibody revealed the involvement of p65 subunit in ET-mediated activation of NF-kB (Lane-3). The addition of cold competitor (ET + COMP) reduced, in a dose-dependent manner, the NF-kB/DNA-binding activity with complete obliteration of the specific band at 200X. The free probe (FP) denotes the faster migrating unbound radioisotope.
 (a)
(a) (b)
(b)
Figure 4. DNA-binding activity of NF-kB in response to ET/thymulin. (a) Thymulin reduced, in a dose-dependent manner, ET-mediated NF-kB activation in the HC, as compared with saline (Lane-1) or thymulin alone (Lanes-2 and 3). The number relative to the lanes indicates assigned animals per lane at each time point. (b) Densitometric analysis shown in the histogram indicates the dose-dependent inhibition of NF-kB activation mediated by ET, with effectiveness at all dose ranges of thymulin. The free probe (FP) denotes the faster migrating unbound radioisotope. ***P < 0.001, as compared to saline. n = 3 - 5, which represents the number of independent experiments in duplicate.
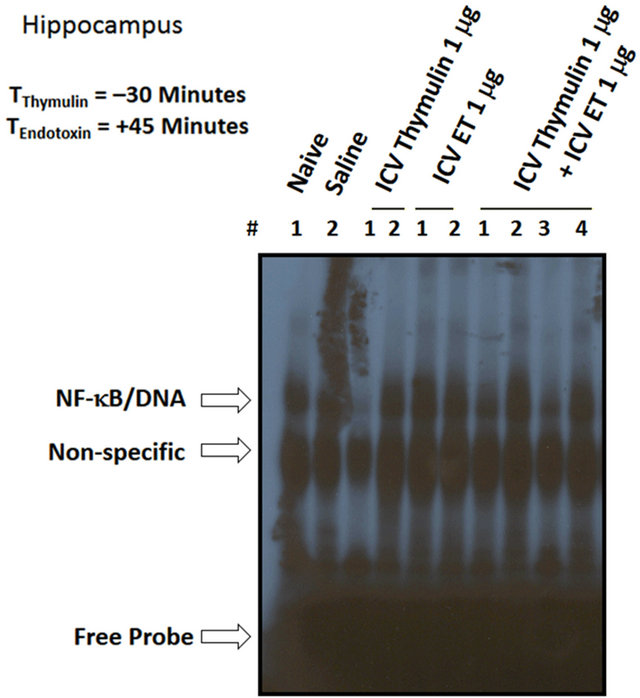
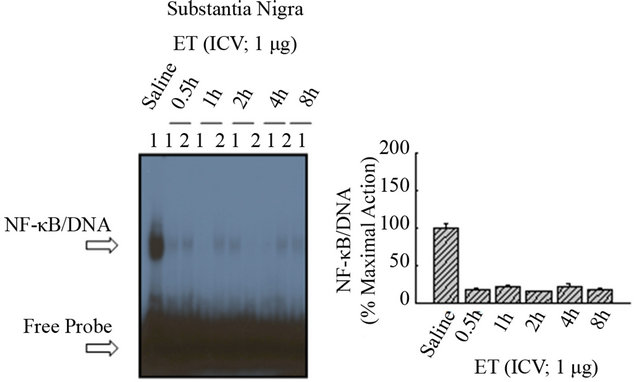
by ET, with effectiveness at all dose ranges of thymulin. These results are duplicated under varying conditions showing effect of saline and ICV ET (Figure 5(a)), in the presence of thymulin (Figure 5(b)). As shown in Figure 6(a), ET did not induce at anyof the time points NF-κB/DNA activation in the diencephalon, as compared with saline (Lane-1). Densitometric analysis shown in the histogram (Figure 6(b)) indicates the inactivity of NF-κB mediated by ET. Similarly, as shown in Figure 6(c), ET did not induce at any of the time points NF-κB/ DNA activation in the Substantia nigra, as compared with saline (Lane-1). Densitometric analysis shown in the histogram (Figure 6(d)) indicates the inactivity of NF-κB mediated by ET.
3.5. Measurement of the Intraperitoneal (IP) Effect of ET on the Nuclear Localization of NF-κB Subunits
Further to ICV analysis of the effect of ET and its reversal by thymulin, IP injections of ET (25 mg; 15 minutes) upregulated the nuclear localization of NF-κB subunits in
 (a)
(a) (b)
(b)
Figure 5. DNA-binding activity of NF-kB in response to ET/thymulin. (a) Naïve and saline treated animals are shown, where thymulin reduced ET-mediated NF-kB activation in the HC. (b) Densitometric analysis shown in the histogram indicates the inhibition of NF-kB activation mediated by ET (1 mg; ICV), with effectiveness of thymulin (1 mg; ICV). The free probe (FP) denotes the faster migrating unbound radioisotope. **P < 0.01, as compared to saline. n = 3 - 4, which represents the number of independent experiments in duplicate.
 (a)
(a)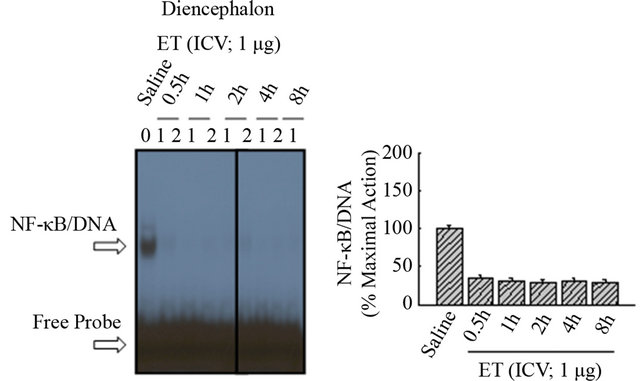 (b)
(b)
Figure 6. The effect of ET on NF-kB in the diencephalon and substantia nigra. (a) ET did not induce at any of the time points NF-kB/DNA activation in the diencephalon, as compared with saline (Lane-1). (b) Densitometric analysis shown in the histogram indicates the inactivity of NF-kB mediated by ET. (c) ET did not induce at any of the time points NF-kB/DNA activation in the Substantia nigra, as compared with saline (Lane-1). (d) Densitometric analysis shown in the histogram indicates the inactivity of NF-kB mediated by ET.
the liver (Figure 7). The number relative to the lanes indicates assigned animals per lane at each point. The bactin distribution depicts semi-quantitative loading in parallel lanes.
3.6. Analysis of the Effect of ET on I(B-( Phosphorylation and Cytosolic Accumulation
As shown in Figure 8, IP ET (15 minutes) upregulated the phosphorylation of IκB-a, thereby decreasing its cytosolic accumulation. The b-actin distribution depicts semi-quantitative loading in parallel lanes (Figure 8).
3.7. Measurement of the Effect of IP ET on NF-(B DNA-Binding Activity
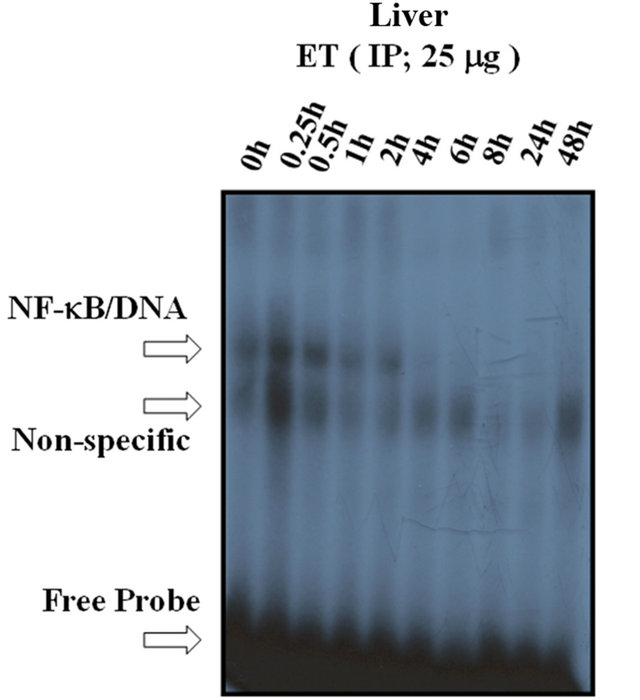
To indicate whether the IP ET is capable of upregulating the DNA-binding activity of NF-κB, a time-dependent curve was analyzed. As shown in Figure 9(a), maximal activation of NF-κB by IP ET in the liver was observed at 0.25 h (15 minutes), declining thereafter. Densitometric analysis of this effect is shown in Figure 9(b).
3.8. Measurement of the IP Role of Thymulin on the ICV Effect of ET on NF-(B DNA-Binding Activity
Trying to understand whether peripheral and central
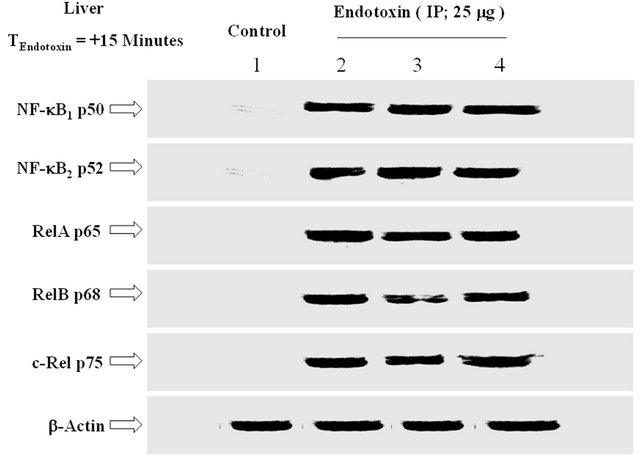
Figure 7. The effect of IP injection of ET on the expression of NF-kB subunits in the liver. IP injections of ET (25 mg; 15 minutes) upregulated the nuclear localization of NF-kB subunits in the liver. The number relative to the lanes indicates assigned animals per lane at each point. The b-actin distribution depicts semi-quantitative loading in parallel lanes. n = 3, which represents the number of independent experiments in duplicate.
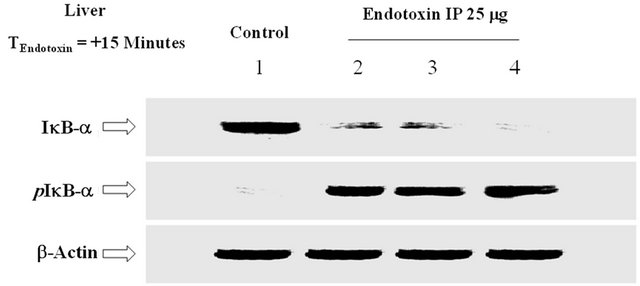
Figure 8. The effect of IP ET on the phosphorylation of IkB-a. ET upregulated the phosphorylation of IkB-a and decreased its cytosolic accumulation. The bactin distribution depicts semi-quantitative loading in parallel lanes. n = 3, which represents the number of independent experiments in duplicate.
 (a)
(a)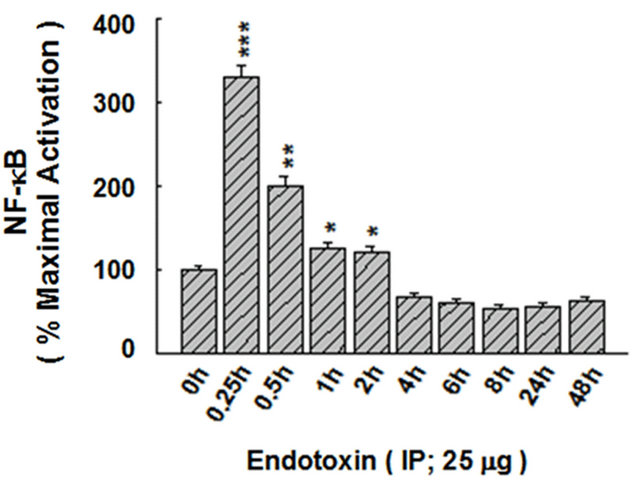 (b)
(b)
Figure 9. The time-response curve of NF-kB DNA-binding activity in response to IP ET. (a) Maximal activation of NF-kB by IP ET in the liver was observed at 0.25 h (15 minutes), declining thereafter. (b) Densitometric analysis is shown with the histogram. The free probe (FP) denotes the faster migrating unbound radioisotope. *P < 0.05, **P < 0.01, ***P < 0.001, as compared to control (0 h). n = 3, which represents the number of independent experiments in duplicate.
mechanisms may by inter-conjugated with the anti-inflammatory role of thymulin, a series of experiments were designed to tackle the issue. Interestingly, pretreatment with thymulin (5 mg; IP) for 30 minutes prior to treatment with ET (1 mg; ICV) reduced ET-induced DNA-binding of NF-κB in the HC (Figure 10), as compared with ET alone (Figures 3(a) and 5(a)).
4. DISCUSSION
The present study indicatesthat ICV injection of ET regulates the nuclear translocation and activation of NF- κB within specific compartments in the brain, an effect particularly localized to the hippocampus. Additionally, thymulin attenuated the ET-induced response, with specific involvement of the transduction pathway implicating the major cytosolic inhibitor of NF-κB, IκB-α. The immunomodulatory potential of thymulin in the CNS has yet to be ascertained in terms of unraveling the cellular and molecular pathways associated with this effect [1, 14,25]. Hence, deciphering the role of intracellular pathways may shed light on the mechanism of action of thymulin in the CNS. There is a wealth of data, however, that correlate the anti-inflammatory actions of thymulin with various mechanisms. For example, it was shown that the growth hormone-releasing activity of thymulin is receptor-mediated and involves calcium (Ca2+), cAMP and inositol phosphates [29]. Furthermore, thymulin has been reported to modulate intracellular cAMP in pathophysiologic conditions [21]. This is corroborated by the report that indicated that cAMP dampened LPS-induced release of TNF-α in macrophages via a specific molecular pathway [30], and that the aforementioned mecha-
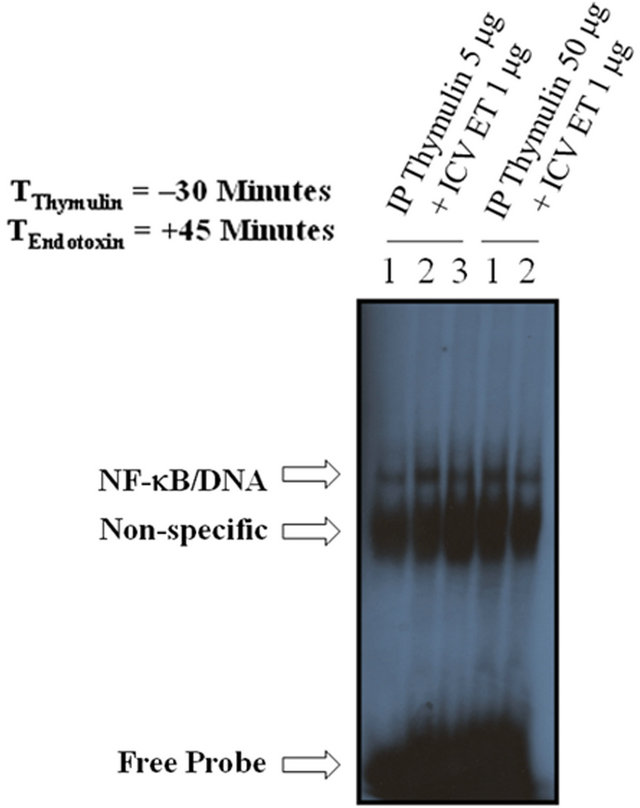
Figure 10. The effect of IP thymulin pretreatment on ICV ET-mediated NF-kB DNA-binding activity in the hippocampus. Thymulin (5 mg; IP) reduced ICV ET-induced DNA-binding of NF-kB in the HC, as compared with ET alone (Figure 3(a)). The free probe (FP) denotes the faster migrating unbound radioisotope. n = 2 - 3, which represents the number of independent experiments in duplicate.
nism may implicate NF-κB and MAPK [31]. Of interest, it has been reported that thymulin addition was capable of inhibiting monocrotaline-induced pulmonary hypertension by modulating the release of IL-6 and suppressing the MAPKp38 pathway [32].
Further and in sync with the aforementioned, thymulin may have the efficacy of down-regulating an inflammatory signal via the NF-κB pathway. Therein,it is reported that thymulinis capable of suppressing the nuclear translocation of various NF-κB subunits in the brain, notably RelA (p65), the major transactivating member of the Rel family [1,3,14,25]. Whether this inhibitory effect on the translocation of NF-κB is mediated via the nuclear localization sequence (NLS) has yet to be ascertained, but this jibes with the notion that the effect of thymulin on the NF-κB pathway is likely involving an upstream regulator, namely the IκB kinase (IKK) [28].
The results therein reported affirm that thymulin can regulate the phosphorylation of IκB-α in the hippocampus, an observation corroborated elsewhere [1-5]. Of particular significance is the observation that thymulin can modulate the NF-κB pathway via the down regulation of the nuclear translocation of various subunits, in addition to down-regulating the DNA-binding activity, an effect dose-dependently amplified.Whether the attenuating effects of thymulin are due to the usage of supraphysiologic concentrations cannot be ruled out; however, definitive reports from our laboratory have affirmed that in vitro and in vivo analysis of the effect of thymulin demanded high concentrations as opposed to circulating femtograms levels. This could be partially explained that tissue levels of thymulin that are biologically effective are essentially higher [3-5].
According to the best of my knowledge and after having carefully combed the MedLine literature for a potential involvement of the NF-κB in the regulatory effect of thymulin, I have observed only a bunch of references shedding albeit a faint light on the integral role of thymulin in regulating an anti-inflammatory response via the NF-κB pathway. For example, IKKb-/- radiation chimeras exhibited elevated circulating TNF-α and IKKb-/- thymocytes displayed increased TNF-α sensitivity, an early indicator for apoptosis [28,31]. This observation is reinforced with another report indicating a suppressor mechanism of thymulin on TNF-a-induced apoptosis in the mouse pancreatic b-cell line [33]. Furthermore, and interestingly, that the observation that centralized actions of ET are counteracted by localized actions of thymulin can lead me to suggest specificity in its actions, although I cannot rule out the influence of the animal model we’re using in our laboratory for various behavioral studies [19]. However, what’s evident is that ET induction of the NF-kB pathway is localized to the hippocampus and not any other area assessed in this study.
The hippocampus is a major component of the brain where it belongs to the limbic system and plays important role in long-term memory and spatial navigation [1]. Pathophysiologically, for instance, in Alzheimer’s disease the hippocampus is one of the first regions of the brain to suffer damage; memory problems and disorientation appear among the first symptoms. Damage to the hippocampus can also result from oxygen starvation (hypoxia), encephalitis, or medial temporal lobe epilepsy [1]. Whether the anti-inflammatory effect of thymulin can be neuroprotective beyond the anti-inflammatory conventional concept has yet to be ascertained, however.
Of note, ICV effect of ET and its reversal by thymulin is not only particularly localized to the hippocampus, but also not involving the diencephalon or substantia nigra (Data not shown). The diencephalon is the part of the forebrain that contains such important structures as the thalamus, hypothalamus and the posterior portion of the pituitary gland [1]. The hypothalamus performs numerous vital functions, most of which relate directly or indirectly to the regulation of visceral activities by way of other brain regions and the autonomic nervous system [19]. However, this does not explain why systemic treatment with thymulin can reduce a central inflammatory response localized to the CNS at specific compartments (hippocampus); has that has major repercussions to the routes involved with the effect of thymulin is worth pursuing.
As that also applies to a non-responsiveness of the substantia nigra, I can theorize that given the fact that the brain is immunologically privileged [1], the relative nonimmune responsiveness of the brain has been attributed to a lack of lymphatic drainage, the presence of the bloodbrain barrier (BBB), the lack of constitutive expression of the major histocompatibility complex (MHC) cluster and the presence of chemical mediators or cofactors purported as capable of inhibiting lymphocyte traffic during inflammation (neuronal cell death and inflammation). This evasion of systemic immunological recognition confers a privilege property that is so unique and, in many ways, plays a major role in shaping the grounds for modified neuroimmune interactions, and hence the observation of the rather limited role of an inflammatory signal or its counteraction by thymulin [1].
In corroboration with the above-mentioned, our group has previously shown that the sympathetic efferent fibers are technically involved with ET-induced localized inflammatory hyperalgesia and cytokine production in peripheral tissues, an effect abrogated by localized and systemic administration of thymulin [19]. It is likely that the bidirectional influence of neuroimmune interactions is a major factor in mediating the anti-inflammatory response of thymulin centrally and peripherally. What is significant is the unequivocal involvement of the IkB/ NF-kB pathway as an integral component of the immunomodulatory effectiveness of thymulin in regulating systemic and peripheral inflammation [1]. This is also reinforced with the observation that thymulin reverses inflammatory hyperalgesia and modulates the increased concentration of proinflammatory cytokines induced by ICV ET [13].
In summary, this report presents a novel immunomodulatory potential of thymulin in vivo at the level of the CNS. These results are highlighted as follows: 1) stereotypic localization led to specific intracerebroventricular injection of ET onto the CNS, with or without pretreatment with thymulin; 2) treatment with ET differentially upregulated the expression and nuclear localization of NF-κB subunits in the HC, an effect attenuated by ICV pretreatment with thymulin; 3) thymulin modulated the phosphorylation of IκB-a in the HC by upregulating the cytosolic accumulation of IκB-a and downregulating pIκB-α; 4) the DNA-binding activity of RelA (p65) was upregulated in the HC, an effect reduced by thymulin; 5) ET did not upregulate the DNA-binding activity of NF-κB in the diencephalon (DE) or substantia nigra (SN); 6) Intraperitoneal (IP) injections of ETupregulated the expression of NF-κB subunits in the liver and reduced the cytosolic accumulation of IκB-α by inducing pIκB-α; and 7) IP pretreatment with thymulin followed by ICV injection of ET reduced the DNAbinding activity of NF-κB in the HC. These results indicate that ET regulates the nuclear translocation/activation of NF-κB subunits within specific compartments in the brain, an effect particularly localized to the hippocampus. Additionally, thymulin attenuated the ET-induced response, with particular involvement of the transduction pathway implicating IκB-α [1,24,25,34].
5. ACKNOWLEDGEMENTS
The author’s workis, in part, supported by the Anonymous Trust (Scotland), the National Institute for Biological Standards and Control (England), the Tenovus Trust (Scotland), the UK Medical Research Council (MRC, London), the National Institutes of Health (NIH) and the Wellcome Trust (London). The author would like to sincerely thank Professors Nayef E. Saadé (American University of Beirut, Lebanon) and Bared Safieh-Garabedian (Queen Mary University of London, UK) for their technical support for this study. Parts of this work were undertaken while the author was a PhD candidate at the University of Dundee, in collaboration with the American University of Beirut, and were presented at the Neuroimmunology meeting in Edinburgh, Scotland, UK in 2001. Dr. John J. Haddad held the distinguished Georges John Livanos fellowship (London, UK) and the National Institutes of Health postdoctoral fellowship (NIH; UCSF).
REFERENCES
- Haddad, J.J. (2008) The regulation of neuroimmuneendocrine interactions: Mechanisms, molecular pathways unraveled and the pivotal role of cytokines—A unsung putative bidirectional interdependence between the immune and neuroendocrine interfaces. Current Immunology Reviews, 4, 134-158. doi:10.2174/157339508785160723
- Welsh, C.J., Steelman, A.J., Mi, W., Young, C.R., Storts, R., Welshm Jr., T.H. and Meagher, M.W. (2009) Neuroimmune interactions in a model of multiple sclerosis. Annals of the New York Academy of Science, 1153, 209- 219. doi:10.1111/j.1749-6632.2008.03984.x
- Haddad, J.J., Saadé, N.E. and Safieh-Garabedian, B. (2002) Cytokines and neuro-immune-endocrine interactions: A role for the hypothalamic-pituitary-adrenal revolving axis. Journal of Neuroimmunology, 133, 1-19. doi:10.1016/S0165-5728(02)00357-0
- Bach, J., Bardenne, M., Pleau, J. and Rosa, J. (1977) Biochemical characterization of a serum thymic factor. Nature, 266, 55-57. doi:10.1038/266055a0
- Millington, G. and Buckingham, J.C. (1992) Thymic peptides and neuroendocrine-immune communication. Journal of Endocrinology, 133, 163-168. doi:10.1677/joe.0.1330163
- Dardenne, M. (1999) Role of thymic peptides as transmitters between the neuroendocrine and immune systems. Annals of Medicine, 2, 34-39.
- Dardenne, M., Pleau, J.M. and Bach, J.F. (1980) Evidence of the presence in normal serum of a carrier of the serum thymic factor (FTS). Journal of Immunology, 17, 83-94.
- Dardenne, M., Pleau, J.M., Savino, W., Prasad, A.S. and Bach, J.F. (1993) Biochemical and biological aspects of the interaction between thymulin and zinc. Progress in Clinical and Biological Research, 380, 23-32.
- Prasad, A.S. (2008) Clinical, immunological, anti-inflammatory and antioxidant roles of zinc. Experimental Gerontology, 43, 370-377. doi:10.1016/j.exger.2007.10.013
- Prasad, A.S. (2007) Zinc: Mechanisms of host defense. Journal of Nutrition, 137, 1345-1349.
- Safieh-Garabedian, B., Ahmed, K., Khamashta, M.A., Taub, N.A. and Hughes, G.R. (1993) Thymulin modulates cytokine release by peripheral blood mononuclear cells: A comparison between healthy volunteers and patients with systemic lupus erythematosus. International Archives of Allergy and Immunology, 101, 126-131. doi:10.1159/000236509
- Safieh-Garabedian, B., Jalakhian, R.H., Saadé, N.E., Haddad, J.J., Jabbur, S.J. and Kanaan, S.A. (1996) Thymulin reduces hyperalgesia induced by peripheral endotoxin injection in rats and mice. Brain Research, 717, 179-183. doi:10.1016/0006-8993(95)01532-9
- Safieh-Garabedian, B., Ochoa-Chaar, C.I., Poole, S., Massaad, C.A., Atweh, S.F., Jabbur, S.J. and Saadé, N.E. (2003) Thymulin reverses inflammatory hyperalgesia and modulates the increased concentration of proinflammatory cytokines induced by i.c.v. endotoxin injection. Neuroscience, 121, 865-873. doi:10.1016/S0306-4522(03)00500-1
- Haddad, J.J., Land, S.C., Saadé, N.E. and Safieh-Garabedian, B. (2000) Immunomodulatory potential of thymulin-Zn2+ in the alveolar epithelium: Amelioration of endotoxin-induced cytokine release and partial amplification of a cytoprotective IL-10-sensitive pathway. Biochemical and Biophysical Research Communication, 274, 500-505. doi:10.1006/bbrc.2000.3155
- Saadé, N.E., Atweh, S.F., Jabbur, S.J., Dardenne, M., Bach, J.F. and Safieh-Garabedian, B. (2003) A thymulin analogue peptide with powerful inhibitory effects on pain of neurogenic origin. Neuroscience, 119, 155-165. doi:10.1016/S0306-4522(03)00072-1
- Hadley, A.J., Rantle, C.M. and Buckingham, J.C. (1997) Thymulin stimulates corticotrophin release and cyclic nucleotide formation in the rat anterior pituitary gland. Neuroimmunomodulation, 4, 62-69.
- Safieh-Garabedian, B., Poole, S., Haddad, J.J., Massaad, C.A., Jabbur, S.J. and Saadé, N.E. (2002) The role of the sympathetic efferents in endotoxin-induced localized inflammatory hyperalgesia and cytokine upregulation. Neuropharmacology, 42, 864-872. doi:10.1016/S0028-3908(02)00028-X
- Dardenne, M., Saadé, N. and Safieh-Garabedian, B. (2006) Role of thymulin or its analogue as a new analgesic molecule. Annals of the New York Academy of Science, 1088, 153-163. doi:10.1196/annals.1366.006
- Safieh-Garabedian, B., Dardenne, M., Pléau, J.M. and Saadé, N.E. (2002) Potent analgesic and anti-inflammatory actions of a novel thymulin-related peptide in the rat. British Journal of Pharmacology, 136, 947-955. doi:10.1038/sj.bjp.0704793
- Reggiani, P.C., Morel, G.R., Cónsole, G.M., Barbeito, C.G., Rodriguez, S.S., Brown, O.A., Bellini, M.J., Pléau, J.M., Dardenne, M. and Goya, R.G. (2009) The thymusneuroendocrine axis: Physiology, molecular biology, and therapeutic potential of the thymic peptide thymulin. Annals of the New York Academy of Science, 1153, 98-106. doi:10.1111/j.1749-6632.2008.03964.x
- Mutchnick, M.G., Good, A.E., Barlas, N. and Trainin, N. (1982) Thymic humoral factor effect on intracellular lymphocyte cAMP in patients with ankylosing spondylitis. Journal of Rheumatology, 9, 627-629.
- Brown, O.A., Sosa, Y.E., Dardenne, M., Pléau, J.M. and Goya, R.G. (2000) Studies on the gonadotropin-releasing activity of thymulin: Changes with age. Journal of Gerontology, 55, B170-B176. doi:10.1093/gerona/55.4.B170
- Rinaldi-Garaci, C., Jezzi, T., Baldassarre, A.M., Dardenne, M., Bach, J.F. and Garaci, E. (1985) Effect of thymulin on intracellular cyclic nucleotides and prostaglandins E2 in peanut agglutinin-fractionated thymocytes. European Journal of Immunology, 15, 548-552. doi:10.1002/eji.1830150604
- Haddad, J.J. and Land, S.C. (2000) O2-evoked regulation of HIF-1a and NF-kB in perinatal lung epithelium requires glutathione biosynthesis. American Journal of Physiology Lung Cellular and Molecular Physiology, 278, L492-L503.
- Haddad, J.J., Olver, R.E. and Land, S.C. (2000) Antioxidant/pro-oxidant equilibrium regulates HIF-1a and NFkB redox sensitivity. Evidence for inhibition by glutathione oxidation in alveolar epithelial cells. Journal of Biological Chemistry, 275, 21130-21139. doi:10.1074/jbc.M000737200
- Yang, J.P., Merin, J.P., Nakano, T., Kato, T., Kitade, Y. and Okamoto, T. (1995) Inhibition of the DNA-binding activity of NF-kB by gold compounds in vitro. FEBS Letters, 361, 89-96. doi:10.1016/0014-5793(95)00157-5
- Kanaan, S.A., Saadé, N.E., Haddad, J.J., Abdelnoor, A.M., Atweh, S.F., Jabbur, S.J. and Safieh-Garabedian, B. (1996) Endotoxin-induced local inflammation and hyperalgesia in rats and mice: A new model for inflammatory pain. Pain, 66, 373-379. doi:10.1016/0304-3959(96)03068-0
- Senftleben, U., Li, Z.W., Baud, V. and Karin, M. (2001) IKKb is essential for protecting T cells from TNFa-induced apoptosis. Immunity, 14, 217-230. doi:10.1016/S1074-7613(01)00104-2
- Brown, O.A., Sosa, Y.E., Dardenne, M., Pléau, J. and Goya, R.G. (1999) Growth hormone-releasing activity of thymulin on pituitary somatotropes is age dependent. Neuroendocrinology, 69, 20-27. doi:10.1159/000054399
- Wall, E.A., Zavzavadjian, J.R., Chang, M.S., Randhawa, B., Zhu, X., Hsueh, R.C., Liu, J., Driver, A., Bao, X.R., Sternweis, P.C., Simon, M.I. and Fraser, I.D. (2009) Suppression of LPS-induced TNF-a production in macrophages by cAMP is mediated by PKA-AKAP95-p105. Science Signaling, 2, ra28. doi:10.1126/scisignal.2000202
- Qi, X.F., Kim, D.H., Yoon, Y.S., Li, J.H., Song, S.B., Jin, D., Huang, X.Z., Teng, Y.C. and Lee, K.J. (2009) The adenylyl cyclase-cAMP system suppresses TARC/CCL17 and MDC/CCL22 production through p38 MAPK and NF-kB in HaCaT keratinocytes. Molecular Immunology, 46, 1925-1934. doi:10.1016/j.molimm.2009.03.018
- Henriques-Coelho, T., Oliveira, S.M., Moura, R.S., Roncon-Albuquerque Jr., R., Neves, A.L., Santos, M., Nogueira-Silva, C., La Fuente Carvalho, F., Brandão-Nogueira, A., Correia-Pinto, J. andLeite-Moreira, A.F. (2008) Thymulin inhibits monocrotaline-induced pulmonary hypertension modulating interleukin-6 expression and suppressing p38 pathway. Endocrinology, 149, 4367-4373. doi:10.1210/en.2008-0018
- Yasuda, J., Nishioka, W., Sakudo, A., Yama, S., Setoguchi, R., Saeki, K., Matsumoto, Y., Awaya, A. and Onodera, T. (2003) Suppressor mechanism of serum thymic factor on tumor necrosis factor-a-induced apoptosis in the mouse pancreatic beta-cell line. Biochemical and Biophysical Research Communication, 311, 501-505. doi:10.1016/j.bbrc.2003.10.025
- Haddad, J.J. and Abdel-Karim, N.E. (2011) NF-kB cellular and molecular regulatory mechanisms and pathways: Therapeutic pattern or pseudoregulation? Cellular Immunology, 271, 5-14. doi:10.1016/j.cellimm.2011.06.021
Abbreviations
AEBSF: 4-(2-aminoethyl)-benzene sulfonyl fluoride-HCl; AR: Ankyrin repeats; CMC: Carboxymethyl cellulose; CNS: Central nervous system; DE: Diencephalon; DTT: Dithiothreitol; ECL: Enhanced chemiluminescence; EMSA: Electrophoretic mobility shift assay; EBV: Epstein-Barr virus; ET: Endotoxin; FTS: Facteur thymique serique; HC: Hippocampus; HTLV: Human Y-lymphotropic virus; HPA: Hypothalamus-pituitary axis; ICV: Intracerebroventricular; IKK: IκB kinase; IP: Intraperitoneal; NF-κB: Nuclear factor-κB; NLS: Nuclear localization sequence; PAT: Peptide analog of thymulin; PBS: Phosphate buffered saline; REL: Rel homology domain; SN: Substantia nigra; TF: Thymic factor.
NOTES
*Retrospect affiliation: Severinghaus-Radiometer Research Laboratories, Department of Anesthesia and Perioperative Care, Faculty of Medicine, University of California, San Francisco, CA, USA.

