Journal of Analytical Sciences, Methods and Instrumentation
Vol. 3 No. 2 (2013) , Article ID: 32759 , 5 pages DOI:10.4236/jasmi.2013.32013
Assessment of Commercial Specific IgE Assay for Detection of Allergens in Allergic Patients
![]()
Allergy &Immunology Unit Ain, Shams University Hospitals, Cairo, Egypt.
Email: mohamedyousef91@yahoo.com, dr.hishamsanad@gmail.com
Copyright © 2013 Mohamed Yousef Attia, Hesham Sanad Mohamed. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Received February 18th, 2013; revised April 10th, 2013; accepted May 14th, 2013
Keywords: Specific IgE; ELISA; RAST; Elimination Test
ABSTRACT
Allergy is a serious health problem throughout the world, affecting people of all ages. Allergic diseases such as asthma, rhinitis and atopic dermatitis are becoming an epidemic in our country, the cost of investigating these diseases is increasing and becoming expensively high. There are many ways to explore allergenic antibodies to assess the presence the amount of specific IgE as: Skin test (Prick), Specific IgE (ELISA), RAST Sp.IgE and Elimination Challenge method. Skin test produces pain, local and anaphylactic reaction and patient discomfort, other procedure with highly cost to patient. So, we have built up an evidence to use it in our allergy and immunology clinic has suitable cost for all patients based about for the other tests but it was very simple, accurate, cheap and does not produce any problems to patients. Our test depends on ELISA technique, it measures the quantitative amount of the following different allergens: As regard food allergens: Milk, Eggs, Banana, Maize, Fish, Chocolate, Wheat, Nuts, Strawberry, Shrimps, Spices, with drug as Aspirin. As regard inhalants as: House dust, Mite, Mixed Pollens, Mixed Moulds, Hay dust, Wool, Latex and Cat Hair. 150 allergic patient results of our test compared with specific IgE national kits (ELISA), Sp.IgE (RAST), Skin test and elimination challenge test. The statistical evaluation results as regard senistivity respectively: 88.9%, 89.6%, 91.2%, 71.4%, 93.1%. As regard specificity the results: 93.1%, 94.7%, 95.3%, 65.5%, 91.6%, the latter results showed that our test was in line with all tests. Our test was probably in food, drug and inhalant allergens, it can be also noted that the cheapest and most commercial technique was used, so as to be available immediately in standard laboratory and its reagents, plate and other requirements were prepared locally in our laboratory. Now it was applying successfully in allergy and immunology unit Ain Shams university hospitals in Egypt.
1. Introduction
The prevalence of allergy appears to be increasing. Hypersensitivity reactions to different allergens account for significant morbidity and mortality [1]. The accurate diagnosis of allergic disease using allergen-specific IgE can be detected by skin prick testing and by blood specific IgE testing (i.e., serum RAST) [2-4]. However, a food elimination/challenge trial is a reliable way to confirm a food allergy suggested by [3]. Skin testing remains an essential diagnostic tool in modern allergy practice. A significant variability has been reported regarding technical procedures using extracts of Foods and inhalant allergens extract (1:10 wt/vol)., interpretation of results, Reaction, pain introduced and documentation [1,5]. Several reports indicated a number of analytical measurements are used to promote more accurate diagnosis and better management of allergic individuals measure different subsets of the IgE antibody response [6].
The aim of this study is to evaluate sensitivity, specificity and validity of a modification ELISA specific IgE, whereas all its reagents and plates were used made locally in our laboratory by comparing with other national tests as: Skin test, Specific IgE, RAST test and elimination challenge test.
2. Material and Method
In the present work, 150 patients were selected from different sectors ages and gender, suffering from allergy. Investigations were carried out at allergy & Immunology clinic Ain-Shams Hospitals (in period from Feb. 2009 to April 2012).
2.1. Consents
Discussion with the patients as regards the benefits of the study was done and informed consents were provided.
For each patient, the following investigations were conducted:
1) Clinical examination and Routine investigations to exclude any side disease might cause allergy 2) Skin prick test: using allergen extract: Epicutaneous prick method was done using allergen extracts. All allergen extracts were prepared in Ain Shams Allergy and Immunology Extract Unit by aqueous vaccine, Positive (histamine) and negative (coca solution) controls were included in the prick test and patients with dermographism were excluded [7].
3) Specific IgE test (National kits) for allergens was done for patients. It was done by AlaSTAT Micro plate Allergen specific IgE (RIDASCREEN Spezifisches IgE, Germany) as used by [8,9].
4) Specific IgE by RAST technique [3,9].
150 allergic patient results of our test compared with specific IgE national kits (ELISA), Sp.IgE (RAST), Skin test and elimination elimenation challenge test.
2.2. Commercial Specific IgE Assay
This was done according to [10] and Modified by Dr. Mohamed yousef & Dr. Hesham Sanad (2012).
Extraction of Allergens Were Used in Coating Microplate: As protocol Followed in Allergy & Immunology Unit Ain Shams University Univeristy Hospitals.
Many allergens were used in our test according to the methods of: [7,8] as the following: as regard food allergens: Milk, Eggs, Banana, Maize, Fish, Chocolate, Wheat, Nuts, Strawberry, Shrimps, Spices, with drug as Aspirin. As regard inhalants as: House dust, Mite, Mixed Pollens, Mixed Moulds, Hay dust, Wool, Latex and Cat Hair.
2.2.1. Ligand-Coated Microplate
A 96-well polystyrene microplate, the plate consists of twelve removable strips mounted in a frame. Each strip includes eight ligand-coated flat-bottom wells. Well positions are indexed by a system of letters and numbers (A through H, 1 through 12) embossed on the left and top edges of the frame. The Antigen Coated Procedure by Pipetting 300 μL of each specific antigen in labeled wells (Foods: Milk, Eggs, Banana, Maize, Fish, Chocolate, Wheat, Nuts, Strawberry, Shrimps, Spices, Aspirin & Inhalants as: House dust, Mite, Mixed Pollens, Mixed Moulds, Hay dust, Wool, Latex and Cat Hair) and 100 μL of human albumin for fixation of antigen in the wall and bottom of wells and forand Left for 72 hours for completely coated in the wells. The plate was washed in washer three times using 400 ml. distilled water. The plate was put in incubator at 37˚C to dry and packaged in a zip-lock foil bag, with desiccant. Store refrigerated and protected from moisture, stable at 2˚C - 8˚C until the expiration date marked on the bag.
2.2.2. Materials Provided
1) Microplate coated with different labelled allergens.
2) Enzyme Conjugate: The allergen/IgE complexes thus linked to the microplate wells are reacted with horseradish peroxidase-labeled monoclonal anti-IgE during a third 1-hour incubation, after which excess enzyme label is washed away.
3) Buffered Wash Solution Concentrate: One vial containing 85 mL of a concentrated (10%) buffered saline solution, with surfactants and preservative. Using a transfer container, the contents of the vial were diluted with distilled water for a total volume of 850 mL. Store 2˚C - 8˚C.
4) TMB Substrate Solution: One brown vial containing 55 mL of 3,3',5,5'-tetra methyl benzidine (TMB) in buffered hydrogen peroxide solution, ready to use. Store at 2˚C - 8˚C.
5) IgE Calibrators: One set of six vials, labeled A through F, each containing 1.6 mL, with preservative. The calibrators contain, respectively, 0.35, 0.7, 3.5, 17.5, 52.5, and 100 kilo units of IgE per liter (KU/L). Store at 2˚C - 8˚C.
6) Stop Solution: vail contain Hydrochloric acid (1 M HCL).
3. Specimen Collection
The patient need not be fasting, and no special preparations are necessary. Blood was collected by venipuncture into plain tubes avoiding hemolysis and the serum was separated from the cells. Samples may be stored refrigerated at 2˚C - 8˚C for 2 days or up to 2 months frozen at −20˚C. Samples were allowed to come to room temperature before assayed.
4. Procedure
All components must be at room temperature (15˚C - 28˚C) before use.
1) 50 mL of each calibrator was added into the wells prepared.
2) 50 mL of the Individuals Serum was pipetted to the specified wells.
3) 50 mL of the Congugate was pipetted into all wells of Calibrators and serum wells 4) The plate was rotated on the micromix for 45 min. and incubated at 37˚C.
5) Decanted, then the plate was washed 4 times with the microwash, each time with 300 mL buffered wash solution. Before TMB substrate was added, the plate was wrapped up with absorbent paper to stroke off all residual droplets.
6) 100 mL of TMB substrate solution was added to all wells.
7) The Plate was incubated for 15 min.
8) 50 mL of Stopping solution was added to all wells 9) The microplate was read, immediately for 5 minutes in the microplate reader at 450 nm.
4.1. Calculation
A standard curve was plotted using log-log graph paper. The average mOD/min. of each calibrator on the vertical axis was plotted against concentration on the horizontal axis. A straight line segments connected adjacent plotted values, then the allergen-specific IgE concentration for the patient samples was estimated by interpolation.
4.2. Expected Values
Quantitative values and interpretation of class results are provided in the table below:
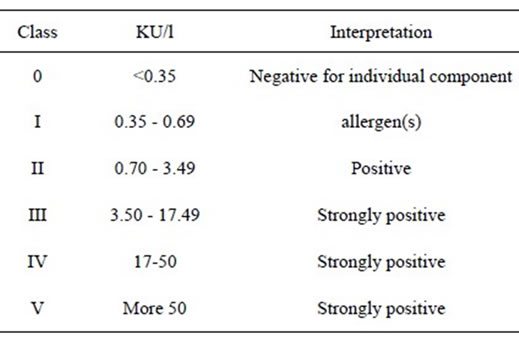
4.3. Sensitivity
Sensitivity is unique as a result of the high IgE-binding capacity of the system, and also because the soluble matrix is able to support allergens which are carbohydrates, in addition to proteins and nucleic acids.
4.4. Specificity
The system is not subject to interference from high total IgE level and other nonspecific binding problems which affect solid-phase systems. See Tables 1-4.
5. Results
Statistical analysis: Data were analyzed using the SPSS program Version 15 as follows.
6. Discussion
There are many ways to explore allergenic antibodies to assess the presence the amount of specific IgE as: Skin test (Prick), Specific IgE (ELISA), RAST Sp.IgE and Elimination Challenge method [3,11-14]. Skin test produces pain, local and anaphylactic reaction and patient discomfort, other procedure with highly cost to patient. So, we have built up an evidence to use it in our allergy and immunology clinic has suitable cost for all patients based about for the other tests but it was very simple, accurate, cheap and does not produce any problems to patients. Our test depends on ELISA technique, it measures the quantitative amount of the following different allergens: As regard food allergens: Milk, Eggs, Banana, Maize, Fish, Chocolate, Wheat, Nuts, Strawberry, Shrimps, Spices, with drug as Aspirin. As regard inhalants as: House dust, Mite, Mixed Pollens, Mixed Moulds, Hay dust, Wool, Latex and Cat Hair. 150 allergic patient results of our test compared with specific IgE national

Table 1. Comparison between the value of National sp.IgE, Egyptian commercial sp.IgE, Skin test and RAST in diagnosis of allergens in allergic patients.

Table 2. The validity of Egyptian commercial sp.IgE test in comparision to elimination-challenge test (confirmatory).
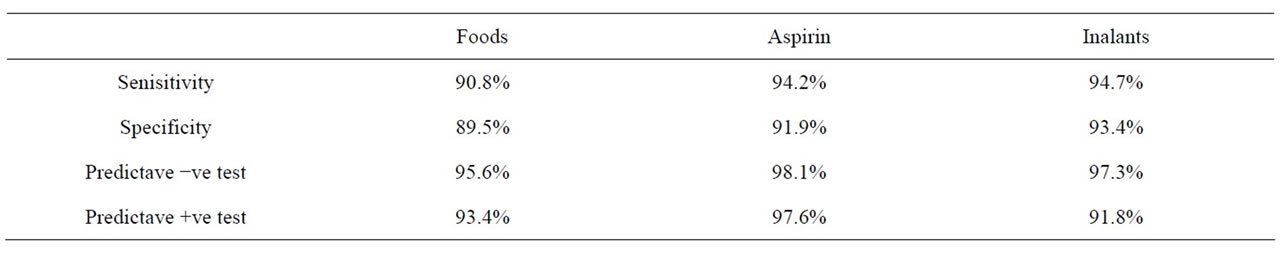
Table 3. Validity of Egyptian commercial sp.IgE test in comparision to elimination-challenge test in foods, aspirin & inhalants.

Table 4. Comparison between National sp.IgE and Egyptian commercial sp.IgE as regard many variables.
kits (ELISA), Sp.IgE (RAST), Skin test and elimination challenge test.
The statistical evaluation results as regard sensitivity respectively: 88.9%, 89.6%, 91.2%, 71.4%, 93.1%. As regard specificity the results: 93.1%, 94.7%, 95.3%, 65.5%, 91.6%, the latter results showed that our test was in line with all tests. In conclusion: our test was probably in food, drug and inhalant allergens, it can be also noted that the cheapest and most commercial technique was used, so as to be available immediately in standard laboratory and its reagents, plate and other requirements were prepared locally in our laboratory. Now it was applying successfully in allergy and immunology unit Ain Shams university hospitals in Egypt.
REFERENCES
- R. Asero, G. Mistrello, R. Ariano, G. Colombo, M. E. Conte, M. Crivellaro, M. De Carli, F. D. Torre, F. Emiliani, F. L. Rizzini, R. Longo, D. Macchia, P. Minale, F. Murzilli, F. Nebiolo, O. Quercia, G. E. Senna and D. Villalta, “Shrimp Allergy in Italian Adults: A Multicenter Study Showing a High Prevalence of Sensitivity to Novel High Molecular Weight Allergens,” International Archives of Allergy and Immunology, Vol. 157, 2012, pp. 3- 10. doi:10.1159/000324470
- B. Manuel, I. Victor, M. María, H. Pinto, F. Carballada, C. Clara and C. Jerónimo, “Seafood Hypersensitivity in Mite Sensitized Individuals: Is Tropomyosin the Only Responsible Allergen?” Annals of Allergy, Vol. 106, No. 3, 2011, pp. 223-229.
- A. J. Douglass and E. R. O’Hehir, “Diagnosis, Treatment and Prevention of Allergic Disease: The Basics,” Medical Journal Australia, Vol. 185, No. 4, 2006, pp. 228-233.
- D. J. Hill, R. G. Heine and C. S. Hosking, “The Diagnostic Value of Skin Prick Testing in Children with Food Allergy,” Pediatric Allergy and Immunology, Vol. 15, No. 5, 2004, pp. 435-441. doi:10.1111/j.1399-3038.2004.00188.x
- C. A. Yang, et al., “Measurement of IgE Antibodies to Shrimp Tropomyosin Is Superior to Skin Prick Testing with Commercial Extract and Measurement of IgE to Shrimp for Predicting Clinically Relevant Allergic Reactions after Shrimp Ingestion,” The Journal of Allergy and Clinical Immunology, Vol. 125, No. 4, 2010, pp. 872-878.
- A. L. Lopata, R. E. O’Hehir and S. B. Lehrer, “Shellfish Allergy,” Clinical & Experimental Allergy, Vol. 40, No. 6, 2010, pp. 850-858. doi:10.1111/j.1365-2222.2010.03513.x
- R. G. Slavin and R. E. Reisman, “Expert Guide to Allergy and Immunology,” Fulcrum Data Services, Philadelphia, 2001.
- K. R. Krishnan, E. L. Peterson and G. Wegienka, “Pattern of Allergen-Specific IgE Sensitization Relative to Total Serum Ig Concentration in Young Adults,” Annals of Allergy, Asthma & Immunology, Vol. 105, No. 5, 2010, pp. 401-403. doi:10.1016/j.anai.2010.09.014
- R. Hamilton, “Proficiency Survey-Based Evaluation of Clinical Total and Allergen-Specific IgE Assay Performance,” Archives of Pathology & Laboratory Medicine, Vol. 134, No. 7, 2010, pp. 975-982.
- El-Shami and Aloba, “Liquid Phase in Vitro Allergen IgE Assay with Situimmobilization,” Advanced Biological Sciences, Vol. 74, 1989, pp. 191-201.
- C. Garcia-Ara, J. Boyano-Wang, J. H. Godbold and H. A. Sampson, “Correlation of Serum Allergy (IgE) Tests Performed by Different Assay Systems,” The Journal of Allergy and Clinical Immunology, Vol. 121, No. 5, 2008, pp. 1219-1224. doi:10.1016/j.jaci.2007.12.1150
- S. Celik-Bilgili, A. Mehl, A. Verstege, U. Staden, M. Nocon, K. Beyer, et al., “The Predictive Value of Specific Immunoglobulin E Levels in Serum for the Outcome of Oral Food Challenges,” Clinical & Experimental Allergy, Vol. 35, No. 3, 2005, pp. 268-273. doi:10.1111/j.1365-2222.2005.02150.x
- J. M. Diaz-Pena, F. Martin-Munoz, M. Reche-Frutos and M. Martin-Esteban, “Specific IgE Levels in the Diagnosis of Immediate Hypersensitivity to Cows’ Milk Protein in the Infant,” The Journal of Allergy and Clinical Immunology, Vol. 107, No. 1, 2001, pp. 185-190. doi:10.1067/mai.2001.111592
- R. G. Hamilton and A. N. Franklin, “In Vitro Assays for the Diagnosis of IgE-Mediated Disorders,” The Journal of Allergy and Clinical Immunology, Vol. 114, No. 2, 2004, pp. 213-225. doi:10.1016/j.jaci.2004.06.046

