International Journal of Organic Chemistry
Vol.07 No.01(2017), Article ID:74722,25 pages
10.4236/ijoc.2017.71006
“Anti-Michael” and Michael Additions in the Reactions of 2-Arylmethyliden-1,3-Indandiones with 2-Aminothiophenol
Jessica J. Sánchez García1, Alberto D. Hernández-Suzan1, Elena Martínez-Klimova1, Marcos Flores-Alamo1, Teresa Ramírez Apan2, Elena I. Klimova1*
1Departamento de Química Orgánica, Universidad Nacional Autónoma de México, Facultad de Química, Cd. Universitaria, Ciudad de México, México
2Instituto de Química, Universidad Nacional Autónoma de México, Facultad de Química, Cd. Universitaria, Ciudad de México, México

Copyright © 2017 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY 4.0).
http://creativecommons.org/licenses/by/4.0/



Received: October 25, 2016; Accepted: March 12, 2017; Published: March 15, 2017
ABSTRACT
A novel 2-indano[2,3b]-2-ferrocenyl- and 2-indano[2,3b]-2-(p-methoxy- phenyl)[1,5]benzo-2,5-dihydrothiazepine 5a,b (addition Michael/cyclization) (~30.32%), indano[2,3b]-2-ferrocenyl- and 2-(p-methoxyphenyl)[1,4] benzothiazine 4a,b (addition “anti-Michael”/cyclization) (~45.43%), respectively, were obtained by the condensation of 2-ferrocenyl-and 2-(p-methoxy- phenyl)methyliden-1,3-indandiones 1a,b with o-aminothiophenol 2 in the presence of AcOH and HCl. A new “anti-Michael” addition reaction of 1,4- bis-heteronucleophile 2 into 2-arylmethyliden-1,3-indandiones was reported. As a result of this reaction the product 1a,b was obtained. The structures of the resultant compounds were elucidated by IR, 1H and 13C NMR spectroscopy, mass spectrometry, elemental and X-ray diffraction analysis. The in vitro antitumor activity of the obtained products was researched using the following human cancer cell lines: glioblastoma (CNS U-251), prostatic adenocarcinoma (PC-3), chronic myelogenous leukemia (K562), colorectal adenocarcinoma (HCT-15), mammary adenocarcinoma (MCF-7), and small cell lung cancer (SKLU) and the sulforhodamine B (SRB) method. Among these new compounds some thiazine and thiazepine derivatives showed compelling in vitro antitumor effects on cell lines K-562, HCT-15, SKLU-1 and MCF-7.
Keywords:
Ferrocene, Addition “Anti-Michael”, Reaction Cyclization, 1,5-Thiazepines, 1,4-Thiazines, Antitumor Cell Lines

1. Introduction
The common strategy for the construction of the 1,5-benzothiazepine moiety is the reaction of 1,3-diarylprop-2-enones with o-aminothiophenol or 1,3-difunc- tional three-carbon building blocks. Among them, α,β-unsaturated carbonyl compounds such as 1,2-enones and 1,2-ynones are best suited for Michael addition and subsequent cyclocondensation [1] . The various reported methodologies involve the use of inorganic supports such as alumina, silica gel, AcOH, trifluoroacetic acid, HCl, piperidine, BF3Et2O, etc. to improve the reaction efficiency [2] [3] [4] . Likewise, the related 1,5-benzothiazepines display a comparable spectrum of biological activity. The 1,5-benzothiazepine framework has been identified as a pluripotent pharmacophore with derivatives encompassing CNS- acting agents [5] , anti-HIV [6] , anti-tuberculosis (TB) [7] , anticancer drugs [8] , antimicrobial [9] , calmodulin antagonists [4] , enzyme inhibitors [11] , antifungal [4] [10] , antibacterial [12] , anti-inflammatory and analgesic agents [4] . Also, it is expected that new active benzo-1,5-thiazepines and other related derivatives could be used in the treatment of serious human diseases like Alzheimer’s diabetes [4] .
Ferrocene compounds are known to possess many chemotherapeutic properties [13] . The incorporation of a ferrocenyl-substituent into a benzo-1,5-thiaze- pine molecule will expand the spectrum of valuable characteristics. Ferrocenyl- substituted 1,5-benzothiazepines have not been extensively studied. The first synthesis of 2-ferrocenyl-4-(4-chlorophenyl)-1,5-benzothiazepine on the base of 1-(4-chlorophenyl)-3-ferrocenyl-2-propenone was reported in 2010 by Willy and Müller [14] . Recently, in 2015, Klimova et al. have published the synthesis of various 2- and 4-ferrocenyl-1,5-benzothiazepines as well as their spectroscopic and structural characteristics [15] . However, The use of 2-ferrocenyl-methyli- den-1,3-diones in the synthesis of polycyclic systems with seven-membered heterocycles, such as dihydro-1,5-benzothiazepines and 1,5-benzothiaze-pines, has not been described until now. Therefore, the synthesis of polycyclic ferrocenyl-1,5-benzothiazepines has received considerable attention.
As a continuation of our previous investigations [15] in this field, the present work examines the possibility of synthesizing tetracyclic ferrocenyldihydro- 1,5-benzothiazepines starting from 2-ferrocenyl- and 2-anisylmethyliden-1,3- indandiones 1a,b and o-aminothiophenol 2 in the presence of acetic or hydrochloric acid.
2. Material and Methods
2.1. Chemistry
Solvents and reagents were purchased from Aldrich and used without further purification. Column chromatography was carried out on alumina (Brockmann activity III). The 1H and 13C NMR spectra were recorded on a Unity Inova Varian spectrometer (300 and 75 MHz) for solutions in CDCl3 with Me4Si as the internal standard. The IR spectra were measured with an FTIR spectrophotometer (Spectrum RXI Perkin-Elmer instruments) using KBr pellets. The mass spectra were obtained on a Varian MAT CH-6 instrument (EI MS, 70 eV). Elementar Analysensysteme LECO CHNS-900 was used for elemental analyses. The following reagents were purchased from Aldrich: ferrocenecarbaldehyde, 99%; p-anisaldehyde, 98%; 1,3-indandione, 97%; 2-aminothiophenol, 99%.
For general information, all experimental data, and copies of the NMR spectra and UV/Vis spectra, see the Supporting Information.
Condensation of 2-arylmethylidene-1,3-indandiones (1a,b) with 2-ami- nothiophenol (2). Tipical Procedure [16] . A mixture of the chalcones (2.5 mmol), 2-aminothiophenol (0.5 g, 4 mmol), AcOH (0.5 mL), HCl conc. (0.1 ml), Et3N (0.1 mL) in methanol (50 mL) was heated to reflux (~60˚C - 65˚C) and stirred until complete dissolution of the enones 1a,b occurred (~6 - 8 h). The organic layer was concentrated, and the residue was chromatographed on alumina (Brockmann activity III, hexane-ether, 3:1) to give 2-arylthiazoles 3a,b (~9% - 11%) [17] [18] [19] , ferrocenyl- and anisylthiazines 4a,b (43% - 45%), ferrocenyl-and anisylthiazapines 5a,b (30% - 32%), and indeno[2,1-b:2,3-b]bis [[1,4]benzothiazine 6 (~10%).
2-Ferrocenylbenzothiazole (3a): Orange crystals: Yield 0.14 g (9%); mp 111˚C - 112˚C (lit. [17] mp 112˚C). 1H NMR (300 MHz, CDCl3,TMS): δ = 4.14 (s, 5 H, C5H5), 4.48 (m, 2 H, C5H4), 4.99 (m, 2 H, C5H4), 7.33 (td, 1 H, J = 1.2, 8.1 Hz, o-C6H4), 7.43 (td, 1 H, J = 1.2, 8.1 Hz, o-C6H4), 7.80 (d, 1 H, J = 8.1 Hz, o-C6H4), 7.95 (d, 1 H, J = 8.1 Hz, o-C6H4) ppm. MS (EI, 70 eV) m/z 319 [M]+. Anal calcd for C17H13FeNS: C, 64.00; H, 4.10; N, 4.40; S, 10.00; Found: C, 63.89; H, 4.26; N, 4.31; S, 9.84.
Indano[2,3b]-2-ferrocenylmethyl[1,4]benzothiazine (4a): Orange crystals: Yield 1.02 g (45%); mp 163˚C - 164˚C, IR (KBr): ν = 467, 481, 511, 729, 762, 812, 887, 999, 1042, 1104, 1163, 1216, 1250, 1344, 1441, 1450, 1552, 1599, 1629, 1718, 2944, 3052, 3081 cm−1. 1H NMR (300 MHz, CDCl3, TMS): δ = 2.81 (d, 1 H, J = 13.8 Hz, CH2), 3.00 (d, 1 H, J = 13.8 Hz, CH2), 3.92 (s, 5 H, C5H5), 3.68 (m, 1 H, C5H4), 3.71 (m, 1 H, C5H4), 3.75(m, 1 H, C5H4), 3.76 (m, 1 H, C5H4), 7.20 (td, 1 H, J = 1.2, 7.2 Hz, C6H4), 7.30 (dd, J = 1.2, 7.8 Hz, 1 H, C6H4), 7.42 (td, 1 H, J = 1.2, 7.8 Hz, C6H4), 7.53 (td, 1 H, J = 1.2, 7.2 Hz, C6H4), 7.64 (dd, 1 H, J = 1.2, 7.8 Hz, C6H4), 7.69 (dd, 1 H, J = 1.2, 7.2 Hz, C6H4), 7.76 (dd, 1H, J = 1.2, 7.2 Hz, C6H4), 8.02 (dd, 1 H, J = 1.2, 7.8 Hz, C6H4) ppm. 13C NMR (75 MHz, CDCl3, TMS): δ = 49.25 (CH2), 68.51 (C5H5), 67.75, 67.71, 69.26, 69.51 (C5H4), 80.21 (CipsoFc), 123.51, 123.30, 126.93, 127.08, 128.16, 128.93, 135.83, 138.30 (2 C6H4), 62.12, 122.52, 129.64, 132.58, 142.93, 145.31 (6 C), 160.19 (C=N), 195.50 (C=O) ppm. MS (EI, 70 eV): m/z 449 [M]+. Anal calcd. for C26H19FeNOS; C, 69.51; H, 4.26; N, 3.12; S, 7.12; Found: C, 69.29; H, 4.34; N, 3.27; S, 7.03.
2-Indeno[2,3b]-2-ferrocenyl[1,5]benzo-2,5-dihydro-thiazepine (5a): Orange crystals: Yield 0.68 g (30%); mp 184˚C - 185˚C, IR (KBr): ν = 481, 497, 507, 733, 761, 817, 893, 1003, 1078, 1105, 1179, 1212, 1224, 1358, 1376, 1429, 1454, 1478,1540, 1578, 1594, 1619, 1681, 1701, 1735, 3008, 3187, 3361 cm−1. 1H NMR (300 MHz, CDCl3, TMS): δ = 4.16 (s, 5 H, C5H5), 3.41 (m, 1 H, C5H4), 3.74 (m, 1 H, C5H4), 4.00 (m, 1 H, C5H4), 4.26 (m, 1 H, C5H4), 5.41 (s, 1 H, CH), 6.94 (td, 1 H, J = 1.2, 7.5 Hz, C6H4), 7.09 (dd, J = 1.2, 7.8 Hz, 1 H, C6H4), 7.16 (dd, 1 H, J = 1.2, 7.8 Hz, C6H4), 7.27 (td, 1 H, J = 1.2, 7.8 Hz, C6H4), 7.33 (td, 1 H, J = 1.2, 7.5 Hz, C6H4), 7.38 (dd, 1 H, J = 1.2, 7.5 Hz, C6H4), 7.42 (td, 1 H, J = 1.2, 7.8 Hz, C6H4), 7.48 (bs, 1 H, NH), 7.56 (dd, 1 H, J = 1.2, 7.8 Hz, C6H4) ppm. 13C NMR (75 MHz, CDCl3, TMS): δ = 42.56 (CH), 68.93 (C5H5), 66.11, 67.04, 67.15, 68.05 (C5H4), 91.11 (CipsoFc), 115.46, 121.38, 121.76, 124.78, 128.65, 130.27, 131.34, 136.94 (2 C6H4), 147.66, 152.69, 154.55, 156.08, 172.44, 178.08 (6 C), 181.45 (C=O) ppm. MS (EI, 70 eV): m/z 449 [M]+. Anal calcd. for C26H19FeNOS: C, 69.51; H, 4.26; N, 3.12; S, 7.12; Found: C, 69.04; H, 4.95; N, 3.54; S, 7.15.
2-(p-Methoxyphenyl)benzothiazole (3b): Yellow crystals: Yield 0.13 g (11%); mp 120˚C - 122˚C (lit. [18] mp 120˚C). 1H NMR (300 MHz, CDCl3, TMS): δ = 3.86 (s, 3 H, CH3), 6.97 (d, 2 H, J = 9.0 Hz, p-C6H4), 7.34 (t, J = 7.5 Hz, 1 H, o-C6H4), 7.46 (t, 1 H, J = 8.1 Hz, o-C6H4), 7.85 (d, 1 H, J = 7.5 Hz, o-C6H4), 8.01 (d, 1 H, J = 8.1 Hz, o-C6H4), 8.03 (d, 2 H, J = 9.0 Hz, p-C6H4) ppm. MS (EI, 70 eV): m/z 241 [M]+. Anal calcd for C14H11NOS: C, 69.70; H, 4.60; N, 5.80; S, 13.26; Found: C, 69.58; H, 4.81; N, 5.63; S 13.04.
Indano[2,3b]-2-[(p-methoxyphenyl)methyl][1,4]benzothiazine (4b): Yellow crystals: Yield 0.40 g (43%); mp 109˚C - 110˚C, IR (KBr): ν = 467, 481, 511, 729, 762, 812, 887, 999, 1042, 1104, 1163, 1216, 1250, 1344, 1441, 1450, 1552, 1599, 1629, 1718, 2944, 3052, 3081 cm−1. 1H NMR (300 MHz, CDCl3,TMS): δ = 2.96 (d, 1 H, J = 13.5 Hz, CH2), 3.13 (d, 1 H, J = 13.5 Hz, CH2), 3.62 (s, 3 H, CH3), 6.53 (d, 2 H, J = 8.7 Hz, p-C6H4), 6.75 (d, 2 H, J = 8.7 Hz, p-C6H4), 7.21 (t, J = 7.5 Hz, 1 H, o-C6H4), 7.35 (t, 1 H, J = 8.1 Hz, o-C6H4), 7.44 (d, 1 H, J = 7.5 Hz, o-C6H4), 7.53 (d, 1 H, J = 8.1 Hz, o-C6H4), 7.66 (t, 1 H, J = 7.5 Hz, o- C6H4),7.69 (t, 1 H, J = 7.5 Hz, o-C6H4), 7.72 (d, 1 H, J = 7.8 Hz, o-C6H4), 8.01 (d, 1 H, J = 7.8 Hz, o-C6H4) ppm. 13C NMR (75 MHz, CDCl3,TMS): δ = 39.43 (CH2), 55.11 (CH3), 113.37, 130.66 (p-C6H4), 123.28, 123.49, 127.05, 127.26, 128.05, 129.14, 132.77, 136.08 (2 o-C6H4), 49.52, 122,75, 125.91, 138.21, 143.09, 145.20, 158.54, 159.87 (8 C), 199.38 (C=O) ppm. MS (EI, 70 eV): m/z 372 [M]+. Anal calcd for C23H17NO2S: C, 74.38; H, 4.62; N, 3.77; S, 8.62; Found: C, 74.21; H, 4.74; N, 3.56; S, 8.93.
2-Indeno[2,3b]-2-(p-methoxyphenyl)[1,5]benzo-2,5-dihydrothiazepine (5b): Orange crystals: Yield 0.30 g (32%); mp 126˚C - 127˚C, IR (KBr): ν = 481, 496, 622, 760, 892, 980, 1078, 1105, 1179, 1212, 1224, 1357, 1454, 1478, 1538, 1577, 1593, 1619, 1680, 1701, 3055, 3186, 3361 cm−1. 1H NMR (300 MHz, CDCl3, TMS): δ = 4.55 (s, 3H, CH3), 6.05 (s, 1H, CH), 6.97 (d, 2 H, J = 9.0 Hz, p-C6H4), 8.02 (d, 2 H, J = 9.0 Hz, p-C6H4), 6.59 (td, 1 H, J = 1.5, 7.5 Hz, o-C6H4), 6.69 (dd, 1 H, J = 1.2, 7.5 Hz, o-C6H4), 7.13 (m, 3 H, o-C6H4), 7.36 (td, 1 H, J = 1.2, 7.8 Hz, o-C6H4), 7.46 (td, 1 H, J = 1.5, 7.5 Hz, C6H4), 7.82 (bs, 1 H, NH),7.84 (dd, 1 H, J = 1.2, 7.8 Hz, o-C6H4) ppm. 13C NMR (75 MHz, CDCl3,TMS): δ = 53,12 (CH), 58.24 (CH3), 119.72, 136.44 (p-C6H4), 122.76, 123.82, 128.02, 128.76, 131.87, 133.04, 135.16, 137.52 (2 o-C6H4), 122,98, 124.06, 136.23, 138.91, 141.21, 144.63, 148.36, 155.17 (8 C), 197.34 (C=O) ppm. MS (EI, 70 eV): m/z 371 [M]+. Anal calcd for C23H17NO2S: C, 74.38; H, 4.62; N, 3.77; S, 8.62. Found: C, 74.56; H, 4.43; N, 3.89; S, 8.54.
Indano[2,1-b:2,3-b]bis[[1,4]benzothiazine (6): Yellow crystals: Yield 0.32 g (10%); mp 200˚C - 201˚C (lit.[19]mp 201˚C - 203˚C). IR (KBr): ν = 635, 738, 755, 985, 1174, 1218, 1246, 1269, 1329, 1351, 1460, 1562, 1606, 1667, 1682, 3064, 3111 cm−1. 1H NMR (300 MHz, CDCl3, TMS): δ = 7.35 - 8.10 (m, 12 H,3 o-C6H4).13C NMR (75 MHz, CDCl3,TMS): δ =56.21 (Cspiro), 124.25, 124.49, 127.08, 127.26, 129.85, 130.16 (3 o-C6H4), 125,98, 128.84, 134.29 (6 C), 157.86 (2 C=N) ppm. MS (EI, 70 eV): m/z 356 [M]+. Anal calcd for C21H12N2S2: C, 70.76; H, 3.39; N, 7.86; S, 17.99; Found: C, 69.40; H, 4.10; N, 8.20; S, 18.30.
Reaction of 1,3-indandione with 2-aminothiophenol 2. This was carried out analogously using of 1,3-indandione (5 mmol), 2-aminothiophenol (12 mmol), methanol (70 mL), AcOH (1.0 mL), HCl conc. (0.2 mL) and Et3N (0.2 mL).The reaction mixture was performed as described above, the precipitate was filtered off and dried on a filter to give 0.85 g of a yellowish product, subsequent chromatography on Al2O3 (hexane-dichloromethane, 1:4) gave 2,2-bis-(2-ami- nophenylthio)indan-1,3-dione 8. The filtrate was concentrated, and the residue was chromatographed on Al2O3 (hexane-dichloromethane, 2:1) to give indeno [2,1-b:2,3-b]bis[[1,4]benzothiazine 6, yield 0.73 g (41%), m.p.201˚C; and 2,2-bis- (2-aminophenylthio)indan-1,3-dione 8.
2,2-bis-(2-aminophenylthio)indan-1,3-dione (8): Yellow crystals: Yield 0.67 g (34%), mp 178˚C - 179˚C, IR (KBr): ν = 675, 742, 761, 898, 943, 1181, 1234, 1252, 1271, 1317, 1363, 1458, 1581, 1609, 1667, 1682, 3386, 3419 cm−1. 1H NMR (300 MHz, CDCl3, TMS): δ = 4.14 (s, 4 H, 2 NH2), 7.71-8.02 (m, 10 H, 2o-C6H4 + o-C6H2), 9.66 (d, 2 H, J = 8.1 Hz, o-C6H2).MS (EI, 70 eV): m/z 392 [M]+. Anal calcd. for C21H16N2O2S2: C, 64.28; H, 4.11; N, 7.14; S, 16.31. Found: C, 64.45; H, 4.03; N, 7.29; S, 16.17.
2.2. X-Ray Analysis
Crystals of 4a and 5a were obtained by crystallization from dichloromethane; crystals of 6 were obtained by crystallization from chloroform. Data were obtained on an Oxford Diffraction Gemini Adiffractometer with a CCD area detector, and the CrystAlisPro and CrysAlis RED software packages were used for data collection and data integration [20] . The structures were solved using SHELXS-97 [21] and refined by full-matrix least-squares on F2 with SHELXL-97. [22] Weighted R factors, Rw, and all goodness-of-fit indicators, S, were based F2. The observed criterion of (F2 > 2σF2) was used only for calculating the R factors. All non-hydrogen atoms were refined with anisotropic thermal parameters in the final cycles of refinement. Hydrogen atoms were placed in ideal positions, with C-H distances of 0.93 and 0.98Å for aromatic and satured carbon atoms, respectively. The isotropic thermal parameters of the hydrogen atoms were assigned the values of Uiso = 1.2 times the thermal parameters of the parent non- hydrogen atom.
Crystal data for C26H19FeNOS (4a): M = 449.33 gmol-1, monoclinic P21/n, a = 7.5018(2), b = 13.2011(5), c = 20.1153(7) Å, α = 90˚, β = 93.922(3), γ = 90˚, V = 1987.39(12) Å3, T = 130(2) K, Z = 4, ρ = 1.502 Mg/m3, wavelength 0.71073 Å, F(000) = 928, absorption coefficient 0.883 mm-1, index ranges −8 ≤ h ≤ 10, −13 ≤ k ≤ 18, −25≤ l ≤ 25, scan range 3.414˚ ≤ θ ≤ 29.589˚, 4695 independent reflections, Rint = 0.0273, 10332 total reflections, 271 refinable parameters, final R indices [I > 2σ(I)] R1 = 0.0331, wR2 = 0.0710, R indices (all data) R1 = 0.0468, wR2 = 0.0766, goodness-of-fit on F2 1.014, largest difference peak and hole 0.353/ −0.300 eÅ−3.
Crystal data for C26H19FeNOS.CH2Cl2 (5a): M = 534.26 g∙mol−1, monoclinic P 21/n, a = 11.3274(9), b = 17. 8011(11), c = 11.9213(9) Å, α = 90, β = 109.584 (9), γ = 90˚, V = 2264.8(3) Å3, T = 130(2) K, Z = 4, ρ = 1.567 Mg/m3, wavelength 0.71073 Å, F(000) = 1096, absorption coefficient 1.016 mm-1, index ranges −15 ≤ h ≤ 9, −16 ≤ k ≤ 24, −16 ≤ l ≤ 16, scan range 3.628˚ ≤ θ ≤ 29.514˚, 5308 independent reflections, Rint = 0.0499, 10868 total reflections, 301 refinable parameters, final R indices [I>2σ(I)] R1 = 0.0565, wR2 = 0.1406, R indices (all data) R1 = 0.0733, wR2= 0.1597, goodness-of-fit on F2 1.052, largest difference peak and hole 1.401/−1.182 eÅ−3.
Crystal data for C21H12N2S2 (6): M = 356.45 g∙mol−1, monoclinic C2/c, a = 22.1186(19), b = 9.4445(6), c = 16.5628(12) Å, α = 92.750(9), β = 109.546(9), γ = 90˚, V = 3260.6(5) Å3, T = 130(2) K, Z = 8, ρ = 1.452 Mg/m3, wavelength 0.71073 Å, F(000) = 1472, absorption coefficient 0.332 mm−1, index ranges −30 ≤ h ≤ 20, −12 ≤ k ≤ 12, −22 ≤ l ≤ 21, scan range 3.521 ≤ θ ≤ 29.469o, 3841 independent reflections, Rint = 0.0334, 7716 total reflections, 226 refinable parameters, final R indices [I > 2σ(I)] R1= 0.0407, wR2 =0.0820, R indices (all data) R1 = 0.0668, wR2 = 0.0895, goodness-of-fit on F2 1.012, largest difference peak and hole 0.298/ −0.284 eÅ−3.
2.3. Cytotoxicity Assay
The compounds were screened in vitro against human cancer cell lines HCT-15 (human colorectal adenocarcinoma), MCF-7 (human mammary adenocarcinoma), K562 (human chronic myelogenous leukemia), U251 (human glioblastoma), PC-3 (human prostatic adenocarcinoma), SKLU-1 (human lung adenocarcinoma). The cell lines were supplied by the National Cancer Institute (USA). The human tumor cytotoxicity was determined using the protein-binding dye sulforhodamine B (SRB) in the microculture assay to measure the cell growth, as is described in the protocols established by the NCI [23] [24] . The cell lines were cultured in the RPMI-1640 medium supplemented with 10% fetal bovine serum, 2 mM L-glutamine, 10,000 units/ml penicillin G sodium, 10 µg/ml streptomycin sulfate, 25 µg/ml amphotericin B (Gibco) and 1% non-essential amino acids (Gibco). The cultures were maintained at 37˚C in a humidified 5% CO2 atmosphere. As determined using trypan blue, the viability of the cells used in the experiments exceeded 95%.
The cells were removed from the tissue culture flasks by treatment with trypsin and diluted with fresh media. 100-ml cell suspension aliquots, containing 5000 - 10,000 cells per well, were transferred into 96-well microtiter plates (Costar) and incubated at 37˚C for 24 h in a 5% CO2 atmosphere.
Stock solutions of the test compounds initially dissolved in DMSO (20 mM) were prepared and further diluted in the medium to produce the desired concentrations. 100-ml aliquots of the diluted solutions of the test compounds were added to each well. The cultures were exposed to the compound at concentrations 50 µM for 48 h. After the incubation period, the cells were fixed to a plastic substratum by the addition of 50 µl of cold 50% aqueous trichloroacetic acid. The plates were incubated at 4˚C for 1 h, washed with tap H2O, and air-dried. The cells fixed with trichloroacetic acid were stained by the addition of 0.4% SRB. Free SRB solution was removed by washing with 1% aqueous acetic acid. The plates were air-dried, and the bound dye was solubilized by the addition of 100 µL of 10 Mm un buffered Tris base. The plates were placed on a shaker for 5 min prior to analysis. The optical densities were determined using a Ultra Microplated Reader (Elx 808: Bio-Tek Instruments, Inc., Winooski, VT, USA) at a test wavelength of 515 nm.
3. Results and Discussion
3.1. Chemistry
The starting compounds 1a,b were prepared by condensation of the respective ferrocenyl- and anisylcarbaldehydes with 1,3-indandione under standard conditions [25] [26] [27] . It was found that o-aminothiophenol 2 reacted with chalcones 1a,b on refluxing condition in methanol in the presence of acetic and hydrochloric acids to give indeno[2,3b]-2-ferrocenylmethyl- and indano[2,3b]-2- anisylmethyl[1,4]benzo-2,3-dihydrothiazines 4a,b (~43% - 45%), and 2-inde-no [2,3b]-2-ferroceny-and 2-p-anisyl[1,5]benzo-2,5-dihydrothiazepines 5a,b (~30% - 32%), respectively. (Scheme 1)
In all cases, the reactions were accompanied by fragmentation [28] of the starting compounds 1a,b to form 2-ferrocenyl- and 2-anisylbenzothiazoles 3a,b (~8% - 11%) [20] [21] respectively, and indano[2,1-b:2,3-b] bis[[1,4]-benzo- thiazine6 (~10%) [19] .
The structures of compounds 3a, b, 4a, b, 5a,b and 6 were isolated by column chromatography on alumina and established based on the data from IR and NMR spectroscopy, mass spectrometry and elemental analysis (see Experimental part). The 1H NMR spectrum of ferrocenyl-1,4-thiazine 4a contains two characteristic doublets for two protons of the methylene group (δ 2.81, 3.00, J =

Scheme 1. Reactions of 2-arylmethylidenindan-1,3-dione with o-aminothiophenol.
13.8 Hz), one singlet for the protons of unsubstituted C5H5 ring of ferrocene, and multiplets for protons of two o-C6H4 groups. The presence in the 13C NMR spectrum of compound 4a of one signal for one methylene group (δ 42.25), one ferrocenyl fragment [δ 68.51 (C5H5)], one CipsoFc carbon atom (δ 80.21), one C=O group (δ 195.50), one C=N fragment (δ 160.19), and six quaternary carbon atoms (δ 62.12, 122.52, 129.64, 132.58, 142.93, 145.31) corroborates completely the suggested structure. The spectroscopic data (1H and 13C NMR) suggest that compound 4b represent also structure of anisyl-1,4-benzothiazine.
The spatial structure of 4a was elucidated by X-ray diffraction analysis of a single crystal obtained by crystallization from dichloromethane. The general view of the molecule 4a is shown in Figure 1 and the main geometrical parameters are given in Table 1. Data from X-ray analysis proved the structure of 4a as indano[2,3b]-2-ferrocenylmethyl[1,4]benzothiazines.
Table 1. Selected bond lengths and bond angles for compounds 4a, 5a and 6.
Figure 1. X-ray crystal structure of 4a.
The 1H NMR spectrum of 5a contains characteristic signals for one proton of the CH group in δ 5.41ppm, one proton of the NH group in δ 4.26 ppm, one singlet for the protons of the unsubstituted C5H5 ring of one ferrocene δ 4.16 ppm and multiplets for the eight protons of two o-C6H4 groups. The data from 13C NMR spectroscopy of this compound are in full accord with the proposed structure. And X-ray diffraction analysis was performed on a single crystal grown by crystallization from dichloromethane. The general view of the molecule 5a is shown in Figure 2 and the main geometrical parameters are given in Table 1. Data from the X-ray analysis demonstrated that 5a is 2-indeno [2,3b]- 2-ferrocenyl[1,5]benzo-2,5-dihydrothiazepine.
The structures of 2-ferrocenyl- and 2-p-anisylbenzothiazoles 3a,b and indano[2,1-b:2,3-b]bis[[1,4] benzothiazine6 were also confirmed by 1H and 13C NMR spectroscopy, mass spectrometry, and data from literature [17] [18] [19] . These facts confirm the assumed structures.
The spatial structure of single crystals of indano[2,1-b:2,3-b]bis[1,4] benzo- thiazine 6 prepared by crystallization from chloroform was determined by X-ray diffraction analysis (Figure 3). The principal geometric parameters of this molecule with a “three-petal” system of fused two six- and one five-membered rings and a quaternary carbon atom C(1) are listed in Table 1. The bond lengths C(1)-S(1) (1.8196Å), and C(1)-S(2) (1.8191 Å) are somewhat larger, and the N(1)-C(1)(1.277Å) and N(2)-C(1) (1.279 Å) bond lengths are somewhat shorter than the standard values [24] .
The results of this study indicate cyclocondensation reactions of 2-arylmethy- lidenindan-1,3-dione 1a,b with o-aminothiophenol2 and breakage of the Cα=Cβ multiple bond [28] in the initial 1a,b compounds subjected to the action of 1,4-bis-nucleophile 2. In this regard, these interactions can be viewed as a step
Figure 2. X-ray crystal structure of 5a.
Figure 3. X-ray crystal structure of 6.
wise process consisting of several stages: 1) Michael addition of the molecule of a o-aminophenol 2 to substrates 1a,b gave intermediates 7a,b; 2) Intramolecular cyclization of 7a,b into tetracyclic thiazepines 5a,b; 3) Breaking of the σ-Cα- Cβ bond in the addition intermediates 7a,b (Scheme 2); 4) “Anti- Michael” addition of o-aminophenol 2 to substrates 1a,b gave intermediates 7a,b; 5) Intramolecular cyclization of 7a,b into tetracyclic thiazines 4a,b (Scheme 3).
To confirm the latter statement, we performed a specially aimed synthesis of compound 6, using indan-1,3-dione and o-aminothiophenol 2 as the initial substrates (Scheme 4). The products 6 and 8 were obtained in yields ~41% and 34%, respectively.
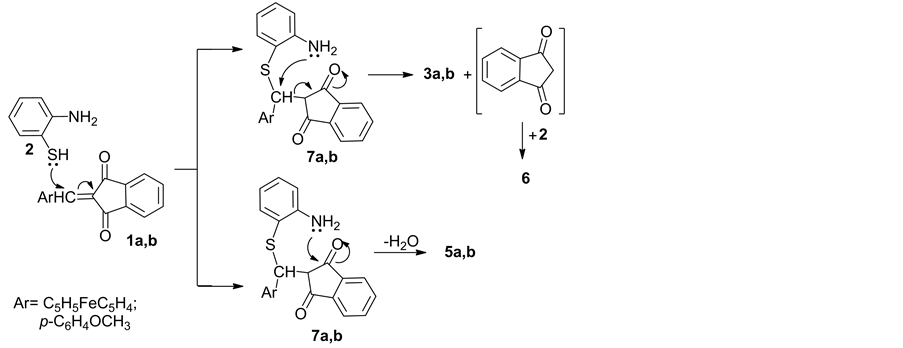
Scheme 2. The mechanism proposed for the process leading to indano[2,3b]-2-ferroce- nylmethyl[1,4]benzothiazines and 2-indeno[2,3b]-2-ferrocenyl[1,5]benzo-2,5-dihydro- thiazepines.
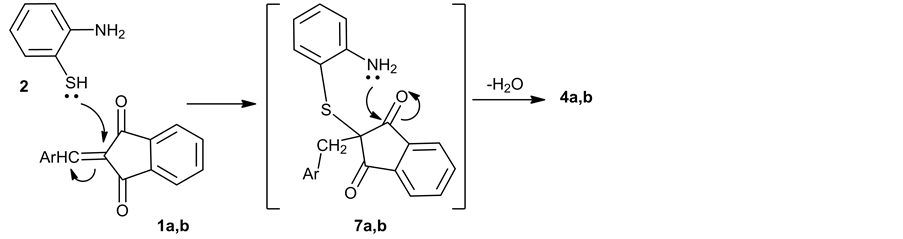
Scheme 3. Formation of the compounds 4a,b.

Scheme 4. Reaction of 1,3-indandione with 2-aminothiophenol 2.
3.2. Cytotoxicity of the Benzothiazepines
In this study, cytotoxicity assays of the four benzothiazepine compounds 4a,b and 5a,b (Figure 4) were performed as described in the Experimental Section by using different cancer cell lines, including human glioblastoma (CNS U251), human prostatic adenocarcinoma (PC-3), human chronic myelogenous leukemia (K562), human colorectal adenocarcinoma (HCT-15), human mammary adenocarcinoma (MCF-7), and small cell lung cancer (SKLU) and the protein- binding dye sulforhodamine B (SRB) assay in microculture to determine cell growth [24] . The initial cytotoxic screening data listen in Table 2, show excellent activities specifically toward K-562, HCT-15, and SKLU-1 tumor cell lines. From those data, we observe good values of cell growth inhibition, 4b being the most active compound (Table 2).
Figure 4. Variations in the structure of benzothiazepines.
Table 2. Inhibition of the Growth (%) of Human Tumor Cell Lines for 4a, b and 5a, b at 25 µM in DMSOa.
aResults express mean ± standard error (SEM) obtainedfrom 3 independentexperimentsperformedat 48 h.
The results of the cytotoxic screening demonstrate that the presence of the substituent ferrocene for compounds 4a,5a and anisole compounds 4b,5b, have the influence in the cytotoxic activity disappears, obtaining good activity for (K- 562, HCT-15 and SKLU-1) tumor cell lines.
4. Conclusion
Reactions of 2-ferrocenyl- and 2-(p-methoxyphenyl) methylidenindandiones with 2-aminothiophenol in a MeOH medium in the presence of AcOH/HCl gave novel indano[2,3b]-2-ferrocenyl-and 2-[(p-methoxyphenyl)methyl][1,4] benzothiazines 4a,b (products of “Anti-Michael”-addition/cyclization) and 2-indeno [2,3b]-2-ferrocenyl-and 2-(p-methoxyphenyl)[1,5]benzo-2,5-dihydrothiaze-pines 5a,b respectively (products of Michael-addition/cyclization). These new compounds were obtained in ~30% - 45% yields. The reactions take place only via breakage of the ArCα = Cβ double bond with the formation of derivatives of arylcarbaldehydes(2-ferrocenyl- and 2-p-methoxyphenylbenzothiazoles 3a,b), as well as indandione (indano [2,1-b:2,3-b]bis[1,4]benzothiazine 6). The obtained compounds were structurally characterized by elemental analysis, IR, 1H and 13C NMR spectroscopy, mass-spectrometry, and single crystal X-ray diffraction analysis. The synthesized compounds 4a,b and 5a,b were evaluated for their in vitro anticancer activities against six human tumor cell lines: U-251, PC-3, K-562, HCT-15, MCF-7, SKLU-1. The compound 4b showed high activity against three tumoral cell lines (K-562, HCT-15 and SKLU-1).
Acknowledgements
This work was supported by the DGAPA (Mexico, grant IN 215015).
Cite this paper
García, J.J.S., Hernández-Suzan, A.D., Martínez-Klimova, E., Flores-Alamo, M., Apan, T.R. and Klimova, E.I. (2017) “Anti-Michael” and Michael Additions in the Reactions of 2-Arylmethyliden-1,3-Indandiones with 2- Aminothiophenol. International Journal of Organic Chemistry, 7, 57-81. https://doi.org/10.4236/ijoc.2017.71006
References
- 1. Bariwal, J.B., Upadhyay, K.D., Manvar, A.T., Trivedi, J.C., Singh, J.S., Jain, K.S. and Shah, A.K. (2008) 1, 5-Benzothiazepine, a Versatile Pharmacophore: A Review. European Journal of Medicinal Chemistry, 43, 2279-2290.
https://doi.org/10.1016/j.ejmech.2008.05.035 - 2. Hekmatshoar, R., Sadjadi, S., Shiri, S., Heravi, M.M. and Beheshtiha, Y.S. (2009) Green Protocol for Synthesis of 1,5-Benzodiazepines and 1,5-Benzothiazepines in the Presence of Nanocrystalline Aluminum Oxide. Synthetic Communications, 39, 2549-2259.
https://doi.org/10.1080/00397910802657925 - 3. Nardi, M., Cozza, A., Maiuolo, L., Oliverio, M. and Procopio, A. (2008) Ga (OTf) 3-Promoted Condensation Reactions for 1, 5-Benzodiazepines and 1, 5-Benzothia- zepines. Tetrahedron Letters, 49, 5302-5308.
https://doi.org/10.1016/j.tetlet.2008.06.082 - 4. Bl-Bayouki, K.A. (2013) Benzo[1,5]thiazephine: Synthesis, Reactions, Spectroscopy and Applications. Organic Chemistry International, 1, 1-71.
https://doi.org/10.1155/2013/210474 - 5. Nikalje, A.P. and Vyawahare, D. (2011) Facile Green Synthesis of 2,4-substituted- 2,3-dihydro-1,5 Benzothiazepine Derivatives as Novel Anticonvulsant and Central Nervous System (CNS) Depressant Agents. African Journal of Pure and Applied Chemistry, 5, 422-428.
- 6. Pommier, Y., Garafalo, A., Brizzi, A., Campiani, G., Fiornini, I. and Nacci, V. (1999) Thiazolothiazepine Inhibitors of HIV-1 Integrase. Journal of Medicinal Chemistry, 42, 3334-3341.
- 7. Upadhyay, K., Manvar, A., Rawal, K., Joshi, S., Trivedi, J., Chaniyara, R. and Shah, A. (2012) Evaluation of Structurally Diverse Benzoazepines Clubbed with Coumarins as Mycobacterium tuberculosis Agents. Chemical Biology & Drug Design, 80, 1003-1008.
https://doi.org/10.1111/j.1747-0285.2012.01436.x - 8. Arya, K. and Dandia, A. (2008) The Expedient Synthesis of 1,5-benzothiazepines as a Family of Cytotoxic Drugs. Bioorganic & Medicinal Chemistry Letters, 18, 114- 119.
https://doi.org/10.1016/j.bmcl.2007.11.002 - 9. Singh, G., Kumar, N., Yadav, A.K. and Mishra, A.K. (2002) Syntheses of Some New 1,5-Benzothiazepine Derivatives and Their Ribofuranosides as Antimicrobial Agents. Heteroatom Chemistry, 13, 620-625.
https://doi.org/10.1002/hc.10051 - 10. Dandia, A., Singh, R., Singh, D., Laxkar, A. and Sivpuri, A. (2010) Regioselective Synthesis of Diltiazem Analogue Pyrazolo[4,3-c][1,5]benzothiazepines and Antifungal Activity. Phosphorus, Sulfur and Silicon, 185, 2472-2479.
https://doi.org/10.1080/10426501003713064 - 11. Ansari, F.I., Kalsoom, S., Zaheer-ul-Haq, Ali, Z. and Jabeen, F. (2012) In Silico Studies on 2,3-dihydro-1,5-benzothiazepines as Cholinesterase Inhibitors. Medicinal Chemistry Research, 21, 2329-2339.
https://doi.org/10.1007/s00044-011-9754-6 - 12. Saini, R.K., Joshi, Y.C. and Joshi, P. (2008) Solvent-Free Synthesis of Some 1,5- Benzothiazepines and Benzodiazepines and Their Antibacterial Activity. Phosphorus, Sulfur and Silicon, 183, 2181-2190.
https://doi.org/10.1080/10426500701852661 - 13. Kopf-Maier, P. and Kopf, H. (1987) Non-Platinum Group Metal Antitumor Agents. History, Current Status, and Perspectives. Chemical Reviews, 87, 1137-1152.
https://doi.org/10.1021/cr00081a012 - 14. Willy, B. and Müller, T.J.J. (2010) Three-Component Synthesis of benzo[b][1,5]thiazepines via Coupling-Addition-Cyclocondensation Sequence. Molecular Diversity, 14, 443-453.
https://doi.org/10.1007/s11030-009-9223-z - 15. Klimova, M.A., Gallardo Vega, J.J., Sánchez García, M., Flores-Alamo, J.M. and Méndez Stivalet, J. (2015) 4-Aryl-2-ferrocenyl- and 2-Aryl-4-ferrocenyl- 2,3-dihydro-1,5-benzothiazepines with Potentially Biological Activities: Synthesis, Characterization, X-ray Diffraction Studies. Journal of Heterocyclic Chemistry, ID JHET-15-0038.
https://doi.org/10.1002/jhet.2519 - 16. Katritzky, A.R., Odens, H.H. and Zhang, S. (2001) Novel Syntheses of 2,3-Dihy- dro-1,5-benzothiazepin-4(5H)-ones and 2H-1,4-Benzothiazin-3(4H)-ones. The Journal of Organic Chemistry, 66, 6792-6796.
https://doi.org/10.1021/jo0101959 - 17. Salisova, M., Prokesova, M., Kubrikanova, M. and Toma, S. (1993) Reactions of Ferrocenecarboxylic Acid and Omega Ferrocenyl-Omega-Oxoalkanoic Acids with 2-Aminothiophenol. Chemical Papers—Chemicke Zvesti, 47, 183-185.
- 18. Arslan, H. and Algül, Ö. (2007) Synthesis and Ab Initio/DFT Studies on 2- (4-methoxyphenyl)benzo[d]thiazole. International Journal of Molecular Sciences, 8, 760-776.
https://doi.org/10.3390/i8080760 - 19. Roth, H.J. and Kok, W. (1976) Zur Kenntnis der Ninhydrin-Reaktion, 3. Mitt. Reaktion mit Dimethoxyanilinen und reaktiven Aromaten. Archiv der Pharmazie, 309, 81-86.
https://doi.org/10.1002/ardp.19763090202 - 20. CrysAlis, C.C.D. and CrysAlis, R. (2009) Crystal Structure Solution. Oxford Difraction, Abingdon.
- 21. Sheldrick, G.M. (1990) SHELXS-97, Crystal Structure Solution. University of Göttingen, Göttingen.
- 22. Sheldrick, G. (1997) M.SHELXS-97, Crystal Structure Refinament. University of Göttingen, Göttingen.
- 23. Monks, A., Scudiero, D., Skehan, P., Shoemaker, R., Paul, K., Vistica, D., Hose, C., Langley, J., Cronise, P., Vaigro-Wolff, A., Gray-Goodrich, M., Campbell, H., Mayo, J. and Boyd, M. (1991) Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. Journal of the National Cancer Institute, 83, 757-766.
https://doi.org/10.1093/jnci/83.11.757 - 24. Eicher, S. Hauptmann, A. Speicher, (2012) The Chemistry of Heterocycles. Structures, Reactions, Synthesis and Applications. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, 529-545.
- 25. Stankovic, E., Elecko, P. and Toma, S. (1996) Knoevenagel Condensation of Ferrocenecarbaldehyde with Some Methylene Active Reagents on Inorganic Supports. Chemical Papers, 50, 68-71.
- 26. Cooke, G., Palmer, H.M. and Schulz, O. (1995) Novel Ferrocene Derivatives From Precursors Derived From Alumina-Catalysed Knoevenagel Reactions of Ferrocenecarboxaldehyde. Synthesis, 1995, 1415-1418.
https://doi.org/10.1055/s-1995-4125 - 27. Wu, D., Ren, Z., Cao, W. and Tong, W. (2005) Solvent-Free Synthesis of 2-Aryli- deneindan-1,3-diones in the Presence of Magnesium Oxide or Silica Gel Under Grinding. Synthetic Communications, 35, 3157-3162.
https://doi.org/10.1080/00397910500282968 - 28. Sánchez García, J.J., Gallardo Vaga, M.A., Flores-Álamo, M., Méndez Stivalet, J.M. and Klimova, E.I. (2015) 1,4-dinitrogen Nucleophiles in the Breaking of the Cα=Cβ Multiple Bond in 2-ferrocenylmethylidene-β-dicarbonyl Compounds. Simple Method for the Preparation of the Al(III) Complexes with β-diketones and β-ketoesters. Journal of Applied Chemical Science International, 2, 147-158.
Supplementary Material
The crystallographic data for 4a, 5a and 6 have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication under the CCDC numbers 1423550, 1423551 and 1423552. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre, 12, Union Road, Cambridge DB2 1EZ, UK; Fax: (internat.) +44-1223/336-033; E-mail: deposit@ccdc.cam.ac.uk].
For general information, all experimental data and copies of the NMR spectra and UV/V are Spectra.
The 1H and 13C NMR spectra were recorded on a Unity Inova Varian spectrometer (300 and 75 MHz) for solutions in CDCl3 with Me4Si as the internal standard. The IR spectra were measured with an FTIR spectrophotometer (Spectrum RXI Perkin-Elmer instruments) using KBr pellets. The mass spectra were obtained on a Varian MAT CH-6 instrument (EI MS, 70 eV). Elementar Analysensysteme LECO CHNS-900 was used for elemental analyses.
Compound 3a “2-Ferrocenylbenzothiazole”
Figure S1. 1H NMR (300 MHz, CDCl3, TMS) spectrum of compound 3a.
Compound 3b “2-(p-Methoxyphenyl)benzothiazole ”
Figure S2. 1H NMR (300 MHz, CDCl3, TMS) spectrum of compound 3b.
Compound 4a “Indano[2,3b]-2-ferrocenylmethyl[1,4]benzothiazine”
Figure S3. 1H NMR (300 MHz, CDCl3, TMS) spectrum of compound 4a.
Figure S4. 13C NMR (75 MHz, CDCl3, TMS) spectrum of compound 4a.
Figure S5. IR (KBr) spectrum of compound 4a.
Figure S6. Mass Spectrometry spectrum of compound 4a.
Compound 4b Indano[2,3b]-2-[(p-methoxyphenyl) methyl][1,4]benzo- thiazine
Figure S7. 1H NMR (300 MHz, CDCl3, TMS) spectrum of compound 4b.
Figure S8. 13C NMR (75 MHz, CDCl3, TMS) spectrum of compound 4b.
Figure S9. IR (KBr) spectrum of compound 4b.

Figure S10.Mass Spectrometry spectrum of compound 4b.
Figure S11.1H NMR (300 MHz, CDCl3, TMS) spectrum of compound 5a.
Figure S12.13C NMR (75 MHz, CDCl3, TMS) spectrum of compound 5a.
Figure S13.IR (KBr) spectrum of compound 5a.
Figure S14.Elemental Analysis spectrum of compound 5a.

Figure S15. Mass Spectrometry spectrum of compound 5a.
Figure S16.1H NMR (300 MHz, CDCl3, TMS) spectrum of compound 5b.
Figure S17.13C NMR (75 MHz, CDCl3, TMS) spectrum of compound 5b.

Figure S18.IR (KBr) spectrum of compound 5b.
Figure S19.IR (KBr) spectrum of compound 6.
Submit or recommend next manuscript to SCIRP and we will provide best service for you:
Accepting pre-submission inquiries through Email, Facebook, LinkedIn, Twitter, etc.
A wide selection of journals (inclusive of 9 subjects, more than 200 journals)
Providing 24-hour high-quality service
User-friendly online submission system
Fair and swift peer-review system
Efficient typesetting and proofreading procedure
Display of the result of downloads and visits, as well as the number of cited articles
Maximum dissemination of your research work
Submit your manuscript at: http://papersubmission.scirp.org/
Or contact ijoc@scirp.org






















