 Journal of Environmental Protection, 2011, 2, 489-501 doi:10.4236/jep.2011.25057 Published Online July 2011 (http://www.scirp.org/journal/jep) Copyright © 2011 SciRes. JEP Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes Masafumi Tateda1, Seisou Suzuki1, Youngchul Kim2, Bandunee Champika Liyanage Athapattu3 1Department of Environmental Engineering, Toyama Prefectural University, Imizu, Japan; 2Department of Environmental Engineer- ing, Hanseo University, Seosan-si, Korea; 3Department of Civil Engineering, The Open University of Sri Lanka, Nawala, Sri Lanka. Email: tateda@pu-toyama.ac.jp Received February 22nd, 2011; revised April 5th, 2011; accepted May 19th, 2011. ABSTRACT The behaviors of Cu, Pb, and Zn during the endothermic burning of heterogeneous wastes were investigated using a variety of operational parameters, i.e., the mixed waste ra tio, burning temperature, and burning time , to obtain funda- mental knowledge to generate an optimal burning operation and recycling strategy for bottom ash. Changing these parameters had no impact on the Cu content of the ash, whereas the Pb content depended on the burning temperature and the mixed ratio, and the Zn conten t was affected by all three parameters. It was found in this study that the optimal burning conditions were a temperature of 1100˚C, a time of 15 minutes, and either the current waste conditions or waste conditions with doub le the waste plastic and wood content. Keywords: Heavy Metals, Endothermic Burning, Portioning Behavior, Industrial Waste, Ash 1. Introduction and Methods Japan has two categories of waste: general waste mainly from residential areas, and industrial waste. Industrial waste accounts for almost 90% of the waste generated, reaching about 400 million tons annually. For treatment of both types of waste, Japan primarily uses incineration. In fact, over 80% of the generated general waste, ap- proximately 40 million tons annually, is incinerated. A variety of metal elements are present in the resultant in- cineration ashes, especially in the fly ash or fly and bot- tom mixed ash; therefore, intermediate treatment meth- ods to reduce their environmental impact are nationally designated. The disposal of ashes treated by either solidi- fication in cement or melting fu sion into landfills and the recycling of those ashes as commercial materials have a serious impact on health and economics due to the spread of toxic materials (i.e., heavy metals) into the environ- ment and the waste of non-recycled metal resources [1,2]. Burning is also one of the nation ally designated interme- diate treatment options for ash recycling (Figure 1). This method is commonly used by private companies that generate a large amount of ash to avoid cost-prohibitive ash disposal in landfill sites. Since ash burning is an en- dothermic process, fuel is necessary to generate the heat required. Normally a synthesized fuel or refused paper & plastic fuel (RPF) is used instead of kerosene to reduce the operating costs. Alternatively, the ash is burned with other combustible waste such as plastic and wood, which results in the mixed burning of ash, plastics, and paper or wood. As mentioned earlier, ash contains metal elements and the effect of heterogeneous burning conditions on the be- havior of those metal elements has not yet been investi- gated. The purpose of this study is to investigate the be- haviors of Cu, Pb, and Zn, as representative elements with negative health and environmental impacts, under heterogeneous waste burning with varying burning tem- perature, burning time, and mixed waste ratio. Under- standing the behaviors of metals in heterogeneous condi- tions is essential in order to determine the appropriate conditions for b urning operations and to op timize a recy- cling strategy for the bottom ash from endothermic burning. 2. Materials and Methods 2.1. Waste Samples The following six samples were used as samples for burning: automobile shredder residue (ASR) fly and bot- tom ashes, fly and bottom ashes from the incineration of waste plastics and woods, waste plastic residue from RPF  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes 490 Figure 1. Designated intermediate treatment methods for fly ash or fly and bottom ash, as determined by the central Japanese government. production, and the benthic sludge of rivers. The ASR fly and bottom ashes were obtained from the incineration of ASR by a rotary kiln. 2.2. Methods for the Analysis of the Total Content of Elements in the Waste Samples The conditions of the boiling extraction method for ele- ment extraction [3], which is the standard Japanese me- thod, were examined to d etermine the optimal conditio ns for element extraction from the ash samples after incin- eration of the mixed waste. The selected conditions are shown in Table 1. An inductively coupled plasma–mass spectrometer (ICP-MS) was used for elemental analysis, and hydro- chloric acid (conc. 35% - 37%, Kanto Kagaku), nitric acid (60% - 61%, Kanto Kagaku) and deionized water were used as solvents. The standard method for boiling extraction was as follows: The ash sample (1 ± 0.01 g, wet base) was placed in a beaker and mixed with hydro- chloric acid (1:4 HCl:H2O) and nitric acid (1 + 1 HNO3:H2O) and boiled for 30 minutes. After cooling, the solution was transferred to a 100 mL volumetric flask, which was accurately filled with deionized water. Next, the solution was filtered using a 1 µm cellulose acetate filter (Advantec). A 10 mL aliquot was diluted to 50 mL in a volumetric flask using deionized water. The solu tion was then analyzed by ICP-MS (HP4500, Yokokawa). 2.3. Quantitative Analysis of Cu, Pb, and Zn Content Cu, Pb, and Zn heavy metals, which are present in rela- tively high concentrations and cause considerable harm to the environment and human health, were selected for analysis using an atomic absorption spectrophotometer (AAS; A-2000, Hitachi). The samples were pre-treated Table 1. Pretreatment condition. Altered conditions Details Standard (SD) Boiling extraction using nitric a ci d and hydrochlor i c acid Altered condition 1 (AC1)Double concentration of hydrochlor ic acid of SD Altered condition 2 (AC2)Double concentration of nitric ac id o f SD Altered condition 3 (AC3)Double concentrations of hydrochloric and nitric aci ds of SD Altered condition 4 (AC4)Double boiling time of SD Altered condition 5 (AC5)Double boiling time and concentration of hydrochloric acid of SD with AC1, which uses double the concentration of hy- drochloric acid as compared to the standard (see Table 1). For degradation of the waste samples, two methods were tested: degradation at 600˚C combustion or drying at 105˚C. For the first method, the waste samples were burned at 600˚C for 60 minutes and the ash was collected for total content analysis for the metals. For the second method, the sample was prepared by drying it at 105˚C without burning and then analyzed for the total content of the metals. The ash or dry samples (2 - 5 g) were trans- ferred into a flask and hydrochloric (60 mL) and nitric (30 mL) acids were added, and the solution was heated until it reduced to about 5 mL. After cooling, 20 mL of hydrochloric acid (1 + 5 HCl:H2O) was added to the so- lution, which was further heated for 5 - 6 minutes. After cooling again, the solution was diluted to 100 mL in a volumetric flask using deionized water. The diluted solu- tion was filtered through a 1 µm glass fiber filter and the filtrate was analyzed using an AAS. 2.4. Quantitative Analysis of Cu, Pb, and Zn in Ash The content of heavy metals, i.e., Cu, Pb, and Zn, in ashes burned under d ifferent conditions was analyzed v ia the following procedure. The AC1 pretreatment, which uses a double concentration of hydrochloric acid as compared to the standard, was used. The parameters that were investigated in this study w ere waste sample mixed ratio, burning temperature, and burning time. Different waste samples types were prepared with varying mixed ratios, as summarized in Table 2. Burning temperatures were either 900˚C, 1000˚C, or 1 100˚C and burning times were either 15, 30, or 60 minutes in an electric furnace (KDF S80, Eyela). All possible combinations of parame- ters were performed and the sample descriptions are shown in Table 3. Ash samples (2 - 5 g) were transferred into a flask, hydrochloric (60 mL) and nitric (30 mL) acids were added, and the solution was h eated until it was reduced to about C opyright © 2011 SciRes. JEP  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes491 Table 2. Mixed status of burning samples for designing the experimental preparation (%). Type AType B Type C Sample type Current condition of waste Double of ASR content Double of waste plastic & wood content bottom ash (BA) 11.22 22.44 11.22 ASR fly ash (FA) 6.12 12.24 6.12 bottom ash (BA) 2.04 2.04 4.08 Waste plastic & wood fly ash (FA) 1.02 1.02 2.04 Waste plastic residue from RPF production (RPF-R) 67.34 50.02 64.36 Benthic sludge of rivers (BSR) 12.24 12.24 12.24 Table 3. Combination of experimental conditions and sam- ple IDs. Burning temperature (˚C) Burning time (minutes) Sample type in Table 2 Sample ID A 915A B 915B 15 C 915C A 930A B 930B 30 C 930C A 960A B 960B 900 60 C 960C A 1015A B 1015B 15 C 1015C A 1030A B 1030B 30 C 1030C A 1060A B 1060B 1000 60 C 1060C A 1115A B 1115B 15 C 1115C A 1130A B 1130B 30 C 1130C A 1160A B 1160B 1100 60 C 1160C 5 mL. After cooling, 20 mL of hydrochloric acid (1 + 5 HCl:H2O) was added to the solution, which was further heated for 5 - 6 minutes. After cooling again, the solution was diluted to 100 mL using deionized water in a volu- metric flask. The diluted solutio n was filtered through a 1 µm glass fiber filter and the filtrate was analyzed using an AAS (A-2000, Hitachi). 3. Results 3.1. Effect of the Chemical Pretreatment on the Content Analysis Figures 2(a)-(f) show the efficacy of each of the pre- treatment methods and the amount of each selected ele- ment. The heavy metals were almost undetectable in the samples of waste plastic residue from RPF production (RPF-R) and the benthic river sludge (BSR) using SD and AC3 methods. The AC1 method resulted in the most balanced detection of the Cu, Zn and Pb metals and other elements, and is therefore considered to be the optimal pretreatment method for analyzing heavy metals con- tained in these samples. The amount of Cu, Zn, and Pb in each sample after pretreatment using the AC1 method is shown in Figure 3. The ASR bottom ash sample (ASR- BA) contained the largest amount of Cu, Zn, and Pb. 3.2. Effect of Sample Preparation on the Cu, Pb, and Zn Content To optimize the detection of the selected heavy metals in the samples, two methods of preparing the samples, i.e., burning at 600˚C or drying at 105˚C, were investigated. Regardless of the waste sample types and the species of heavy metal, the metal content of the burned samples was always larger than that of the dried samples (Figures 4(a)-(i)). Therefore, it is evident that bu rning the samples at 600˚C is a better method for degrading the waste sam- ples for optimal detection of the heavy metals. The rea- son for the lower detection of the metals in the dried samples might be the remnant presence of organic mate- rials. The initial reaction of the organic materials with hydrochloric acid and altering the pH and oxidation-re- duction potential (ORP) did not alter the environment around the heavy metals enough to accelerate the metal extraction from the samples. 3.3. Effect of Varying the Burning Conditions on the Cu, Pb, and Zn Content Figures 5(a)-(c), 6(a)-(c) and 7(a)-(c) show the behav- iors of Zn, Pb and Cu, respectively, under different burn- ing conditions, where Figures 5-7(a), Figures 5-7(b), and Figures 5-7(c) display the effects of varying the tem- perature, burning time and sample type, respectively. 4. Discussion 4.1. Optimal Chemical Pretreatment The metal content of the samples were assessed employ- ing the 6 different pretreatment methods described in Table 1 and Figure 2. High amounts of all metals were detected using both the AC1 and AC4 pretreatment me- thods, although the metal levels detected after the AC4 Copyright © 2011 SciRes. JEP 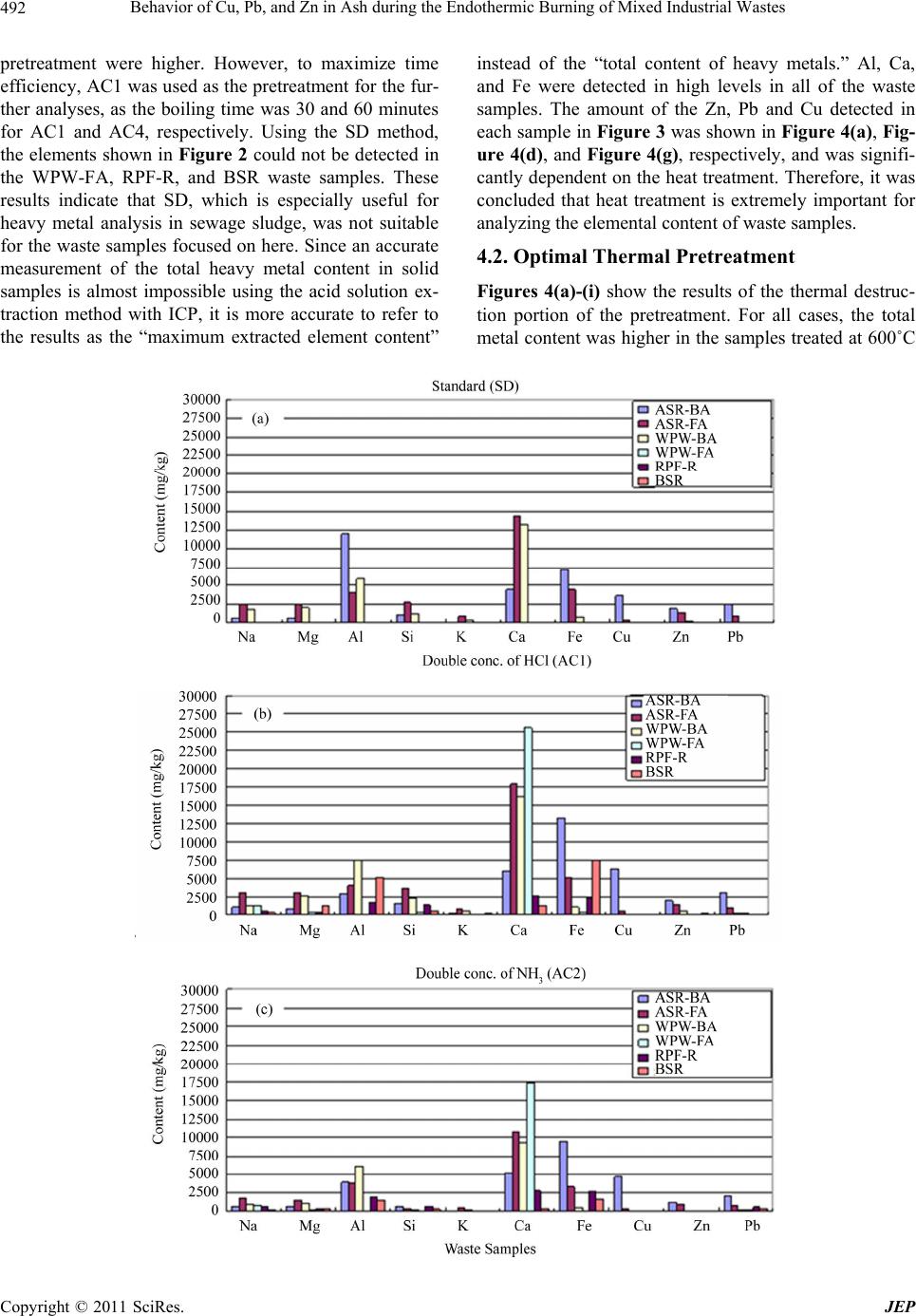 Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes Copyright © 2011 SciRes. JEP 492 pretreatment were higher. However, to maximize time efficiency, AC1 was used as the pretreatment for the fur- ther analyses, as the boiling time was 30 and 60 minutes for AC1 and AC4, respectively. Using the SD method, the elements shown in Figure 2 could not be detected in the WPW-FA, RPF-R, and BSR waste samples. These results indicate that SD, which is especially useful for heavy metal analysis in sewage sludge, was not suitable for the waste samples focused on here. Since an accurate measurement of the total heavy metal content in solid samples is almost impossible using the acid solution ex- traction method with ICP, it is more accurate to refer to the results as the “maximum extracted element content” instead of the “total content of heavy metals.” Al, Ca, and Fe were detected in high levels in all of the waste samples. The amount of the Zn, Pb and Cu detected in each sample in Figure 3 was shown in Figure 4(a), Fig- ure 4(d), and Figure 4(g), respectively, and was signifi- cantly dependen t on the heat treatment. Therefore, it was concluded that heat treatment is extremely important for analyzing the elemental content of waste samples. 4.2. Optimal Thermal Pretreatment Figures 4(a)-(i) show the results of the thermal destruc- tion portion of the pretreatment. For all cases, the total metal content was higher in the samples treated at 600˚C 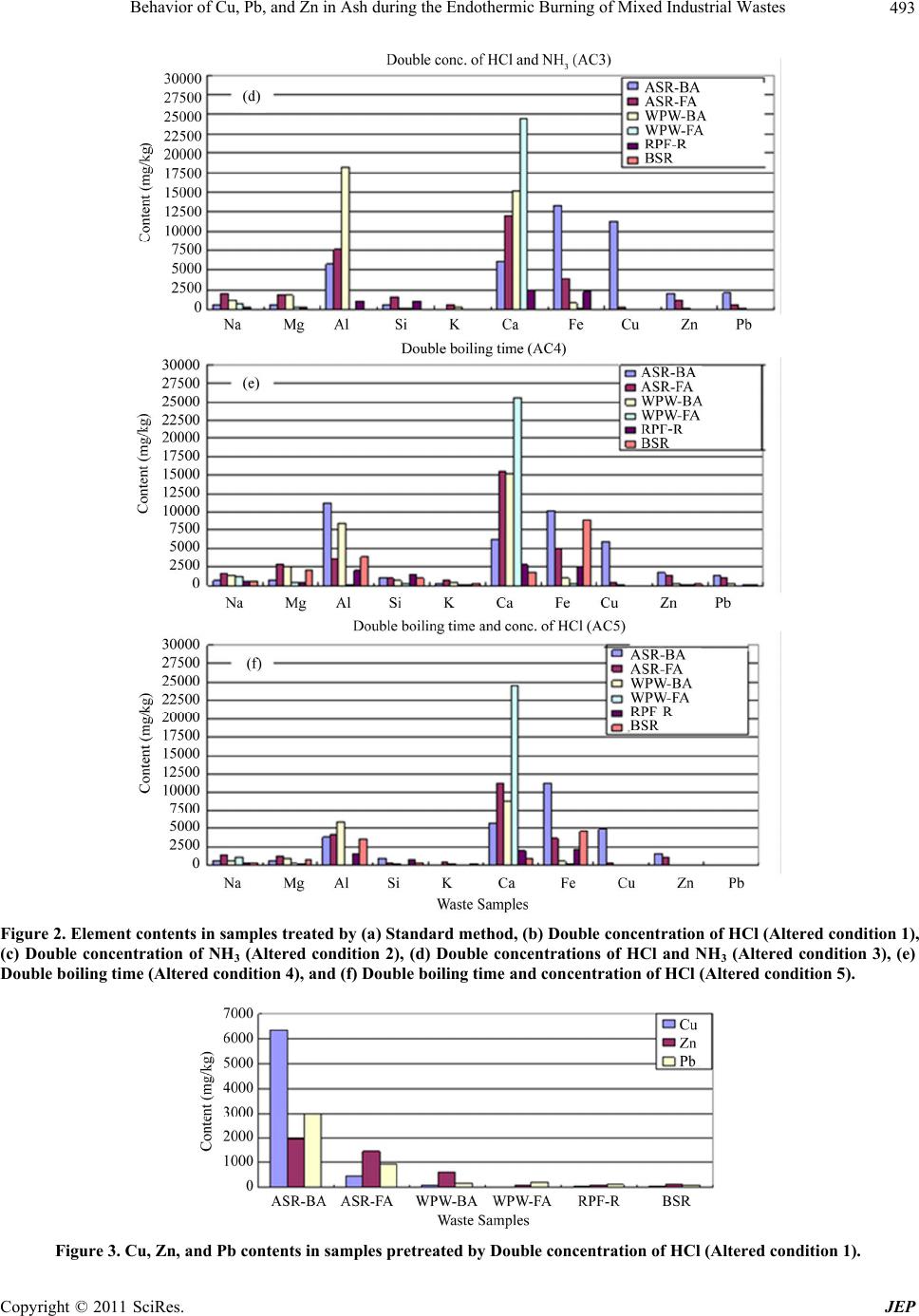 Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes493 Figure 2. Element contents in samples treated by (a) Standard method, (b) Double concentration of HCl (Altered condition 1), (c) Double concentration of NH3 (Altered condition 2), (d) Double concentrations of HCl and NH3 (Altered condition 3), (e) Double boiling time (Altered condition 4), and (f) Double boiling time and concentration of HCl (Altered condition 5). Figure 3. Cu, Zn, and Pb contents in samples pretreated by Double concentration of HCl (Altered condition 1). Copyright © 2011 SciRes. JEP  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes Copyright © 2011 SciRes. JEP 494 Figure 4. Comparison of pretreatment (thermal treatment) on element content: (a)-(c):Type A-C on Zn, (d)-(f):Type A-C on Pb, and (g)-(i):Type A-C on Cu. than those that were dried at 105˚C. As it was established that thermal pretreatment at 600˚C is better than at 105˚C, the effect of thermal pretreatment at 900˚C, 1000˚C, and 1100˚C was also investigated. Since the time of burning or drying was one hour for the results in Figure 4, a comparison of Figure 4 and 960A–C, 1060A–C, and 1100A–C for the detection of Cu, Pb, and Zn (Figures 5- 7) was carried out. The amounts of the elements detected after the 600˚C thermal pretreatment was highest in almost all cases (Figures 8-10). There was the only one exception: the amount of Pb detected after 960B pretreatment was higher than that after 600˚C thermal pretreatment. There- fore, it can be concluded that the 600˚C thermal pretreat- ment was the best overall method to obtain the maximum extraction content of elements from waste samples. 4.3. Behaviors of Cu, Pb, and Zn in Ash Figures 5-7 show the behaviors of Zn, Pb, and Cu, re- spectively, under different operating conditions; the re- sults are summarized in Table 4. The speciation of Cu, Pb, and Zn during municipal solid waste combustion was described as follows [4,5]: Cu, CuCl, CuH, CuO, CuS, Cu2, (CuCl)3, CuCl, CuO, CuO-Al2O3, CuO-Fe2O3, CuS-FeS, CuSO4, Cu2O-Al2O3, Cu2-Fe2O3, Cu2S, Cu5FeS4, Pb, PbCl, PbCl2, PbO, PbS, Pb2, PbCl2, PbO-SiO2, 2Pb-SiO2, PbS, PbSO4, Pb2B2O4, Pb3O4, Zn, ZnCl2 ZnS, ZnO, ZnO-SO2, ZnS, ZnSO4, 2ZnO-SiO2, Zn2SiO4, ZnFe2O4, ZnCr2O4, ZnAl2O4, and ZnO-2ZnSO4. The metallic states (i.e., Zn, Pb, and Cu), mono-oxidized species (i.e., ZnO, PbO, and CuO), and chlorinated species (i.e., ZnCl2, PbCl2, and CuCl2) were chosen as the representative compounds in this study; their melting and boilin g points are listed in Figure 11. According to Verhulst et al. [6], ZnCl2 (solid (s)) con- verts to ZnO(s) at less than 300˚C and forms ZnCl2  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes495 Figure 5. The behavior of Zn depending on the (a) burning temperature, (b) burning time, and (c) sample types. Copyright © 2011 SciRes. JEP  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes 496 Figure 6. The behavior of Pb depending on the (a) burning temperature, (b) burning time, and (c) sample types. C opyright © 2011 SciRes. JEP  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes497 Figure 7. The behavior of Cu depending on the (a) bur ning temperature, (b) burning time, and (c) sample types. Copyright © 2011 SciRes. JEP  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes Copyright © 2011 SciRes. JEP 498 Figure 8. Content of Zn detected after burning at different temperatures. Figure 10. The content of Pb detected after burning at dif- ferent temperatu re s . Table 4. Summary of Zn, Pb, and Cu behaviors. Influence byZn Pb Cu Burning temperature Its detection became small as temperature goes up. High detection at 900˚C No significan t difference Burning time -No difference at 900˚C -Its detection became smaller as time becomes longer. -No significant difference No significan t difference Mixed ratio -Mixed ratio B always shows the highest. -No significant difference between mixed ratios A and C . Mixed ratio B seemed high No significant difference (gas (g)), whereas PbCl2(g) starts to volatilize around 300˚C and PbO(g) and PbCl(g) are predominant above 800˚C. In the case of Cu, CuCl2 is converted to CuO around 700˚C and CuCl(g) is predominant around 900˚C after Cu3Cl3(g) is formed. Generally, the presence of Cl greatly influences the behavior of metals; the volatility of heavy metals increases when they are chlorinated due to their decreased boiling point. Figure 9. The content of Cu detected after burning at dif- ferent temperatu re s .  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes499 Figure 11. Melting and boiling properties of (a) metal, (b) metal oxide, and (c) metal chloride species. The broken line indicates 1100ºC, which was the highest burning tempera- ture in this study. The temperature of ZnO is the sublima- tion point. The effect of Cl on heavy metals was discussed by many researchers [7-10]. According to most studies, Cl exerts a stronger influence on Zn than on Cu or Pb; however, Pb was the most volatile chloride according to a study by Trouvé et al. (1998). Also, Pb and Zn were more likely than Cu to be transferred into th e combustio n gas by forming compounds with chlorine [11]. All re- ports agreed that chlorinated Cu was the most stable. In the current study, the chlorine co ncentrations in the sam- ples were not analyzed because, in reality, the chlorine concentration in waste cannot be controlled. In addition to the chlorine content, the combustion temperature also greatly influences metal partitioning and speciation [12]. Heavy metal partitioning behaviors are also greatly af- fected by the presence of alkaline metals such as Na and K and moisture in the waste [13]. According to Wang et al. (1999), the presence of Na and K increased the parti- tioning of heavy metals into fly ash. All of the analyzed samples contained Na and K (Figure 2), but the behavior of Cu was not influenced by the changes in the mixed ratio. In opposition to the trend reported by Wang et al. (1999), Pb and Zn showed the highest con tents in the ash under the mixed ratio B, which had the highest Na and K concentration. Therefor e, it can be said that the influence of Na and K was not supported by this study. The behavior of Cu behavior has also been shown to be affect by the presence of Ca; the detection level of Cu in the gas phase drastically increased in the presence of limestone with a Ca/S ratio of 1.3 [14]. However, the increase in the detection level disappeared when the ratio doubled to 2.5. From Figure 2, it is evident that the ASR-FA, WPW-BA, and WPW-FA samples contain a relatively large amount of Ca, which could influence Cu detection in the ash analysis. It has also been reported that the presence of organic chloride species decreases the capture of Cu by limestone, while the presence of inorganic chlorides increases it [15]. The influence of Ca on Cu was not directly tested in this experiment. How- ever, according to Table 2, there was no evident impact of Ca on Cu as the Cu detection levels did not differ sig- nificantly upon changes in the mixed ratio (Table 4) al- though Type C did contain double the amount of WPW- BA and WPW-FA, which contained a large amount of Ca (Figure 2). From the results shown in Table 4, it was determined that Cu was the most stable among the three metals as it demonstrated no significant difference in the Cu levels upon changing the burning temperature, burn- ing time, and waste mixed ratio, which is in agreement with the results of Williams [16] and Trouvé et al. (1998). Zn, on the other hand, was the most unstable metal among the three: the content of Zn in the ash decreased with increasing temperature and was also influenced by the burning time at 1000˚C and 1100˚C. Changes to the mixed ratio resulted in a higher Zn content in the ash from sample type B. In contrast, the amount of Pb de- tected was not influenced by th e burning time (Table 4). Weight percentages of 89% - 96% of Cu, 58% - 94% of Pb, and 37% - 86% of Zn remained in the bottom ash [4]. These ranges are consistent with the results of this study. 4.4. Comparison to Environmental Criteria After burning, ash either goes to a lan dfill site or to recy- cling after clearing the criteria for the heavy metal ex- traction test; lower heavy metal conten t in the ash is bet- ter for both purposes. According to the results, the sam- ples with the lowest conten t of heavy metals were 1115A and 1115C. The content of Zn, Pb, and Cu was 3,384 ± 434 mg/kg, 4,013 ± 2228 mg/kg, and 23,570 ± 8,210 mg/kg, respectively, for 1115A, and 5219 ± 58 mg/kg, 4455 ± 414 mg/kg , and 18,271 ± 434 mg/k g, respectiv ely, for 1115C. The heavy metal content in samples 1115A and 1115C were compared to standards related to envi- ronmental issues such as landfill, sea dumping, and com- posting (Table 5). The experimental values were 7.5 to11.6, 261 to 337, and 803 to 891 times larger than the Copyright © 2011 SciRes. JEP  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes Copyright © 2011 SciRes. JEP 500 Table 5. Environmental values. Heavy metals Landfill standard (Japan) Sea dumping (Japan) Soil (Japan) Compost standard (mg/kg) This study (Mean) (mg/kg) (mg/l) (mg/kg) (mg/kg) JapanIndia†USEPA†Canada†Germany† 1115A 1115C Zn - 450 - - 1000 2800 500 400 3,3845,219 Cu - 70 125 - 300 1500 60 100 23,57018,271 Pb 0.3 5 0.01* - 100 300 150 150 4,0134,455 note†:Reference [17]. sea dumping standards for Zn, Cu, and Pb, respectively. When compared with the United States Environmental Protection Agency (USEPA) compost standards, which are relatively high, the experimental values were ap- proximately 1.2 to 1.9, 12 to 16, and 13 to 15 times higher for Zn, Cu, and Pb, respectively. Therefore, al- though the content of heavy metals in ash is greatly re- duced by tuning the burning temperature and time for Zn and Pb, it is still far higher than that allowed by any of the standards described here. 5. Conclusions The behaviors of Cu, Pb, and Zn under the endothermic burning of heterogeneous wastes were investigated by changing the operational parameters, i.e., the mixed waste ratio, burning temperature, and burning time, to obtain fundamental knowledge to generate an approp riate burning operation and recycling strategy for bottom ash. Changing these parameters yielded no significant effect on the Cu content of the ash, whereas the Pb content was influenced by the burning temperature and mixed ratio, and the Zn content was influence by all three parameters. The burning conditions not only influence the partition- ing behavior of metals in thermal treatment reactors, such as incinerators, but also the characteristics of the metals in the ash. Therefore, it is important to understand these effects to plan an effective recycling strategy for incin- eration ash. In this study, the optimal operation condi- tions were 1115A and 1115C, which correspond to a burning temperature of 1100˚C, a burning time of 15 minutes, and either the current waste conditions or the waste condition with double the waste plastic & wood content. It is also important that managers in charge of thermal treatment reactors conduct their own investiga- tion into the partitioning behavior of heavy metals at their plants based on the academic results that have been reported in order to optimize the operations for their re- actors. REFERENCES [1] T. Masafumi, I. Michihiko and F. Masanori, “Loss of Metallic Elements Associated with Ash Disposal and So- cial Impacts,” Resources, Conservation and Recycling, Vol. 19, 1996, pp. 93-108. [2] L. T. Theis and K. H. Gardner, “Environmental Assess- ment of Ash Disposal,” Critical Reviews in Environ- mental Control, Vol. 20, No. 1, 1990, pp. 21-42. doi:10.1080/10643389009388388 [3] Japan Sewage Work Association, “Standard Methods for Sewage Examination Part 2,” Japan Sewage Work Asso- ciation, Tokyo, 1997, pp.215-216. [4] L. Sørum, F. J. Frandsen and J. E. Hustad, “On the Fate of Heavy Metals in Municipal Solid Waste Combustion Part I: Devolatilisation of Heavy Metals on the Grate,” Fuel, Vol. 82, No. 18, 2003, pp. 2273-2283. doi:10.1016/S0016-2361(03)00178-9 [5] Y. Ménard, A. Asthana, F. Patisson, Ph. Sessiecq and D. Ablitzer, “Thermodynamic Study of Heavy Metals Be- havior during Municipal Waste Incineration,” Process Safety and Environmental Protection, Vol. 84, No. B4, 2006, pp. 290-296. [6] D. Verhulst, A. Buekens, P. J. Spencer and G. Eriksson, “Thermodynamic Behavior of Metal Chlorides and Sul- fates under the Conditions of Incineration Furnaces,” En- vironmental Science & Technology, Vol. 30, 1996, pp. 50-56. doi:10.1021/es940780+ [7] T. Nobuo, O. Shigenobu, N. Matsutataro and M. Katu- yuki, “Fundamental Study on Separation of Heavy Metals from Fly Ash by Chlorinated-Volatilization (Enka Ki- hatsu Ho ni Yoru Hibai Chu no Jukinzoku no Bunri ni Kansuru Kisoteki Kenkyu),” Environmental Sanitation Engineering Research, Vol. 8, No. 3, 1994, pp. 185-190. [8] M. Wobst, H. Wichmann and M. Bahadir, “Distribution Behavior of Heavy Metals Investigated in a Labora- tory-Scale Incinerator,” Chemosphere, Vol. 44, No. 5, 2001, pp. 981-987. doi:10.1016/S0045-6535(00)00493-8 [9] K.-S. Wang, K.-Y. Chiang, C.-C. Tsai, C.-J. Sun, C.-C. Tsai and K.-L. Lin, “The Effects of FeCl3 on the Distri- bution of the Heavy Metals Cd, Cu, Cr, and Zn in a Si- mulated Multimetal Incineration System,” Environmental International, Vol. 26, No. 4, 2001, pp. 257-263. doi:10.1016/S0160-4120(00)00115-X [10] G. Trouvé, A. Kauffmann and L. Delfosse, “Comparative Thermodynamic and Experimental Study of Some Heavy Metal Behaviours during Automotive Shredder Residues Incineration,” Waste Management, Vol. 18, 1998, pp. 301-307. doi:10.1016/S0956-053X(98)00040-3 [11] F.-S. Zhang, S.-I. Yamasaki, M. Nanzyo and K. Kimura, “Evaluation and Cadmium and Other Metal Losses from Various Municipal Wastes during Incineration Disposal,”  Behavior of Cu, Pb, and Zn in Ash during the Endothermic Burning of Mixed Industrial Wastes501 Environmental Pollution, Vol. 115, No. 2, 2001, pp. 253- 260. doi:10.1016/S0269-7491(01)00104-X [12] K.-Y. Chiang, K.-S. Wang, F.-L. Lin and W.-T. Chu, “Chloride Effects on the Speciation and Partitioning of Heavy Metal during the Municipal Solid Waste Incinera- tion Process,” Science of the Total Environment, Vol. 203, 1997, pp. 129-140. doi:10.1016/S0048-9697(97)00140-X [13] K.-S. Wang, K.-Y. Chiang, S.-M. Lin, C.-C. Tsai and C.-J. Sun, “Effects of Chlorides on Emissions of Toxic Com- pounds in Waste Incineration: Study on Partitioning Cha- racteristics of Heavy Metal,” Chemosphere, Vol. 38, No. 8, 1999, pp. 1833- 1849. doi:10.1016/S0045-6535(98)00398-1 [14] Lopes Helena M., P. Abelha, N. Lapa, J. S. Oliveira, I. Cabrita and I. Gulyurtlu, “The Behavior of Ashes and Heavy Metals during the Co-Combustion of Sewage Sludge in a Fluidised Bed,” Waste Management, Vol. 23, No. 9, 2003, pp. 859-870. doi:10.1016/S0956-053X(03)00025-4 [15] J.-C. Chen and M.-Y. Wey, “The Effect of Operating Conditions on the Capture of Metals with Limestone dur- ing Incineration,” Environmental International, Vol. 22, No. 6, 1996, pp. 743-752. doi:10.1016/S0160-4120(96)00066-9 [16] P. T. Williams, “Pollutants from Incineration: an Over- view,” In: R. E. Hester and R. M. Harrison, Eds., Waste Incineration and the Environment, Royal Society of Chemistry, London, 1994, pp. 27-52. [17] J. Kurian, S. Esassu, K. Palanivelu and A. Selvam, “Stu- dies on Landfill Mining at Solid Waste Dumpsites in In- dia,” Proceeding Sardinia 2003, Ninth International Waste Management and Landfill Symposium S. Mar- gherita di Pula, CISA Environmental Sanitary Engineer- ing Centre, Cagliari, Italy, 2003. Copyright © 2011 SciRes. JEP
|