International Journal of Organic Chemistry
Vol.04 No.05(2014), Article ID:52833,11 pages
10.4236/ijoc.2014.45035
Preparation of Polyfunctionally Substituted Pyridine-2(1H)-Thione Derivatives as Precursors to Bicycles and Polycycles
Fathi A. Abu-Shanab1,2*, Sayed A. S. Mousa1, Sherif M. Sherif3, Mohamed I. Hassan1
1Department of Chemistry, Faculty of Science, Al-Azhar University, Assiut, Egypt
2Department of Chemistry, Faculty of Science, Gazan University, Gazan, KSA
3Department of Chemistry, Faculty of Science, Cairo University, Giza, Egypt
Email: *fathiabushanab@yahoo.com
Copyright © 2014 by authors and Scientific Research Publishing Inc.
This work is licensed under the Creative Commons Attribution International License (CC BY).
http://creativecommons.org/licenses/by/4.0/



Received 3 October 2014; revised 19 November 2014; accepted 6 December 2014
ABSTRACT
Reaction of acetylacetone with 1 mole of dimethylformamide dimethyl acetal (DMFDMA) affords enamine 2a which reacts with cyanothioacetamide to give pyridinethione 3a. Pyridinethione 3a reacts with methyl iodide, halogenated compounds, aromatic aldehyde and malononitrile/ele- mental sulfur to yiled compounds 7-10 respectively. Reactions of thioether 7 in ethanolic K2CO3, 1 mole DMFDMA and 4-(dimethylamino)benzaldehyde give compounds 11, 13, 14 respectively. Enaminone 12 can be prepared by reaction of compound 11 with DMFDMA. We have demonstrated some reactions in order to show the potential usefulness of the prepared compounds for the preparation of new bipyridyl compounds 15, 16, 18, bicyclic compounds 17 and uncommon tricyclic compounds 20, 21, 22 and 23 respectively using DMFDMA.
Keywords:
Acetyl Acetone, DMFDMA, Malononitrile Dimmer, Bipyridyl, 5-Acetylpyridinethione

1. Introduction
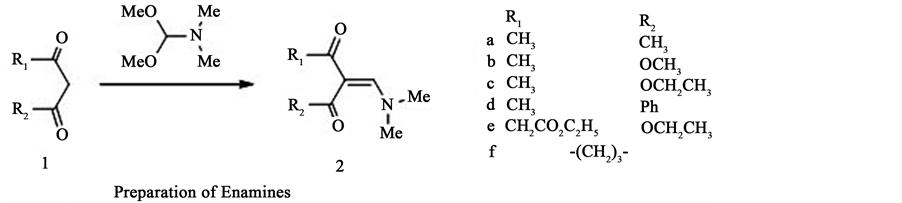
Formamide acetals are useful reagents in organic synthesis; [1] [2] their main application has been used for functional group transformations [3] , but they may also be regarded as one-carbon synthons in the construction of carbon skeletons. One type of reaction, which is potentially valuable for the future purpose, is the reaction of N,N'-dimethylformamide dimethyl acetal (DMFDMA) with 1,3-dicarbonyl compounds 1 to give enamines 2 [2] [4] (Figure 1).
Figure 1. Preparation of Enamines.
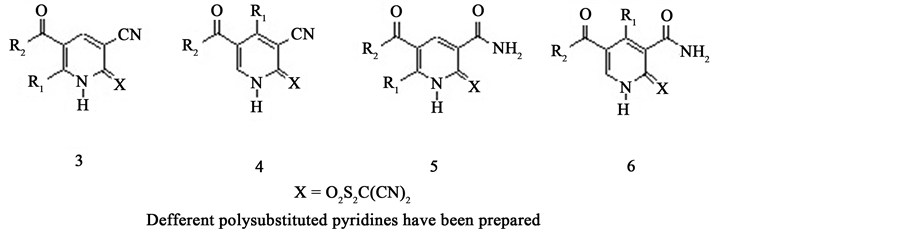
We have reported that enamines 2 were used as precursors in the synthesis of pentasubstituted pyridines 3-6 [5] -[8] (Figure 2).
The treatment of acetyacetone (1a) with dimethyl formamide dimethylacetal (DMFDMA) in dry DMF under nitrogen and stirring over night afforded the corresponding enamine 2a which on treatment with cyanothioacetamide and sodium hydride in dry DMF (in situ) afforded pyridine-2(1H)-thione (3a) [6] , when the emamine 2a was treated with cyanothioacetamide in ethanol and pepridine as a base afforded the pyridine-2(1H)-thione (5a) [7] [12] (Figure 3).
2. Results and Discussion
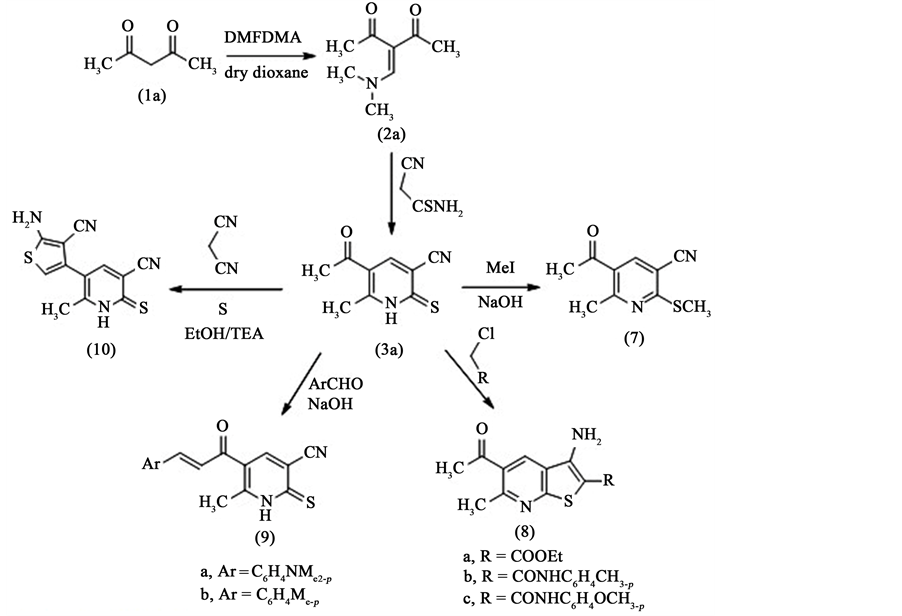
In conjunction of this work, we report here the reaction of acetylacetone 1a with one mole of N,N'-dimetylfor- mamide dimethyl acetal (DMFDMA) in dry dioxane gave the corresponding enamine 2a. The treatment of this enamine (in situ) with cyanothioacetamide in ethanol in the presence of sodium ethoxide under reflux gave 5- acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a with a very good yied [7] , Scheme 1.
We have found that the prepared compound 3a included three functional groups which are thioamido group, cyano group and acetyl group. These functional groups can be used for the preparation of bicyclic or polycyclic compounds of biological interest. Thus, some illustrative reactions designed to demonstrate the potential usefulness of 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a for further heterocyclic synthesis. Therefore, the reaction of 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a with methyl iodide in alcoholic sodium hydroxide afforded the corresponding thioether derivative 7, which in turn is a good intermediate for the preparation of further heterocyclic compounds of biological interest. The structure of the isolated compound 7 is confirmed by spectral analysis. The IR spectrum shows the disappearance of (NH) group. Also, the 1H NMR spectrum shows the disappearance of the thioamide proton and the appearance of a singlet signal corresponding to (SCH3) at δH = 2.63 ppm. Also, the mass spectrum shows the molecular ion peak at m/e 206 which corresponding to the molecular formula (C10H10N2OS). The reaction of 5-acetyl-6-methyl-2-thiox-o-1,2- dihydropyridine-3-carbonitrile 3a with ethyl chloroacetate or chloroacetamides in ethanolic sodium ethoxide afforded the corresponding 5-acetyl-3-amino-6-methylthieno[2,3-b]pyridine derivatives 8a-c in a good yield. The structure of the isolated compounds is confirmed by elemental and spectral analysis. The IR spectrum shows the disappearance of cyano group and appearance of amino group at υmax = 3427, 3328 cm−1 in compound 8a as example beside the other functional groups. Also, the mass spectra show the molecular ion peaks fit to all compounds 8a-c. Also, the 1H NMR spectra show signals fit to the structure of all compounds 8a-c. The presence of acetyl group in 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a is useful for the preparation of fused heterocyclic compounds. So that the reaction of compound 3a with aldehydes like 4-(dimethylamino) benzaldehyde and 4-methylbenzaldehyde in ethanolic sodium hydroxide afforded the corresponding chalcones 9a,b. The structure of the isolated chalcones is confirmed by elemental analysis as well as spectral analysis. The mass spectra show the molecular ion peak fit to all compounds 9a,b. As an example compound 9a shows the molecular ion peak at m/e 323 which corresponding to the molecular formula (C18H17N3OS). Also, the 1H NMR spectra of these compounds 9a,b show the disappearance of the signal corresponding to the methyl of acetyl group and the appearance of two doublets signals corresponding to the two protons of double bond of chalcone. Finally, 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a was treated with malononitrile and sulfur element (Gewald’s reaction) in ethanol in the presence of triethylamine as a base to afford 5-(5-amino-4- cyanothiophen-3-yl)-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 10 in a good yield, Scheme 1. The IR spectrum of compound 10 shows the appearance of amino group at υmax = 3435, 3350 cm−1 beside the other
Figure 2. Different polysubstituted pridines have been prepared.
Figure 3. Tetrasubstituted pyridinethione have been prepared.
Scheme 1.Synthesis of pyridine (1H)-thione derivative 3a and its reactions with MeI, α- chloroketones, aldehydes and malononitrile.
functional groups. Also, 1H NMR spectrum of compound 10 shows singlet signal at δH = 2.45 ppm corresponding to methyl group and singlet signal at δH = 6.95 ppm corresponding to amino group and singlet signal at δH = 7.07 ppm corresponding to CH thiophene ring and singlet signal at δH = 7.2 ppm corresponding to CH pyridine ring.
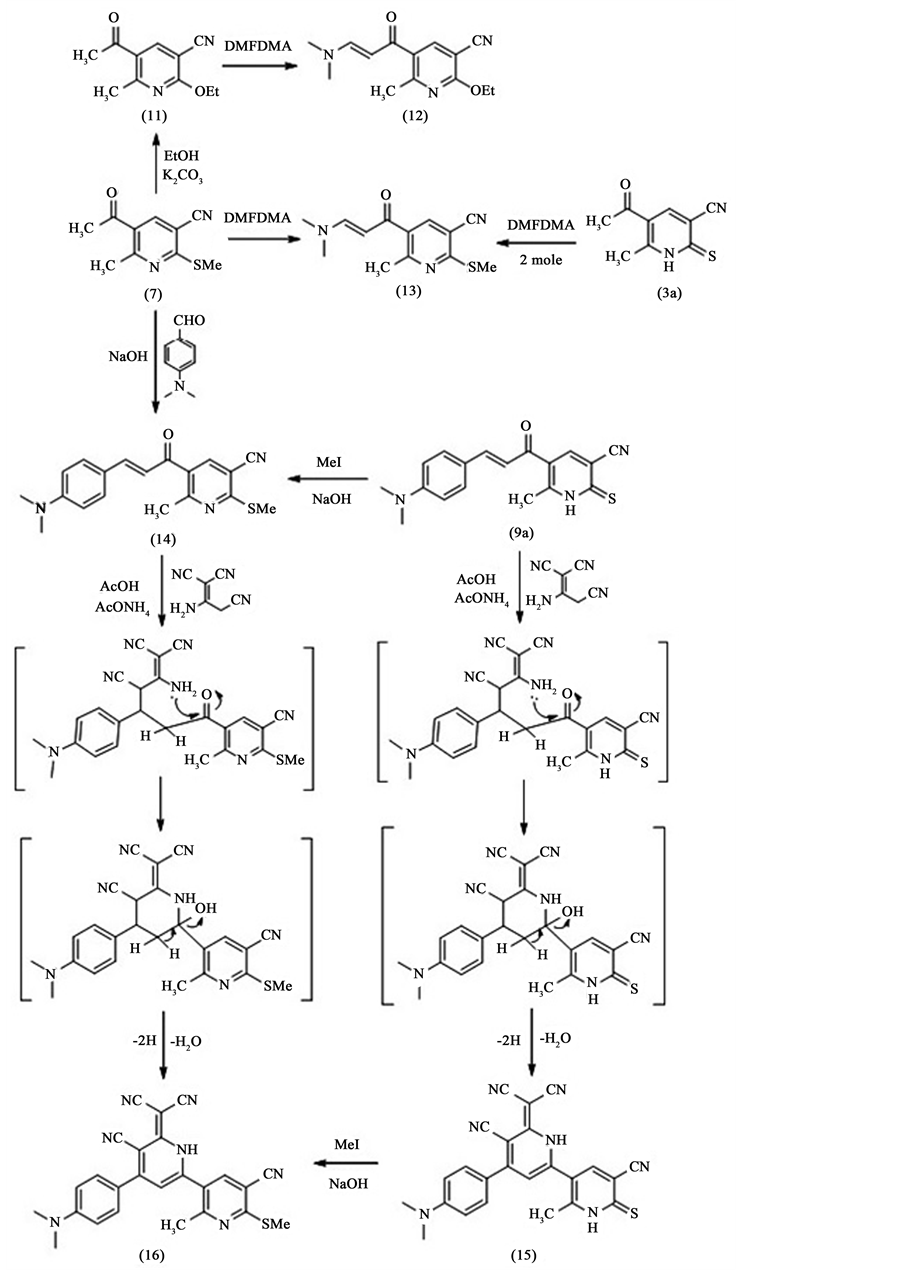
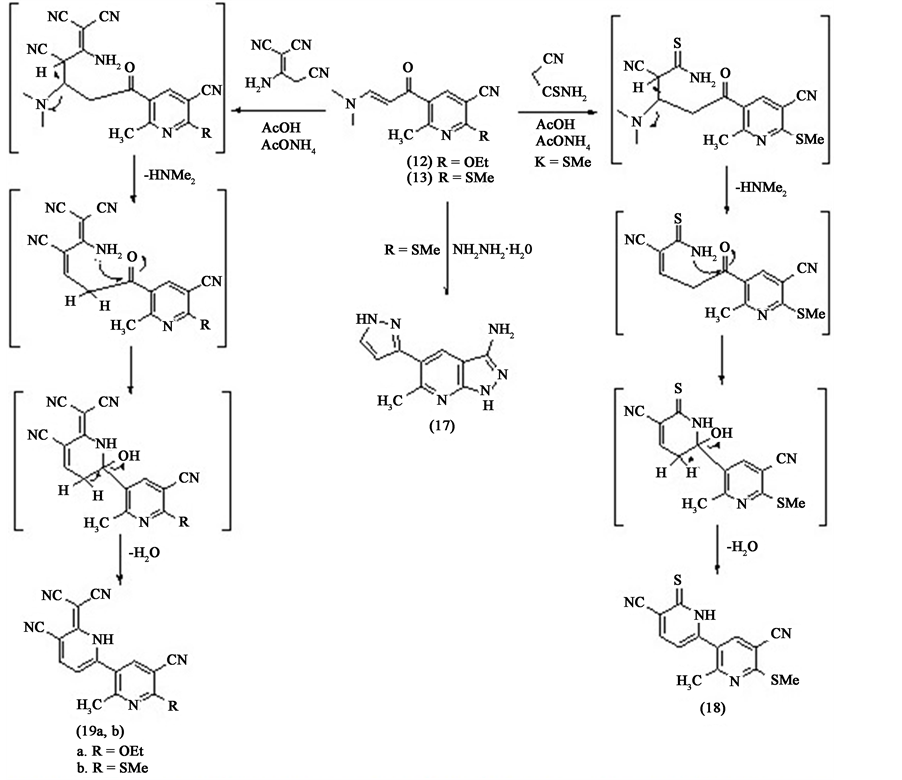
5-Acetyl-6-methyl-2-(methylthio)nicotinonitrile 7 can be used as intermediate for further preparation of heterocyclic compounds. So that compound 7 was treated with potassium carbonate in ethanol to afford 5-acetyl-2- ethoxy-6-methylnicotinonitrile 11. This compound was formed by nucleophilic substitution of SMe by OEt group. The structure of the isolated compound is confirmed by elemental and spectral analyses. The mass spectrum shows the molecular ion peak at m/e 204 corresponding to the molecular formula (C11H12N2O2). Also, the 1H NMR spectrum shows the disappearance of SMe signal and appearance of two signals; a triplet at δH = 1.43 ppm and a quartet at δH = 4.54 ppm corresponding to the OEt moiety, in addition to the rest of signals corresponding to the other protons in the molecule. Compound 11 was reacted with N,N'-dimetylformamide dimethyl acetal (DMFDMA) in dry xylene to give the corresponding enamine 12 in a good yield. The mass spectrum of compound 12 shows the molecular ion peak at m/e 259 which corresponding to the molecular formula (C14H17N3O2). Also, the 1H NMR spectrum of compound 12 shows the disappearance of the singlet signal which is related to the methyl of acetyl group and the appearance of two singlet signals at δH = 2.68 and 3.04 ppm corresponding to the two methyl groups of NMe2 moiety. Consequently the 1H NMR spectrum shows the appearance of two doublets at δH = 6.25 ppm and 7.87 ppm corresponding to the two protons of the enamine double bond.
Enamine 13 can be prepared in a good yield by reaction of 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyri-dine- 3-carbonitrile 3a with two moles of N,N'-dimetylformamide dimethylacetal (DMFDMA) in dry xylene or by the reaction of 5-acetyl-6-methyl-2-(methylthio)nicotinonitrile 7 with one mole of N,N'-dimetylformamide dimethylacetal (DMFDMA) in dry xylene. The structure of the isolated compound is confirmed by elemental and spectral analysis. Whereas the mass spectrum shows the molecular ion peak at m/e 261 which corresponding to the molecular formula (C13H15N3OS). Also, the 1H NMR spectrum of it shows the disappearance of the singlet signal which is related to the methyl of acetyl group and appearance of two singlet signals at δH = 2.62 and 2.64 ppm corresponding to the two methyl groups of NMe2 moiety. Consequently, the 1H NMR spectrum shows the appearance of two doublets at δH = 5.28 ppm and 7.75 ppm corresponding to the two protons of double bond of enamine.
Chalcone 14 can either be prepared by the reaction of compound 7 with (4-(dimethylamino)benzaldehyde) in ethanolic sodium hydroxide or by treatment of compound 9a with methyl iodide in ethanolic sodium hydroxide. The mass spectrum of compound 14 shows the molecular ion peak at m/e 337 corresponding to the molecular formula (C19H19N3OS). Also, the 1H NMR spectrum of compound 14 shows singlet signal at δH = 2.62 ppm corresponding to methyl group and singlet signal at δH = 2.66 ppm corresponding to SCH3 and two singlet signal at δH = 2.9, 3.04 ppm corresponding to NMe2 moiety and appearance of some signals of other protons in molecule.
For preparation of bipyridyl derivatives, we have carried out the reaction of chalcones 5-(3-(4-(dimethyla- mino)phenyl)acryloyl)-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 9a and 5-(3-(4-(dimethylamino) phenyl)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile 14 with malononitrile dimmer [9] in acetic acid and ammonium acetate afforded the corresponding bipyridyl derivatives 6-(dicyanomethylene)-4-(4-(dimethylami- no)phenyl)-2'-methyl-6'-thioxo-1,1',6,6'-tetrahydro-[2,3'-bipyridine]-5,5'-dicarbonitrile 15 and 6-(dicyanomethy- lene)-4-(4-(dimethylamino)phenyl)-2'-methyl-6'-(methylthio)-1,6-dihydro-[2,3'-bipyridine]-5,5'-dicarbonitrile
16 respectively. The reaction proceeds by Michael addition followed by cyclization through condensation as shown in Scheme 2.
The compound 16 can also be obtained by the reaction of 6-(dicyanomethylene)-4-(4-(dimethylami-no) phenyl)-2'-methyl-6'-thioxo-1,1',6,6'-tetrahydro-[2,3'-bipyridine]-5,5'-dicarbonitrile 15 with methyl iodide in alcoholic sodium hydroxide Scheme 2.
The structure of the isolated compounds 15 and 16 is established by elemental and spectral analysis. Whereas the mass spectra of these compounds show the molecular ion peaks at m/e 435 corresponding to the molecular formula (C24H17N7S), and at m/e 449 corresponding to the molecular formula (C25H19N7S) for 15 and 16 respectively. The IR spectra of both compounds 15 and 16 show the disappearance of the carbonyl group and the appearance of NH group. Also, the 1H NMR spectra of these compounds show signals fit to structures 15 and 16.
For further preparation of heterocyclic compounds [10] , we carried out the following reactions. The reaction of enamine 13 with excess hydrazine hydrate in ethanol afforded 6-methyl-5-(1H-pyrazol-3-yl)-1H-pyrazolo[3, 4-b]pyridin-3-amine 17 in a good yield as shown in Scheme 3. The IR spectrum of compound 17 shows the disappearance of the cyano group and the appearance of NH2 and NH groups at υmax at 3405 cm−1, 3329 cm−1 and 3136 cm−1 respectively. Also, the mass spectrum of compound 17 shows the molecular ion peak at m/e 214 corresponding to the molecular formula (C10H10N6). Also, the 1H NMR spectrum of compound 17 shows signals fit to the structure.
Scheme 2.Reactions of tetrasubstitutedpridine 7 with DMFDMA, alcholic K2CO3 and p-N, N- dimethylaminobezaldehyde.
Scheme 3. Reactions of tetrasubstitutedpridine (12,13) with cyanothioacetamide, hydrazine hydrate and malononitrile dimer.
Also, the enamine 13 is treated with cyanothioacetamide in acetic acid and ammonium acetate afforded 2'- methyl-6'-(methylthio)-6-thioxo-1,6-dihydro-[2,3'-bipyridine]-5,5'-dicarbonitrile 18. The reaction is started by Micheal addition of cyanothioacatamide on the double bond followed by elimination of dimethylamine (HNMe2) and cyclization with the carbonyl group. The structure of the isolated compound 18 is confirmed by elemental and spectral analysis. The IR spectrum of compound 18 shows the disappearance of carbonyl group and appearance of NH group at υmax at 3428 cm−1. The mass spectrum of compound 18 shows the molecular ion peak at m/e 298 corresponding to the molecular formula (C14H10N4S2). Also, the 1H NMR spectrum of compound 18 shows the disappearance of protons of NMe2 moiety and appearance of NH proton beside the other protons.
Another type of bipyridyl derivatives 19a,b can be prepared by the reaction of the enamines 12 and 13 with malononitrile dimmer in acetic acid and ammonium acetate. This reaction proceeds by Michael addition of malononitrile dimmer, followed by elimination of dimethylamine (HNMe2) and cyclization through condensation of amino group with carbonyl group as shown in Scheme 3. The mass spectrum of compound 19a shows the molecular ion peak at m/e 328 corresponding to the molecular formula (C18H12N6O), and compound 19b shows the molecular ion peak at m/e 330 corresponding to the molecular formula (C17H10N6S). The IR spectra of the compounds 19a,b shows the disappearance of the carbonyl group and the appearance of NH group beside the other groups. Also, the 1H NMR spectra of compounds 19a,b show the disappearance of protons of NMe2 moiety and appearance of NH proton beside the other protons.
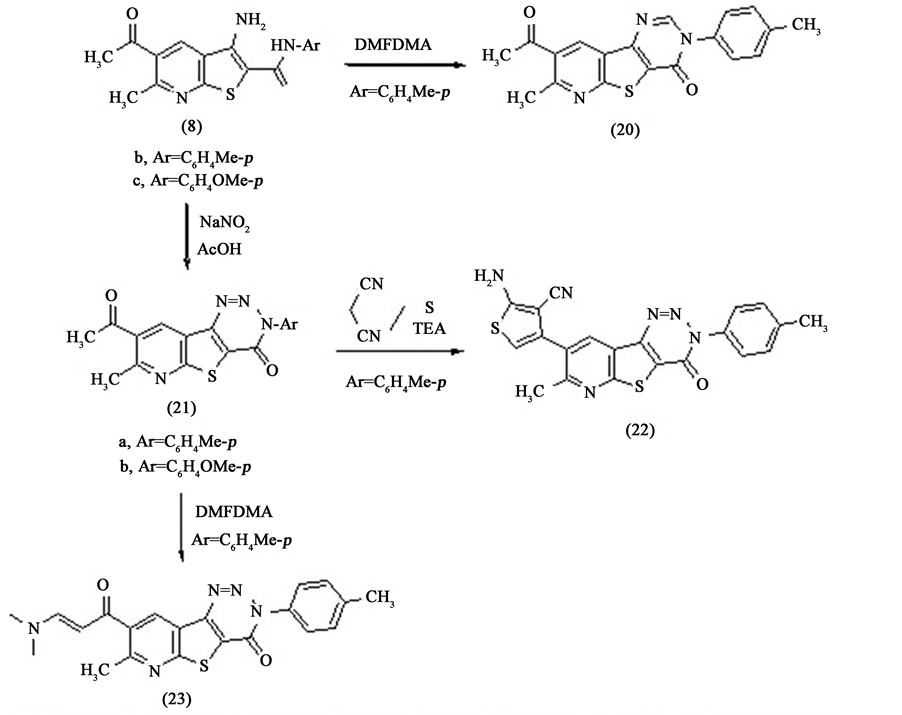
The tricyclic heterocyclic compounds are biologically interest compounds. They are examples of uncommon ring system [11] [12] . Therefore we are interested for the preparation of this type of heterocyclic compound. Thus 5-acetyl-3-amino-6-methyl-N-(p-tolyl)benzo[b]thiophene-2-carboxamide 8b is reacted with N,N'-dime- tylformamide dimethyl acetal (DMFDMA) in dry dioxane afforded 8-acetyl-7-methyl-3-(p-tolyl)pyrido [3',2':4,5] thieno[3,2-d]pyrimidin-4(3H)-one 20. The IR spectrum of compound 20 shows the disappearance of (NH2) and (NH) groups. The mass spectrum of compound 20 shows the molecular ion peak at m/e 349 which corresponding to the molecular formula (C19H15N3O2S). Also, the 1H NMR spectrum of compound 20 shows the appearance of two singlet signals at δH = 8.43 ppm, and 8.52 ppm corresponding to two protons of pyrimidinone and pyridine rings respectively beside other signals for other protons.
For further reaction of 5-acetyl-3-amino-6-methyl-N-substituted[b]thiophene-2-carboxamide 8b,c it reacted with nitrous acid in acetic acid afforded the tricyclic compounds 21a,b in a good yield as shown in Scheme 4. The structures of the compounds 21a,b are established by elemental and spectral analysis. Whereas the IR spectra of both compounds 21a,b show the disappearance of the bands corresponding to (NH2) and (NH) groups. The mass spectrum of the compound 21a as an example shows the molecular ion peak at m/e 350 corresponding to molecular formula (C18H14N4O2S).
Also, the 1HNMR spectra of compounds 21a,b show the disappearance of the signals which corresponding to (NH2) and (NH) groups beside the appearance the other signals for other groups.
We have found that the prepared tricyclic compounds 20 and 21a,b contain acetyl group which is very important for the preparation of new heterocyclic compounds. So that the reaction of 21a with malononitrile and sulphur element in ethanol and triethylamine (Geweld reaction) afforded 2-amino-4-(7-methyl-4-oxo-3-(p- tolyl)-3,4-dihydropyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-8-yl)thiophene-3-carbonitrile 22. The IR spectrum of compound 22 shows the disappearance of the carbonyl group of acetyl moiety and the appearance of amino and cyano groups at υmax at 3427 cm−1 and 2208 cm−1 respectively. Also, the mass spectrum of this compound 22 shows the molecular ion peak at m/e 430 which corresponding to the molecular formula (C21H14N6OS2).
Scheme 4. Reactions of thinopyridine derivatives (8b,c) with DMFDMA and sodium nitrile in acetic.
Also, the compound 21a is treated with N,N'-dimetylformamide dimethyl acetal (DMFDMA) in dry xylene afforded the corresponding enamine 8-(3-(dimethylamino)acryloyl)-7-methyl-3-(p-tolyl)pyrido[3',2':4,5] thie- no[3,2-d][1,2,3]triazin-4(3H)-one 23 in a good yield, Scheme 4. The mass spectrum of compound 23 shows the molecular ion peak at m/e 405 corresponding to molecular formula (C21H19N5O2S). Also, the 1H NMR spectrum of compound 23 shows the disappearance of the methyl of acetyl moiety and appearance instead of it two singlet signals at δH = 3.63 ppm and 3.67 ppm corresponding to (NMe2) moiety. Also, it shows the appearance of two doublet signals at δH = 5.42 ppm and 7.82 ppm respectively corresponding to the double bond protons of enaminone moiety beside signals for other protons.
3. Experimental
All melting points are uncorrected. IR spectra were recorded on a Perkin-Elmer 17,100 FTIR spectrometer as KBr disks. NMR spectra were recorded on a Varian Gemini (400 MHz) spectrometer for solutions in CDCl3 or DMSO-d6 with tetramethylsilane (TMS) as an internal standard unless otherwise. Mass spectra were obtained on Finnigan 4500 (low resolution) spectrometers using electron impact (EI) at Micro-analytical Center Cairo University Giza Egypt.
Preparation of 5-acetyl-3-cyano-6-methylpyridine-2(1H)-thione (3a):
A mixture of acetylacetone (1a) (1.0 g, 10 mmol), dry dioxane (10 mL) and N,N-dimethylformamide dimethyl acetal (1.19 g, 10 mmol) was stirred under dry condition at room for 24 h. In a second flask, a mixture of dry ethanol (15 mL), and sodium metal (0.46 g, 20 mmol) was left under stirring for 10 min. Then cyanothioacetamide (1.0 g, 10 mmol) was added to the mixture. The mixture was left for further 10 min. The contents of the second flask were transferred into the first flask, and the resulting mixture was stirred for further 1 h, followed by converting stirring into reflux for 4 h. After cooling, the mixture was poured onto acidified ice/cold water. The product was recovered by filtration and recrystallised from ethanol as orange crystals (76%), Mp. 232˚ - 233˚, similar to be published before [7] and mixed Mp.
Preparation of 5-acetyl-6-methyl-2-(methylthio)nicotinonitrile (7)
Mixture of 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a (1.92 g, 10 mmol) in ethanol as solvent and sodium hydroxide (0.4 g, 10 mmol) with stirring for 1 hr., and add methyl iodide (0.62 ml, 10 mmol) with stirring until precipitate formed. The product was recovered by filtration and recrystallised from ethanol as white crystals (1.52 g, 74%), Mp. 140˚C - 142˚C; 1H-NMR (CDCl3): δ = 2.54 (3H, s, CH3 py.), 2.63 (3H, s, SCH3), 2.77 (3H, s, CH3CO), 8.07 (1H, s, CH py.); IR (KBr) υ 2227 (CN), 1685 cm−1 (C=O); MS (EI)+: m/z 206 M+; Anal. Calcd for C10H10N2OS (206.27): C, 58.23; H, 4.89; N, 13.58. Found: C, 58.03; H, 4.73; N, 13.41.
General procedure for the preparation of compounds 8a-c
In dry flask, a mixture 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a (1.92 g, 10 mmol) and a-chloro compounds (10 mmol) in ethanol and sodium ethoxide (20 mmol) was left under reflux for two hours. The mixture was left for cooling and poured onto ice cold water. The solid product was recovered by filtration and recrystallised from the proper solvent.
Ethyl 5-acetyl-3-amino-6-methylthieno[2,3-b]pyridine-2-carboxylate (8a): Obtained using ethyl 2-chloroa- cetate (1.06ml, 10 mmol). The product was recrystallised from acetic acid as yellow crystals (2.16 g, 77.7%), Mp. 220˚C - 222˚C; 1H-NMR (DMSO): δ = 1.25 (3H, t, CH3 ethyl), 2.6 (3H, s, CH3 py.), 2.66 (3H, s, CH3CO), 4.25 (2H, q, CH2 ethyl), 7.29 (2H, s, NH2), 8.95 (1H, s, CH py.); IR (KBr) υ 3427, 3328 (NH2), 1679 cm−1 (C=O); MS (EI)+: m/z 278 M+; Anal. Calcd for C13H14N2O3S (278.33): C, 56.10; H, 5.07; N, 10.06, Found: C, 55.96; H, 4.94; N, 9.97.
5-Acetyl-3-amino-6-methyl-N-(p-tolyl)thieno[2,3-b]pyridine-2-carboxamide (8b): Obtained using 2-chloro- N-(p-tolyl)acetamide (1.83 g, 10 mmol). The product was recrystallised from ethanol as yellow crystals (2.7 g, 79%), Mp. 218˚C - 220˚C; 1H-NMR (DMSO) δ = 2.26 (3H, s, CH3 Ar), 2.64 (3H, s, CH3 py.), 2.73 (3H, s, CH3CO), 7.12 (2H, d, Ar), 7.47 (2H, s, NH2), 7.55 (2H, d, Ar), 9.04 (1H, s, CH py.), 9.4 (1H, s, NH); IR (KBr) υ 3428 , 3312 (NH2, NH), 1685 cm−1 (C=O); MS (EI)+: m/z 339 M+; Anal. Calcd for C18H17N3O2S (339.42): C, 63.70; H, 5.05; N, 12.38, Found: C, 63.56; H, 4.93; N, 12.15.
5-Acetyl-3-amino-N-(4-methoxyphenyl)-6-methylthieno[2,3-b]pyridine-2-carboxamide (8c):
Obtained using 2-chloro-N-(4-methoxyphenyl)acetamide (1.99 g, 10 mmol). The product was recrystallised from ethanol as yellow crystals (2.8 g, 79%), Mp. 240˚C - 242˚C; 1H-NMR (DMSO) δ = 2.65 (3H, s, CH3 py.), 2.73 (3H, s, CH3CO), 3.76 (3H, s, CH3O), 6.9 (2H, d, Ar), 7.45 (2H, s, NH2), 7.56 (2H, d, Ar), 9.04 (1H, s, CH py.), 9.4 (1H, s, NH); IR (KBr) υ 3428, 3310, 3251 (NH2, NH), 1680 cm−1 (C=O); MS (EI)+: m/z 355 M+; Anal. Calcd for C18H17N3O3S (355.42): C, 60.83; H, 4.82; N, 11.82, Found: C, 60.76; H, 4.73; N, 11.69.
General procedure for the preparation of compounds 9a,b
A mixture of 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a (1.92 g, 10 mmol) in ethanol as solvent in presence of sodium hydroxide (0.4 g, 10 mmol) with aromatic aldehydes (10 mmol) with stirring for 2hr. then poured onto ice, cold water and acidified with conc. hydrochloric acid until the precipitate was formed. The solid product was recovered by filtration and recrystallised from ethanol.
5-(3-(4-(Dimethylamino)phenyl)acryloyl)-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (9a):
Obtained using 4-(dimethylamino)benzaldehyde (1.49g, 10 mmol). Mp. 140˚C - 142˚C as yellow crystals (2.45 g, 76%); 1H-NMR (CDCl3) δ = 2.65 (3H, s, CH3), 2.79 (6H, s, NMe2), 6.70 (2H, d, Ar), 7.06 (1H, d, CH chalcone), 7.74 (2H, d, Ar), 7.85 (1H, d, CH chalcone), 8.07 (1H, s, CH py.), 13.2 (1H, br., NH); IR (KBr) υ 3437 (NH), 2225 (CN), 1685 cm−1 (C=O); MS (EI)+: m/z 323 M+; Anal. Calcd for C18H17N3OS (323.42): C, 66.85; H, 5.30; N, 12.99, Found: C, 66.69; H, 5.17; N, 12.86.
6-Methyl-2-thioxo-5-(3-(p-tolyl)acryloyl)-1,2-dihydropyridine-3-carbonitrile (9b): Obtained using 4-methyl- benzaldehyde (1.2 g, 10 mmol). Mp. = 240˚C - 242˚C as yellow crystals (2.2 g, 74.8%); 1H-NMR (DMSO) δ = 2.31 (3H, s, CH3 Ar), 2.58 (3H, s, CH3 py.), 7.24 (2H, d, Ar), 7.49 (1H, d, CH chalcone), 7.6 (1H, d, CH chalcone), 7.69 (2H, d, Ar), 8.58 (1H, s, CH py.), 13 (1H, br., NH); IR (KBr) υ 3434 (NH), 2231 (CN), 1659 cm−1 (C=O); MS (EI)+: m/z 294 M+; Anal. Calcd for C17H14N2OS (294.38): C, 69.36; H, 4.79; N, 9.52, Found: C, 69.19; H, 4.68; N, 9.45.
5-(5-Amino-4-cyanothiophen-3-yl)-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile (10)
In dry flask, a mixture 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a (1.92 g, 10 mmol), malononitrile (0.66 g, 10 mmol) and sulfur (0.32 g, 10 mmol) in ethanol and few drops of triethylamine as base was left under reflux for three hours. The mixture was left for cooling then poured onto ice cold water. The product obtained was recrystallised from a mixture of ethanol/DMF (3:1) as brown crystals (1.9 g, 69.8%), Mp. > 300˚C; 1H-NMR (DMSO) δ = 2.45 (3H, s, CH3), 6.95 (2H, s, NH2), 7.07 (1H, s, CH thiophene), 7.2 (1H, s, CH py.); IR (KBr) υ 3435, 3350 (NH2), 3250 (NH), 2210 cm−1 (CN); Anal. Calcd for C12H8N4S2 (272.35): C, 52.92; H, 2.96; N, 20.57, Found: C, 52.85; H, 2.92; N, 20.40.
5-Acetyl-2-ethoxy-6-methylnicotinonitrile (11)
In dry flask, a mixture of 5-acetyl-6-methyl-2-(methylthio)nicotinonitrile 7 (2.06 g, 10 mmol) in ethanol and potassium carbonate was left under reflux for 3hr. after cooling the mixture was poured onto ice cold water. The product was recovered and recrystallised from EtOH/H2O (1:1) as yellowish crystals (1.6 g, 78%), Mp. 78˚C - 80˚C; 1H-NMR (CDCl3) δ = 1.43 (3H, t, CH3 ethyl), 2.66 (3H, s, CH3), 3.04 (3H, s, CH3CO), 4.54 (2H, q, CH2 ethyl), 7.85 (1H, s, CH py.); IR (KBr) υ 2228 (CN), 1688 cm−1 (C=O); MS (EI)+: m/z 204 M+; Anal. Calcd for C11H12N2O2 (204.23): C, 64.69; H, 5.92; N, 13.72, Found: C, 64.51; H, 5.83; N, 13.54.
5-(3-(Dimethylamino)acryloyl)-2-ethoxy-6-methylnicotinonitrile (12)
In dry flask a mixture of 5-acetyl-2-ethoxy-6-methylnicotinonitrile 11 (2.04 g, 10 mmol) in dry xylene as solvent and N,N'-dimethylformamide dimethyl acetal (DMFDMA) (1.32 ml, 10 mmol) was left under reflux for 2 hr., cool and the solvent was evaporated. The product was recovered and recrystallised from EtOH/H2O (1:1) as yellow crystals (1.9 g, 73.3%), Mp. 68˚C - 70˚C; 1H-NMR (CDCl3) δ = 1.3 (3H, t, CH3 ethyl), 2.62 (3H, s, CH3), 2.68, 3.04 (6H, 2s, NMe2), 4.58 (2H, q, CH2 ethyl), 6.25 (1H, d, CH), 7.87 (1H, d, CH), 8.2 (1H, s, CH py.); IR (KBr) υ 2230 (CN), 1684 cm−1 (C=O); MS (EI)+: m/z 259 M+; Anal. Calcd for C14H17N3O2 (259.31): C, 64.85; H, 6.61; N, 16.20, Found: C, 64.56; H, 6.47; N, 16.11.
5-(3-(Dimethylamino)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile (13)
(A) In dry flask, a mixture of 5-acetyl-6-methyl-2-(methylthio)nicotinonitrile 7 (2.06 g, 10 mmol) in dry xylene as solvent and N,N'-dimethylformamide dimethyl acetal (DMFDMA) (1.32 ml, 10 mmol) was left under reflux for 2 hr., cool and poured in dry backer and the solvent was evaporated. The product was recovered and recrystallised from EtOH/H2O (1:1) as yellow crystals (2 g, 76.6%), Mp. 100˚C - 102˚C; (B) In dry flask a mixture of 5-acetyl-6-methyl-2-thioxo-1,2-dihydropyridine-3-carbonitrile 3a (1.92 g, 10 mmol) in dry xylene as solvent and N,N'-dimethylformamide dimethyl acetal (DMFDMA) (2.64 ml, 20 mmol) was left under reflux for 2 hr., cool and poured in dry backer and the solvent was evaporated. The product was recovered and recrystallised from EtOH/H2O (1:1) as yellow crystals (2.1 g, 80.4%), Mp. and mixed Mp. 100˚C - 102˚C; 1H-NMR (CDCl3) δ = 2.62, 2.64 (6H, 2s, NMe2), 2.9 (3H, s, CH3 py.), 3.15 (3H, s, SCH3), 5.28 (1H, d, trans CH), 6.28 (1H, d, cis CH), 7.75 (1H, d, trans CH), 8.07 (1H, s, CH py.), 10.15 (1H, d, cis CH); IR (KBr) υ 2227 (CN), 1685 cm−1 (C=O); MS (EI)+: m/z 261 M+; Anal. Calcd for C13H15N3OS (261.35): C, 59.74; H, 5.79; N, 16.08, Found: C, 59.63; H, 5.45; N, 15.8.
5-(3-(4-(Dimethylamino)phenyl)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile (14)
(A) mixture of 5-(3-(4-(dimethylamino)phenyl)acryloyl)-6-methyl-2-thioxo-1,2-dihydropyridine-3-carboni- trile 9a (3.23 g, 10 mmol) in ethanol as solvent and sodium hydroxide (0.4g, 10mmol) with stirring for 1hr., and add methyl iodide (0.62 ml, 10 mmol) with stirring until precipitate was formed. The product was recovered by filtration and was purified by recrystallised from ethanol as yellow crystals (2.5 g, 74%), Mp. 160˚C - 162˚C; (B) mixture of 5-acetyl-6-methyl-2-(methylthio)nicotinonitrile 7 (2.06 g, 10 mmol) in ethanol as solvent in presence of sodium hydroxide (0.4 g, 10 mmol) with 4-(dimethylamino)benzaldehyde (1.49 g, 10 mmol) with stirring for 2 hr., until precipitate formed and dilute with water. The product was recovered by filtration and purified by recrystallised from ethanol as yellow crystals (2.4 g, 71%), Mp. and mixed Mp. 160˚C - 162˚C; 1H-NMR (CDCl3) δ = 2.62 (3H, s, CH3), 2.66 (3H, s, SCH3), 2.9, 3.04 (6H, 2s, NMe2), 6.83 (2H, d, Ar), 7.46 (2H, d, Ar), 6.67 (1H, d, CH), 7.38 (1H, d, CH), 7.85 (1H, s, CH py.); IR (KBr) υ 2217 (CN), 1648 cm−1 (C=O); MS (EI)+: m/z 337 M+; Anal. Calcd for C19H19N3OS (337.45): C, 67.63; H, 5.68; N, 12.45, Found: C, 67.49; H, 5.62; N, 12.48.
6-(Dicyanomethylene)-4-(4-(dimethylamino)phenyl)-2'-methyl-6'-thioxo-1,1',6,6'-tetrahydro-[2,3'-bipyridine]-5,5'-dicarbonitrile (15)
In dry flask, a mixture 5-(3-(4-(dimethylamino)phenyl)acryloyl)-6-methyl-2-thioxo-1,2-dihydropyridine-3- carbonitrile 9a (3.23 g, 10 mmol) and malononitrile dimmer (1.32 g, 10 mmol) in acetic acid and presence of ammonium acetate was left under reflux for three hours. The mixture was left for cooling and poured onto ice, cold water. The product was recovered by filtration and recrystallisation from ethanol as brown crystals (3.25 g, 74.7%), Mp. 260˚C - 262˚C; 1H-NMR (DMSO) δ = 2.38 (3H, s, CH3), 3.06 (6H, s, NMe2), 6.83 (2H, d, Ar), 7.5 (1H, s, CH py.), 7.93 (2H, d, Ar), 8.21 (1H, s, CH py.), 11.93 (1H, br, NH), 12.4 (1H, br, NH); IR (KBr) υ 3334, 3207 (2NH), 2206 cm−1 (CN); MS (EI)+: m/z 435 M+; Anal. Calcd for C24H17N7S (435.51): C, 66.19; H, 3.93; N, 22.51, Found: C, 66.06; H, 3.78; N, 22.35.
6-(Dicyanomethylene)-4-(4-(dimethylamino)phenyl)-2'-methyl-6'-(methylthio)-1,6-dihydro-[2,3'-bipyridine]-5,5'-dicarbonitrile (16)
(A) In dry flask a mixture of 5-(3-(4-(dimethylamino)phenyl)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile 14 (3.37 g, 10 mmol) and malononitrile dimmer (1.32 g, 10 mmol) in acetic acid acid and ammonium acetate was left under reflux for four hours, cool. The solid product was recovered by filtration and recrystallised from acetic acid as brown crystals (3.4 g, 76%), Mp. 220˚C - 222˚C; (B) mixture of 6-(dicyanomethylene)-4-(4-(di- methylamino)phenyl)-2'-methyl-6'-thioxo-1,1',6,6'-tetrahydro-[2,3'-bipyridine]-5,5'-dicarbonitrile (15) (4.35 g, 10 mmol) in ethanol as solvent in presence of sodium hydroxide (0.4 g, 10mmol) and methyl iodide (0.62 ml, 10 mmol) with stirring until precipitate formed. The product was recovered by filtration and recrystallised from acetic acid as brown crystals (3.2 g, 71.5%), Mp. and mixed Mp. 220˚C - 222˚C; 1H-NMR (DMSO) δ = 2.61 (3H, s, CH3), 2.65 (3H, s, SCH3), 2.99, 3.01 (6H, 2s, NMe2), 6.82 (2H, d, Ar), 7.09 (1H, s, CH py.), 7.73 (2H, d, Ar), 8.66 (1H, s, CH py.), 10.3 (1H, br, NH); IR (KBr) υ 3345 (NH), 2213 cm−1 (CN); MS (EI)+: m/z 449 M+; Anal. Calcd for C25H19N7S (449.54): C, 66.80; H, 4.26; N, 21.81, Found: C, 66.69; H, 4.18; N, 21.66.
6-Methyl-5-(1H-pyrazol-3-yl)-1H-pyrazolo[3,4-b]pyridin-3-amine (17)
In flask a mixture of 5-(3-(dimethylamino)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile 13 (2.61 g, 10 mmol) and excess of hydrazine hydrate was left under reflux for four hours, cool. The solid product was recovered by filtration and recrystallised from ethanol as yellowish crystals (1.6 g, 75%), Mp. 260˚C - 262˚C; 1H- NMR (DMSO) δ = 2.49 (3H, s, CH3), 5.54 (2H, s, NH2), 6.47 (1H, d, CH pyrazole), 7.8 (1H, s, CH py.), 8.3 (1H, d, CH pyrazole), 11.75 (1H, s, NH), 12.91 (1H, s, NH); IR (KBr) υ at 3405, 3329, 3136 cm−1 (NH2, NH); MS (EI)+: m/z 214 M+; Anal. Calcd for C10H10N6 (214.23): C, 56.07; H, 4.71; N, 39.23, Found: C, 55.85; H, 4.56; N, 39.16.
2'-Methyl-6'-(methylthio)-6-thioxo-1,6-dihydro-[2,3'-bipyridine]-5,5'-dicarbonitrile (18)
In dry flask a mixture of 5-(3-(dimethylamino)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile 13 (2.61 g, 10 mmol) and cyanothioacetamide (1 g, 10 mmol) in acetic acid and ammonium acetate was left under reflux for four hours. Cool and poured the mixture onto ice cold water. The product was recovered by filtration and recrystallised from ethanol as brown crystals (2.3 g, 77.1%), Mp. 170˚C - 172˚C; 1H-NMR (DMSO) δ = 2.63 (3H, s, CH3), 2.65 (3H, s, SCH3), 7.7 (1H, d, CH py.), 8 (1H, d, CH py.), 8.14 (1H, s, CH py.), 12.25 (1H, br, NH); IR (KBr) υ = 3428 (NH), 2221 cm−1 (CN); MS (EI)+: m/z 298 M+; Anal. Calcd for C14H10N4S2 (298.39): C, 56.35; H, 3.38; N, 18.78, Found: C, 56.12; H, 3.24; N, 18.58.
General procedure for the preparation of compounds 19a,b
In dry flask, a mixture of 5-(3-(dimethylamino)acryloyl)-2-ethoxy-6-methylnicotinonitrile 12 (2.59 g, 10 mmol) or 5-(3-(dimethylamino)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile 13 (2.61 g, 10 mmol) and malononitrile dimmer (1.32 g, 10 mmol) in acetic acid and ammonium acetate was heated under reflux for four hours, cool. The solid product was recovered by filtration and recrystallised from ethanol
6-(Dicyanomethylene)-6'-ethoxy-2'-methyl-1,6-dihydro-[2,3'-bipyridine]-5,5'-dicarbonitrile (19a):
Obtained using 5-(3-(dimethylamino)acryloyl)-2-ethoxy-6-methylnicotinonitrile 12. Mp. 200˚C - 202˚C as brown crystals (2.4 g, 73.1%); 1H-NMR (DMSO) δ = 1.39 (3H, t, CH3), 2.62 (3H, s, CH3), 4.50 (2H, q, CH2), 7.58 (1H, d, CH py.), 8.48 (1H, d, CH py.), 8.7 (1H, s, CH py.), 11.3 (1H, br, NH); IR (KBr) υ 3330 (NH), 2218 cm−1 (CN); MS (EI)+: m/z 328 M+; Anal. Calcd for C18H12N6O (328.34): C, 65.85; H, 3.68; N, 25.60, Found: C, 65.71; H, 3.52; N, 25.43.
6-(Dicyanomethylene)-2'-methyl-6'-(methylthio)-1,6-dihydro-[2,3'-bipyridine]-5,5'-dicarbonitrile
(19b): Obtained using 5-(3-(dimethylamino)acryloyl)-6-methyl-2-(methylthio)nicotinonitrile 13. Mp. 190˚C - 192˚C as brown crystals (2.3 g, 69.7%); 1H-NMR (DMSO) δ = 2.58 (3H, s, CH3), 2.64 (3H, s, SCH3), 6.5 (1H, d, CH py.), 8.2 (1H, d, CH py.), 8.69 (1H, s, CH py.), 11.31 (1H, br, NH); IR (KBr) υ 3340 (NH), 2212 cm−1 (CN); MS (EI)+: m/z 330 M+; Anal. Calcd for C17H10N6S (330.37): C, 61.80; H, 3.05; N, 25.44, Found: C, 61.63; H, 2.89; N, 25.27.
8-Acetyl-7-methyl-3-(p-tolyl)pyrido[3',2':4,5]thieno[3,2-d]pyrimidin-4(3H)-one (20)
A mixture of 5-acetyl-3-amino-6-methyl-N-(p-tolyl)thieno[2,3-b]pyridine-2-carboxamide 8b (3.39 g, 10 mmol) in dry dioxane and DMFDMA (1.32 ml, 10 mmol) with stirring for 12 hrs. The product was recovered by filtration and recrystallised from acetic acid as gray crystals (2.6 g, 74.5%), Mp. 200˚C - 202˚C; 1H-NMR (DMSO) δ 2.26 (3H, s, CH3 Ar), 2.68 (3H, s, CH3 py.), 2.69 (3H, s, CH3CO), 7.16 (2H, d, Ar), 7.52 (2H, d, Ar), 8.43 (1H, s, CH pyrimidinone), 8.52 (1H, s, CH py.); IR (KBr) υ at 1691, 1649 cm−1 (2C=O); MS (EI)+: m/z 349 M+; Anal. Calcd for C19H15N3O2S (349.41): C, 65.31; H, 4.33; N, 12.03, Found: C, 65.19; H, 4.26; N, 11.95.
General procedure for the preparation of compounds 21a,b
A mixture of N-substituted-5-acetyl-3-amino-6-methylthieno[2,3-b]pyridine-2-carboxamide 8b,c (10 mmol) in acetic acid and sodium nitrite (1.38 g, 20mmol) with stirring for 1 hr. the precipitate was formed and dilute with water. The product was recovered by filtration and recrystallised from ethanol.
8-Acetyl-7-methyl-3-(p-tolyl)pyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-4(3H)-one (21a): Obtained using 5- acetyl-3-amino-6-methyl-N-(p-tolyl)thieno[2,3-b]pyridine-2-carboxamide 8b (3.39g, 10 mmol). Mp. 170˚C - 172˚C as gray crystals (3 g, 85.7%); 1H-NMR (DMSO) δ = 2.4 (3H, s, CH3 Ar), 2.74 (3H, s, CH3 py.), 2.77 (3H, s, CH3CO), 7.4 (2H, d, Ar), 7.54 (2H, d, Ar), 9.17 (1H, s, CH py.); IR (KBr) υ 1700, 1687 cm−1 (2C=O); MS (EI)+: m/z 350 M+; Anal. Calcd for C18H14N4O2S (350.40): C, 61.70; H, 4.03; N, 15.99, Found: C, 61.56; H, 3.94; N, 15.78.
8-Acetyl-3-(4-methoxyphenyl)-7-methylpyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-4(3H)-one (21b): Obtained using 5-acetyl-3-amino-N-(4-methoxyphenyl)-6-methylthieno[2,3-b]pyridine-2-carboxamide 8c (3.55 g, 10
mmol). Mp. = 220˚C - 222˚C as gray crystals (2.9 g, 79.4%); 1H-NMR (DMSO) δ = 2.74 (3H, s, CH3 py.), 2.81 (3H, s, CH3CO), 3.85 (3H, s, CH3O), 7.15 (2H, d, Ar), 7.61 (2H, d, Ar), 9.26 (1H, s, CH py.); IR (KBr) υ 1687 cm−1 (2C=O); Anal. Calcd for C18H14N4O3S (366.40): C, 59.01; H, 3.85; N, 15.29, Found: C, 58.90; H, 3.76; N, 15.17.
2-Amino-4-(7-methyl-4-oxo-3-(p-tolyl)-3,4-dihydropyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-8-yl)thiophene-3-carbonitrile (22):
In dry flask a mixture 8-acetyl-7-methyl-3-(p-tolyl)pyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-4(3H)-one 21a (3.5g, 10 mmol), malononitrile (0.66 g, 10 mmol) and elemental sulfer (0.32 g, 10mmol) in ethanol and few drops of triethylamine as base was heated under reflux for three hours. The mixture was left for cooling and poured onto ice cold water. The product was recovered by filtration and recrystallised from a mixture of ethanol/DMF (3:1) as brown crystals (3 g, 69.7%), M.p 260˚C - 262˚C; IR (KBr) υ 3427 (NH2), 2208 (CN), 1683 cm−1 (C=O); MS (EI)+: m/z 430 M+; Anal. Calcd for C21H14N6OS2 (430.51): C, 58.59; H, 3.28; N, 19.52, Found: C, 58.43; H, 3.14; N, 19.36.
8-(3-(Dimethylamino)acryloyl)-7-methyl-3-(p-tolyl)pyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-4(3H)-one (23):
In dry flask a mixture 8-acetyl-7-methyl-3-(p-tolyl)pyrido[3',2':4,5]thieno[3,2-d][1,2,3]triazin-4(3H)-one 21a (3.5 g, 10 mmol) and DMFDMA (1.32 ml, 10 mmol) in dry dioxane was left under reflux for two hours. The mixture was left for cooling and evaporates the solvent. The product was recovered by filtration and recrystallised from ethanol as brown crystals (2.9 g, 71.6%), Mp. 210˚C - 212˚C; 1H-NMR (DMSO) δ = 2.39 (3H, s, CH3 Ar), 2.66 (3H, s, CH3 py.), 3.63, 3.67 (6H, 2s, NMe2), 5.42 (1H, d, CH), 7.41 (2H, d, Ar), 7.54 (2H, d, Ar), 7.82 (1H, d, CH), 9.12 (1H, s, CH py.); IR (KBr) υ 1693, 16.44 cm−1 (2C=O); MS (EI)+: m/z 405 M+; Anal. Calcd for C21H19N5O2S (405.48): C, 62.21; H, 4.72; N, 17.27, Found: C, 62.08; H, 4.59; N, 17.11.
4. Conclusion
From the biological importance of pyridine-2(1H)-thione derivatives, we have used it in order for the preparation of biologically important bipyridyles, bi- and uncommon tricyclic compounds.
References
- Granik, V.G., Zhidkova, A.M. and Glushkov, R.G. (1977) Advances in the Chemistry of the Acetals of Acid Amides and Lactams. Russian Chemical Reviews, 46, 361. http://dx.doi.org/10.1070/RC1977v046n04ABEH002137
- Abdulla, R.F. and Brinkmeyer, R.S. (1979) The Chemistry of Formamide Acetals. Tetrahedron, 35, 1675-1735.
- Anelli, P.L., Brocchetta, M., Palano, D. and Visigalli, M. (1997) Mild Conversion of Primary Carboxamides into Carboxylic Esters. Tetrahedron Letters, 38, 2367-2368. http://dx.doi.org/10.1016/S0040-4039(97)00350-X
- Malesic, M., Krbavcic, A., Golobic, A., Golic, L. and Stanovenik, B. (1997) The Synthesis and Transformation of Ethyl 2-(2-acetyl-2-benzoyl-1-ethenyl)amino-3-dimethylaminopropenoate. A New Synthesis of 2,3,4-Trisubstituted Pyrroles. Journal of Heterocyclic Chemistry, 34, 1757-1762.
- Abu-Shanab, F.A., Elnagdi, M.H., Aly, F.M. and Wakefield, B.J. (1994) α,α-Dioxoketene Dithioacetals as Starting Materials for the Synthesis of Polysubstituted Pyridines. Journal of the Chemical Society, Perkin, 1, 1449-1452. http://dx.doi.org/10.1039/p19940001449
- Abu-Shanab, F.A., Redhouse, A.D., Thompson, J.R. and Wakefield, B.J. (1995) Synthesis of 2,3,5,6-Tetrasubstituted Pyridines from Enamines Derived from N,N-Dimethylformamide Dimethyl Acetal. Synthesis, 5, 557-560. http://dx.doi.org/10.1055/s-1995-3954
- Abu-Shanab, F.A., Aly, F.M. and Wakefield, B.J. (1995) Synthesis of Substituted Nicotinamides from Enamines De- rived from N,N-Dimethylformamide Dimethyl Acetal. Synthesis, 8, 923-925.
- Abu-Shanab, F.A., Hessen, A.M. and Mousa, S.A.S. (2007) Dimethylformamide Dimethyl Acetal in Heterocyclic Syn- thesis: Synthesis of Polyfunctionally Substituted Pyridine Derivatives as Precursors to Bicycles and Polycycles. Journal of Heterocyclic Chemistry, 44, 787-791. http://dx.doi.org/10.1002/jhet.5570440406
- Carboni, R.A., Conffman, D.D. and Howard, E.G. (1958) Cyanocarbon Chemistry. XI.1 Malononitrile Dimer. Journal of the American Chemical Society, 80, 2838-2840. http://dx.doi.org/10.1021/ja01544a061
- Abu-Shanab, F.A., Sherif, S.M. and Mousa, S.A.S. (2009) Dimethylformamide Dimethyl Acetal as a Building Block in Heterocyclic Synthesis. Journal of Heterocyclic Chemistry, 46, 801-827. http://dx.doi.org/10.1002/jhet.69
- Melani, F., Cecchi, L., Colotta, V., Filacchini, G., Martini, C., Giannicini, G. and Lucacchini, A. (1989) Dipyrazolo[5,4- b:3',4'-d]pyridines. Synthesis, Inhibition of Benzodiazepine Receptor Binding and Structure-Activity Relationships. Farmaco, 44, 585-594.
- Abu-Shanab, F.A.M., Mousa, S.A.S., Eshak, E.A., Sayed, A.Z. and Al-Harrasi, A. (2011) Dimethylformamide Dime- thyl Acetal (DMFDMA) in Heterocyclic Synthesis: Synthesis of Polysubstituted Pyridines, Pyrimidines, Pyridazine and Their Fused Derivatives. International Journal of Chemistry, 1, 207-214.
NOTES
*Corresponding author.