 International Journal of Clinical Medicine, 2011, 2, 185-195 doi:10.4236/ijcm.2011.23031 Published Online July 2011 (http://www.SciRP.org/journal/ijcm) Copyright © 2011 SciRes. IJCM 185 Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury —Supportive Treatments in Cisplatin Nephrotoxicity Ragaa Hamdy Mohamed Salama1*, Nahed Abdel-Mak sou d Abd -El -Hameed2, Sary Khalil Abd-El-Ghaffar3, Zaghloul Thabet Mohammed2, Nagwa Mahmoud Aly Ghandour2 1Department of Medical Biochemistry, Assiut University, Assiut, Egypt; 2Forensic Medicine and Toxicology Faculty of Medicine, Assiut, Egypt; 3 Pathology Department, Veterinary Medicine, Assiut University, Assiut, Egypt. Email: *ragaa_2002@yahoo.com Received August 15th, 2010; revised January 10th, 2011; accepted January 30th, 2011. ABSTRACT Nigella sativa and Matricaria chamomilla are extensively consumed as tea or tonic. Despite their widespread use as a home remedy, relatively few trials evaluated their benefits in nephroprotection. Hence, this study evaluates the nephro- protective effects of supportive treatments (N. sativa, M. chamomilla and vitamin E) in cisplatin nephrotoxicity rat model. Eighty rats divided into 10 groups, of 8 animals each. The first group (G1) injected with saline intrapretoneal (I.P). G2 injected with 5 mg/kg cisplatin I.P on zero day of experiment and repeated 4 times, with 5 days free interval. G3 - G10 received daily supportive treatments, started 5 days before the experiment (–5 day). Concomitantly G4, G6, G8 and G10 injected with 5 mg/kg cisplatin I.P like G2. On day sixteen, animal scarified, serum and/or kidney tissue were used to determine kidney function tests (serum urea, creatinine, NAG, β-GAL), oxidative stress indices (NO, LPO), antioxidant activities (SOD), sulphur compounds (GGT, GSH, total thiols), apoptotic indices (cathepsin D, DNA frag- mentation), two minerals (Ca2+ and Zn2+). Cisplatin caused marked elevation in serum GGT that reduced significantly in group received M. chamomilla with cisplatin (P < 0.001). There is a correlation between GGT and NAG in cisplatin group (r = 0.731, P < 0.05) that may suggest one of possible mechanisms of renal injury by cisplatin. M. chamomilla followed by N. sativa and vitamin E improved the biochemical and pathological renal injury, as determined by increas- ing the body weight, normalizing the kidney functions, decreasing the oxidative stress markers, improving the apoptotic markers, minimizing the pathological changes. Hence, N. sativa and M. chamomilla will be promising nephroprotective agents for reducing cisplatin nephrotoxicity, most probably, by antioxidants effects and inhibition GGT production, respectively. Keywords: Cisplatin, Nephrotoxicity, Nephroprotection, Matricaria chamomilla, Nigella sativa, Vitamin E 1. Introduction Cisplatin is a major antineoplastic drug for the treatment of solid tumors, but it has dose-dependent renal toxicity. It has multiple intracellular effects, causing direct cyto- toxicity with reactive oxygen species as apoptosis, in- flammation and fibrogenesis [1]. Despite all the marvelous advancements in modern medicine, traditional herbal medicine has always been practiced [2]. N. sativa is the most common herbal medicine all over the world for the treatment and pre- vention of a number of diseases. Their seeds/oil has anti-inflammatory, analgesic, antipyretic, antimicrobial, hypoglycemic and antineoplastic activity without sig- nificant adverse effects. This may be related to their cytoprotective and antioxidant actions [3]. M. chamo- milla is a well-known medicinal plant as carminative, analgesic, and anticonvulsant in traditional medicine [4]. It has moderate antioxidant, antimicrobial activities and significant antiplatelet activity in vitro. Animal model studies indicate potent anti-inflammatory action, some antimutagenic and cholesterol-lowering activities, as well as antispasmotic and anxiolytic effects [5]. Its methanolic extract showed potent neuroprotective activ- ity against global cerebral ischemia/reperfusion injury-  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury 186 induced oxidative stress in rats [6]. There are numerous varieties of Chamomile but the most two popular are Roman Chamomile and German Chamomile. German Chamomile which is called Matricaria chamomilla, considered the more potent of the two and received more scientific evaluation. The traditional drug of Matricaria recutita is the dried flower heads [7]. Combination of two or more herbs may be used with chemotherapy to decrease toxic adverse effects, elevate the immune function, and improve the quality of life in the patients. However, this required further studies in human. The aim of the present study is to design cisplatin nephrotoxicity rat model mimics human cycles of cis- platin chemotherapy, then, evaluates the nephroprotec- tive effects of N. sativa, M. chamomilla and vitamin E in this model. 2. Materials and Methods 2.1. Preparation of Supportive Treatments and Cisplatin N. sativa (seeds) and M. chamomilla (dry leaves and flowers) were selected with a fair degree of quality as- surance from faculty of Pharmacy, Pharmacognosy de- partment, Assiut University, Egypt. The identity of the planets was verified by the Center of Medicinal, Aro- matic and Poisonous Plants and a voucher specimen were kept on record in the herbarium of the Faculty of Pharmacy. The dry leaves crushed to powder and ex- tracted according to method described by El-Daly [8]. Twenty five grams of either powder extracted by 95% ethanol (500 ml) with continuous stirring at 4˚C, over- night, this was repeated for three successive days. The pooled extracts (1500 ml), evaporated under reduced pressure using Duché rotary flash evaporator rendering the product alcohol-free. Then, one gram of the product, reconstituted with 20 ml saline so, final concentration was 50 mg/ml and stored at 4˚C. N. sativa oil and Vita- min E purchased from Pharco-Company, Egypt. Eight grams of N. sativa oil (w/v) emulsified with 100 ml 2% polyethylene glycol-400 w/v (final concentration was 80 mg/ml). Vitamin E (90 mg, w/v) dissolved in 30 ml corn oil, so, final concentration was 3 mg/ml as described by El-Daly [8]. Vial of 50 mg Cisplatin, Bristol-Myers Squibb Company, USA, reconstituted in 20 ml saline immediately before injection with final concentration 2.5 mg/ml. 2.2. Experimental Design The study carried out on 80 healthy adult male Spra- gue-Dawley rats, weighing 250 - 350 g. Their ages ranged from 26 - 28 weeks. The animals were housed conventionally in clean cages and fed with standard food and water ad libitum. The animals were housed in groups in 12-hour light/ dark cycle. The care and treat- ments of the animals were approved and performed ac- cording to the guidelines of Animal House and ethical standards of Faculty of Medicine, Assiut University, Egypt. The experiment carried out in 10 groups of 8 animals each (G1 to G10). The first group (G1) was the healthy reference (H.R) group, injected with 1 ml saline I.P in the same way as group 2. The second group (G2) was cisplatin nephrotoxicity rat model, injected with 5 mg/kg cisplatin I.P on the zero day of experiment. The injection repeated 4 times, with 5 days free interval in between (on the day, zeroth, 5th, 10th, 15th) that mimics human regimen. By the 5th day up to 43% of the admin- istered cisplatin is recovered in the urine [9]. G3 and G4 received N. sativa extract (50 mg/kg I.P), G5 and G6 received N. sativa oil (400 mg/kg orally by tubing), G7 and G8 received M. chamomilla extract (50 mg/kg I.P), G9 and G10 received vit. E (6 mg/kg I.P). The injection of the three compounds started 5 days before the ex- periment (–5 day) and continued daily till the end of the experiment. In addition, G4, G6, G8 and G10 received cisplatin injection one hour before supportive treatments in the same way like group 2. On the day sixteenth, ani- mals weighted, blood samples obtained, then animals sacrificed and both kidneys were removed and weighted. One kidney was homogenized in 3 ml ice cold saline using a Glas-Col homogenizer fitted with teflon plunger (Terre Haute, # 099C, USA). Then centrifuged at 4000 × g for 10 min. at 4˚C, supernatant was kept at –70˚C for further biochemical measurements. The second kidney prepared for histopathological examinations. 2.3. Biochemical Analysis 1) Serum samples used for determination of: a) Kidney function tests: serum urea and creatinine using kits from Stanbio, USA. b) Oxidative stress indices: nitric oxide (NO) measured as total nitrite [10] and lipid peroxida- tion (LPO) as Thio Barbituric Acid Reactive Substances [11]. c) Antioxidants: Superoxide dismutase activity (SOD) [12]. d) Sulphur compounds: Gamma glutamyl transferase (GGT) using kit from QCA, Spain and total thiols by chemical method [13]. e) Serum levels of re- lated mineral as Ca2+ and Zn2+ by atomic absorption spectrophotometer (Buck scientific, USA. Model 210 VGP) using nitrous oxide/acetone flame absorption at wave length 422.7 nm and 213.9 nm, respectively. 2) Tissue supernatants were used for determination of: a). Proximal convoluted tubules functional tests such as beta-N-acetylglucosaminidase (NAG), -Galactosidase ( -GAL) [14]. b) Oxidative stress index: Lipid peroxi- dation (LPO) [11]. c) Antioxidants: SOD [12], reduced Copyright © 2011 SciRes. IJCM  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury187 glutathione (GSH) determined by spectrophotometer using chemical method [15]. d) Apoptotic indices: Cathepsin D by chemical methods, as hemoglobin split- ting activity of cathepsin [16], and DNA fragmentation by colorimetric method using diphenylamine (98% v/v glacial acetic acid; 1.5% v/v sulfuric acid; and 0.5% v/v of 1.6% acetaldehyde) [17]. e) Total protein using kit from Stanbio USA. 2.4. Histopathological Examinations Kidney was divided into 2 parts, the 1st part fixed in 10% phosphate buffered formalin and stained by H&E for light microscopic examination. The 2nd part fixed in 5% gluteraldhyde, then, prepared semithin sections stained by toluden blue stain, and examined by light microscope. Representative fields of the semithin sec- tions were selected, then ultrathin sections (70 nm) were cut with diamond knife using a Reichert OMVs ultra microtom for electron microscopic studies using lead citrate, uranyl acetate stain [18], and transmission elec- tron microscope (Jo El JEM, Japan. Model 100 CxII) in Electron Microscope Unit of Assiut University. 2.5. Statistical Analysis Soft ware, SPSS version 16 was used for statistical analysis. Values expressed as mean ± SE. Differences between obtained values carried out by Mann-Whitney U test using G1 as reference group. One way analysis of variance (ANOVA) followed by post hoch test (Tukey HSD) multiple comparison test using G2 as reference group. A P < 0.05 is a criterion for significant. 3. Results For clinical assessment, there was increase in body weight in G1 (3.99%) and in groups received supportive treatment only (G3, G5, G7, G9). Contrary, there was reduction in body weight in all groups received cisplatin plus supportive treatment. The maximum is in G2 that received cisplatin only (7.59%), then G4 (4.55%), G6 (3.98%), G10 (3.75%) and the minimum is G8 that re- ceived combination of cisplatin and M. chamomilla (3.31%). In this study, Cisplatin caused elevation in all kidney function indices. Co-administration of M. chamomilla extract with cisplatin provided the best protection for the kidney by reducing the levels of urea, creatinine, NAG and β-GAL followed by co-administration of vitamin E then N. sativa orally and finally N. sativa extract as shown in Table 1. Determination of oxidative stress and antioxidant indices revealed that cisplatin caused sig- nificant elevation in NO and LPO, significant decrease in SOD and GSH as shown in Tables 2-4, respectively. The best correction achieved in group received cisplatin with vitamin E then groups treated with N.sativa (extract or oil) and cisplatin. However, M. chamomilla extract with cisplatin provided the lowest protection against oxidative stress. Determination of sulpher compounds revealed that cisplatin caused marked elevation in serum GGT. The best reduction in the level of GGT achieved in M. chamomilla with cisplatin treated group (P < 0.001) as shown in Table 4. On the other hand, cisplatin caused marked decrease in total thiols and GSH compared to control (G1), but vit. E showed the best recovery for total thiols and GSH due to its antioxidant action that reserve GSH and total thiols. Co-administration of M. chamo- milla with cisplatin approved better protection from apoptosis, as shown in Figure 2(f), followed by co-ad- ministration with N. sativa oil, vitamin E and finally N. sativa extract as shown in Table 5. In this study, cis- platin caused marked decrease in serum Ca2+ and Zn2+ levels. Co-administration of N. sativa oil with cisplatin approved better protection from cisplatin- induced hypo- calcaemia, followed by M. chamomilla, vitamin E and finally N. sativa extract as shown in Table 6. The histopathological changes demonstrated the pro- tective effect of co-administration of supportive treat- ments in different groups (Figure 2) in comparison to new cisplatin rat model (Figure 1).The intensity of histopathological lesions demonstrated the protective effect of co-administration of supportive treatments in different groups as shown in Table 7. The biochemical and histological results of the rats’ kidney of groups 3, 5, 7, 9 that received supportive treatment only and served as control groups for these compounds revealed no ab- normal changes (data not shown). 4. Discussion The public received information about complementary and alternative medicines (CAMS) inaccurate or incom- plete [19] that, encourage the practitioners and research- ers to evaluate the actual benefits of these agents. N. sativa oil has been shown to possess 67 constituents, many of which are capable of inducing beneficial phar- macological effects in human [20]. By HPLC analysis of N. sativa oil, thymoquinone (TQ), dithymquinone (DTQ), thymohydroquinone, and thymol are considered the main active ingredients [21]. Bisabololoxide A is the principle constituents of some bioactivities of German chamomile such as anti-inflammatory, gastrointestinal and antipru- ritic actions [22]. For clinical assessment of cisplatin nephrotoxicity rat model, there was 7.59% decrease in body weight in cis- platin treated group along the period of experiment (cis- platin 5 mg/kg , 4 doses, time of experiment 21 days); compared to 14% decrease in body weights (cisplatin 15mg/kg, time of experiment 5 days) [23]. In this study, Copyright © 2011 SciRes. IJCM  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury Copyright © 2011 SciRes. IJCM 188 Table 1. Kidney functions in rats treated with cisplatin compared to those treated with cisplatin combinations. G1 G2 G4 G6 G8 G10 Serum Urea (mg/dl) 25.3 ± 1.2 60.9 ± 1.6 51.92 ± 3.4 46.34 ± 5 N.S** 41.53 ± 4.1 N.S** N.S*** 45.53 ± 3.8 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.4* 0.01* 0.0001* 0.006* Creatinine (mg/dl) 0.37 ± 0.01 0.64 ± 0.01 0.57 ± 0.03 0.53 ± 0.03 N.S** 0.46 ± 0.04 P < 0.05** N.S*** 0.53 ± 0.03 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.4* 0.04* 0.0001* 0.03* Tissue NAG (mu/mg protein) 5.07 ± 0.2 8.54 ± 0.4 6.89 ± 0.5 6.8 ± 0.5 N.S** 6.33 ± 0.3 N.S** N.S*** 6.84 ± 0.5 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.02* 0.01* 0.001* 0.02* β-GAL (mu/mg protein) 2.88 ± 0.2 7.93 ± 0.3 6.44 ± 0.6 5.86 ± 0.6 N.S** 5.32 ± 0.4 N.S** N.S*** 5.96 ± 0.5 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.1* 0.01* 0.0001* 0.02* G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin; G8 received Matricaria chamomilla extract + cisplatin; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only supportive treatment not shown as it is within normal range. N. B: * versus G2; ** versus G4; ***versus G6; **** versus G8. ANOVA followed by Tukey test as post ANOVA test was used to compare between group 2 and different groups. Table 2. Oxidative stress in rats treated with cisplatin compared to those treated with cisplatin combinations. G1 G2 G4 G6 G8 G10 Serum NO (µmol/ml) 14.06 ± 0.3 25.52 ± 1.7 19.01 ± 1.8 19.58 ± 1.1 N.S** 22.2 ± 1.8 N.S** N.S*** 19.05 ± 1.4 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.007* 0.02* 0.6* 0.008* LPO (nmol/ml) 0.37 ± 0.004 1.01 ± 0.02 0.76 ± 0.09 0.68 ± 0.09 N.S** 0.85 ± 0.08 N.S** N.S*** 0.66 ± 0.1 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.09* 0.005* 0.6* 0.002* Tissue LPO (nmol/mg protein) 0.24 ± 0.01 0.73 ± 0.02 0.51 ± 0.08 0.48 ± 0.07 N.S** 0.64 ± 0.07 N.S** N.S*** 0.46 ± 0.08 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.07* 0.01* 0.9* 0.01* G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin; G8 received Matricaria chamomilla extract + cisplatin; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only supportive treatment not shown as it is within normal range. N. B: * versus G2; ** versus G4; ***versus G6; ****versus G8. ANOVA followed by Tukey test as post ANOVA test was used to compare between group 2 and different groups.  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury189 Table 3. Levels of SOD in rats treated with cisplatin compared to those treated with cisplatin combinations. G1 G2 G4 G6 G8 G10 Serum SOD (U/ml) 6.215 ± 0.3 3.55 ± 0.1 4.78 ± 0.5 P < 0.05* 4.8 ± 0.5 P < 0.05* N.S** 2.93 ± 0.3 N.S* P < 0.01** P < 0.01*** 5.125 ± 0.6 P < 0.05* N.S** N.S*** P < 0.01**** ANOVA test 0.0001* 0.4* 0.4* 0.9* 0.1* Tissue SOD (U/mg protein) 0.69 ± 0.05 0.175 ± 0.01 0.38 ± 0.09 0.45 ± 0.09 N.S** 0.244 ± 0.08 N.S** P < 0.05*** 0.48 ± 0.1 N.S** N.S*** P < 0.05**** ANOVA test 0.0001* 0.5* 0.1* 0.9* 0.09* G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin; G8 received Matricaria chamomilla extract + cisplatin ; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only supportive treatment not shown as it is within normal range. N.B: * versus G2; ** versus G4; ***versus G6; **** versus G8. ANOVA followed by Tukey test as post ANOVA test was used to compare between group 2 and different groups. Table 4. Sulpher related compound s in rats treated with cisplatin compared to those treated with cisplatin combinations. G1 (N = 20) G2 (N = 12) G4 (N = 14) G6 (N = 14) G8 (N = 18) G10 (N = 14) Serum GGT (U/l) 5.92 ± 0.45 13.9 ± 1.4 12.08 ± 1.4 N.S* 8.24 ± 1.1 P < 0.01* P < 0.05** 7.15 ± 0.7 P < 0.001* P < 0.01** 10.05 ± 1.07 P < 0.05* P < 0.05**** ANOVA test 0.0001* 0.9* 0.001* 0.0001* 0.09* Tissue GSH (μmol/g protein) 4.14 ± 0.07 2.45 ± 0.07 2.77 ± 0.1 N.S* 3.12 ± 0.3 P < 0.05* N.S** 2.64 ± 0.06 N.S* N.S** N.S*** 3.23 ± 0.2 P < 0.05* N.S** N.S*** N.S**** ANOVA test 0.0001* 0.8* 0.02* 0.9* 0.004* Total Thiols (nmol/ml) 10.13 ± 1 3.39 ± 0.3 6.21 ± 1.1 P < 0.01* 4.96 ± 0.5 P < 0.05* N.S** 4.36 ± 0.6 N.S* N.S** N.S*** 5.17 ± 0.5 P < 0.05 N.S** N.S*** N.S**** ANOVA test 0.0001* 0.1* 0.8* 0.9* 0.7* G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin; G8 received Matricaria chamomilla extract + cisplatin; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only supportive treatment not shown as it is within normal range. N.B: * versus G2; ** versus G4; ***versus G6; **** versus G8. ANOVA followed by Tukey test as post ANOVA test was used to compare between group 2 and different groups. there was 1.4 times increase in the percentage of kidney weight to body weight after cisplatin-treatment. This could be explained by the marked decrease in the total body weight in cisplatin treated group. This was in agreement with another studies, where there were 1.7 and 2 times increase respectively, in percentage of kid- ney weight to body weight in cisplatin-treated animals [24,25]. The co-administration of supportive treatment with cisplatin resulted in decrease in this percentage when compared to cisplatin treated groups; this may be due to the relatively less reduction in the total body weights in these groups. The cut-off value for the normal range of blood urea nitrogen is (≤40 mg/dl) and serum creatinine is (≤0.2 mg/dl) based on the values obtained from normal un- treated mice [23]. In this study, urea and creatinine levels were 2.4 and 1.7 times increase in cisplatin-treated group when compared to control goup. The increase in urea r Copyright © 2011 SciRes. IJCM  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury 190 Table 5. Apoptotic markers in rats treated with cisplatin compared to those treated with cisplatin combinations. G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin; G8 received Matricaria chamomilla extract + cisplatin ; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only supportive treatment not shown as it is within normal range. N.B: * versus G2; ** versus G4; ***versus G6; **** versus G8. ANOVA followed by Tukey test as post ANOVA test was used to compare between group (2) and different groups. Table 6. Serum calcium and zinc in rats treated with cisplatin compared to those treated with cisplatin combinations. G1 (N = 20) G2 (N = 12) G4 (N = 14) G6 (N = 14) G8 (N = 18) G10 (N = 14) Ca+2 (ppm/ml) 35.4 ± 0.37 13.5 ± 0.36 19 ± 0.37 P < 0.01* 22.8 ± 0.37 P < 0.001* P < 0.01** 22.2 ± 0.37 P < 0.001* P < 0.01** N.S*** 22.06 ± 0.36 P < 0.001* P < 0.01** N.S*** N.S**** Zn+2 (ppm/ml) 0.68 ± 0.03 0.49 ± 0.03 0.41 ± 0.037 N.S* 0.5 ± 0.036 N.S* N.S** 0.5 ± 0.036 N.S* N.S** N.S*** 0.5 ± 0.037 N.S* N.S** N.S*** N.S**** G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin; G8 received Matricaria chamomilla extract + cisplatin; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only supportive treatment not shown as it is within normal range. N.B: * versus G2; ** versus G4; ***versus G6; **** versus G8. ANOVA followed by Tukey test as post ANOVA test was used to compare between group (2) and different groups. level by 3.7 and 2 times and creatinine level by 2.3 and 4.5 times was reported [24,25]. NAG and β-GAL levels are markers of early-impaired renal function and renal tubular damage [26]. Cisplatin group, in this study, had significant elevation of NAG and β-GAL levels when compared to control group (Table 1) due to dysfunction of tubular epithelial cells induced by increased traffic of proteins in the tubular lumen [27,28]. This was con- firmed by histopathological examinations (Figure 1(a) and (c)). These pathological changes were minimized in groups received cisplatin with supportive treatments es- pecially in group treated with N. sativa oil (G6) or M. chamomilla (G8) (Table 1, Figure 2(c), (d), (f) and (h)). Oxidative stress was implicated in the pathogenesis of cisplatin-induced nephrotoxicity. Oxidative stress, asso- ciated with increased generation of reactive oxygen me- tabolites (ROM) caused lipid peroxidation in kidney, decreased levels of antioxidants and antioxidant enzymes [29]. In this study, oxidative stress induced by cisplatin was manifested by elevation in nitric oxide (NO) and lipid peroxide levels. There was significant elevation in lipid peroxide [25,30], decrease in nitric oxide level [24], decrease in antioxidant as GSH and SOD activities was reported in cisplatin-induced nephrotoxicity [31]. The expression of Cathepsin D, the lysosomal prote- ases, had been shown to increase with protein degradation occurred during apoptosis [32]. In this study, cisplatin induced apoptosis in the form of significant increase in cathepsin D level and DNA fragmentation that is docu- mented by light and electron microscopy in Figures 1(b) and (f). M. chamomilla extract provided significant re- duction in these parameters followed by N. sativa oil and vitamin E then, N. sativa extract as shown in Table 5. The intensity of these apoptotic changes was markedly G1 (N = 20) G2 (N = 12) G4 (N = 14) G6 (N = 14) G8 (N = 18) G10 (N = 12) Catepsin D UEA 0.024 ± 0.005 0.15 ± 0.02 0.102 ± 0.02 N.S* 0.052 ± 0.02 P < 0.01* P < 0.05** 0.046 ± 0.02 P < 0.001* P < 0.05** N.S*** 0.061 ± 0.02 P < 0.01* P < 0.05** N.S*** N.S**** ANOVA test 0.0001* 0.4* 0.0001* 0.0001* 0.002* % of DNA Fragmentation 27.2 ± 0.3 40.9 ± 0.3 36.6 ± 1.8 P < 0.05* 33.5 ± 2.2 P < 0.01* N.S** 31.7 ± 2.4 P < 0.001* N.S** N.S*** 34.5 ± 2.2 P < 0.05* N.S** N.S*** N.S**** ANOVA test 0.0001* 0.4* 0.01* 0.01* 0.05* Copyright © 2011 SciRes. IJCM  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury191 (a) (b) (c) (d) (e) (f) Figure 1. Histopathological changes of new cisplatin nephrotoxicity rat model. The following changes were demonstrated: (a) cystic dilatation of the renal tubules and vacuolar degeneration in the tunica media (arrows) of the blood vessels as well as perivascular edema (double arrows) H&E 10 × 25. (b) evidences of apoptotic renal tubular epithelium cells (arrows ), semithin section with toludin blue stain, 10 × 100. (c) necrosis of renal tubular epithelial cell and the mitochondrial vacuolation (mv) with destruction of their cisternae, electron photomicrograph Lead citrate, uranyl acetate stain, ×10,000. (d) acidophilic tubular casts (arrows) in medullary renal tubules. H&E 10 × 10. (e) karyorrhexis (arrows) and loss of cellular membrane of the renal tubular epithelium, semithin section with toludin blue stain, 10 × 100. (f) sloughed apoptotic renal tubular epithelium cell, conde nsed nucle ar chromatin (N) and c y toplasmic or ganelle s (C) with the prese nce of detached apop- totic bodies (A), electron photomicrograph; lead citrate, uranyl acetate stain, ×14,000. Copyright © 2011 SciRes. IJCM 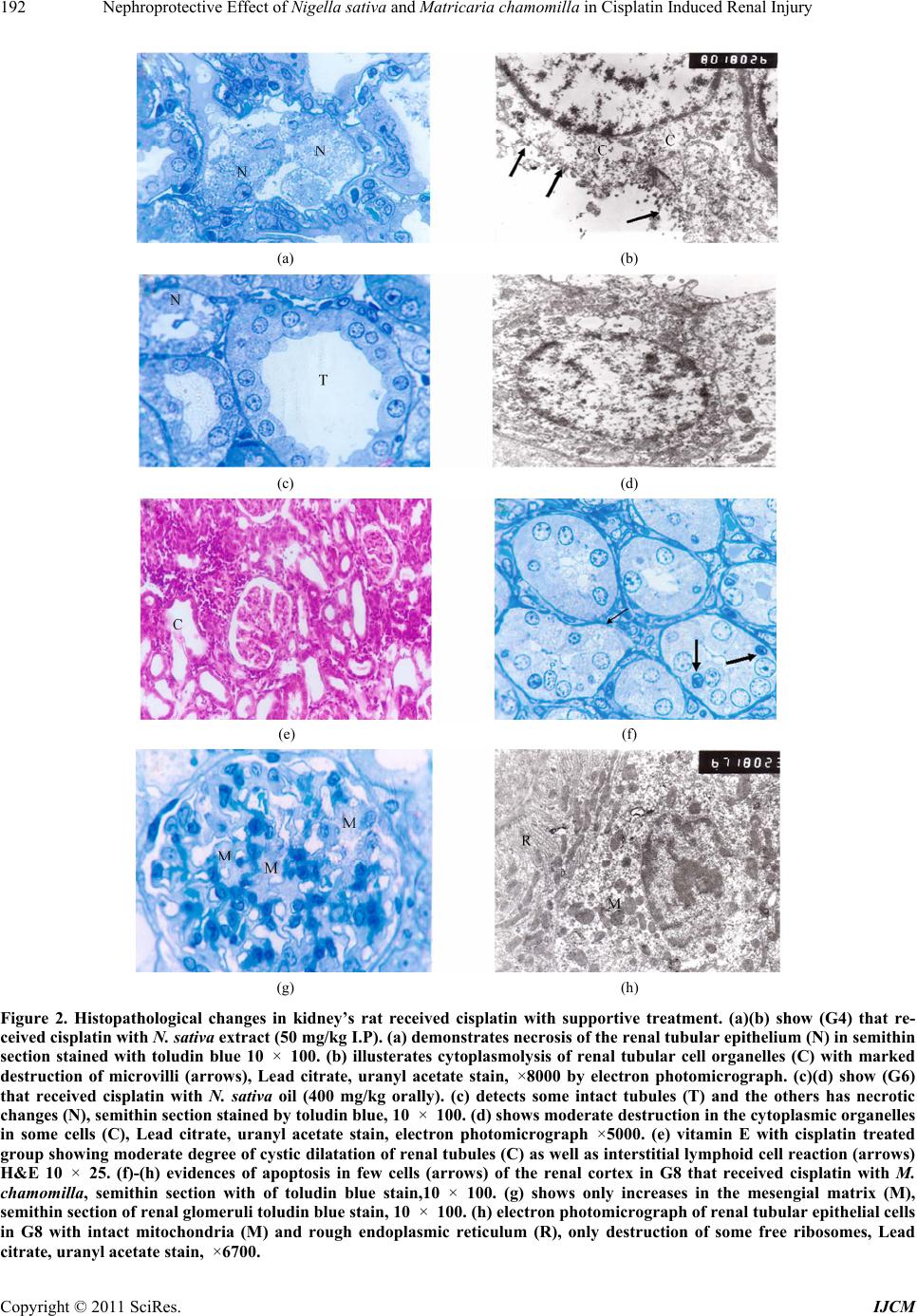 Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury 192 (a) (b) (c) (d) (e) (f) (g) (h) Figure 2. Histopathological changes in kidney’s rat received cisplatin with supportive treatment. (a)(b) show (G4) that re- ceived cisplatin with N. sativa extract (50 mg/kg I.P). (a) demonstrates necrosis of the renal tubular epithelium (N) in semithin section stained with toludin blue 10 × 100. (b) illusterates cytoplasmolysis of renal tubular cell organelles (C) with marked destruction of microvilli (arrows), Lead citrate, uranyl acetate stain, ×8000 by electron photomicrograph. (c)(d) show (G6) that received cisplatin with N. sativa oil (400 mg/kg orally). (c) detects some intact tubules (T) and the others has necrotic changes (N), semithin section stained by toludin blue , 10 × 100. (d) shows moderate destruction in the cytoplasmic organelles in some cells (C), Lead citrate, uranyl acetate stain, electron photomicrograph ×5000. (e) vitamin E with cisplatin treated group showing moderate degr ee of cystic dilatation of renal tubules (C) as well as interstitial ly mphoid cell reaction (arrows) H&E 10 × 25. (f)-(h) evidences of apoptosis in few cells (arrows) of the renal cortex in G8 that received cisplatin with M. chamomilla, semithin section with of toludin blue stain,10 × 100. (g) shows only increases in the mesengial matrix (M), semithin section of renal glomeruli toludin blue stain, 10 × 100. (h) electron photomicrograph of renal tubular epithelial cells in G8 with intact mitochondria (M) and rough endoplasmic reticulum (R), only destruction of some free ribosomes, Lead citrate, uranyl acetate stain, ×6700. Copyright © 2011 SciRes. IJCM  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury Copyright © 2011 SciRes. IJCM 193 Table 7. Intensity of the histopathological lesions in kidneys of rats of different cisplatin treated groups. G2 G4 G6 G8 G10 Apoptosis ++++ +++ ++ + ++ Necrobiosis of tubular epithelial cell ++++ +++ ++ + ++ Cystic dilatation ++++ +++ ++ -ve ++ Necrobiosis of glomerular tuft ++++ +++ ++ + ++ Renal Cortex Interstitial lymphoid cell reaction +++ ++ ++ + ++ Tubular casts ++++ + + -ve + Renal Medulla Angiopathic changes (bl.v.) +++ -ve -ve -ve -ve (++++): Severe and involved all animals. (+++): Severe and involved most of animals. (++): Moderate and involved some animals. (+): Weak and involved few animals. (-ve ): Negative. G1 is the healthy reference group; G2 is cisplatin treated group; G4 received Nigella sativa extract + cisplatin; G6 received Nigella sativa oil + cisplatin ;G8 received Matricaria chamomilla extract + cisplatin; G10 received vit. E + cisplatin. Data of groups 3, 5, 7, 9 that received only sup- portive treatment not shown as it is within normal range. decreased in group treated with cisplatin and M. chamo- milla as shown in Table 7 and Figure 2(f ) . GGT, a key enzyme of GSH metabolism, can modu- late crucial redox-sensitive functions, such as antioxi- dant/antitoxic defenses and cellular proliferative/ apop- totic balance. The mechanisms of GGT involvement in various pathological processes suggest its potential role as therapeutic target and diagnostic/prognostic marker [33]. The highest level of activity is on the luminal sur- face of the proximal tubule cells in the kidney. Its most common physiological substrates are glutathione and glutathione conjugates [34]. The nephrotoxicity of cis- platin was the result of the binding of cisplatin to glu- tathione and the subsequent metabolism of the cis- platin-glutathione complex via a γ-glutamyl transpepti- dase (GGT)-dependent pathway in the proximal tubules [23]. GGT cleaved the gamma-glutamyl group of the glutathione-conjugate, and aminopeptidase cleaved the cysteinyl-glycine bond, resulting in platinum-cysteine- conjugate. Finally the cysteine conjugate was metabo- lized by cysteine-s-conjugate beta-lyase to reactive thiol [35]. Also, they found that cisplatin was nephrotoxic in wild-type mice but not in GGT-deficient mice and the toxicity was specific to the proximal tubule cells. Acivi- cin, an inhibitor of GGT, blocks the nephrotoxicity of cisplatin in rats [36]. In this study, the highest level of GGT occurred in cisplatin group and the lowest level in (G8) that received combination of cisplatin and M. chamomilla as shown in Table 4. There is a correlation between GGT and NAG in cisplatin group (r = 0.731, P < 0.05). Moreover, M. chamomilla doesn’t contain glu- tamic or methionin [37] to help in regeneration of glu- tathione. Also, Chamazulene (one of constituents of M. chamomilla) affected free radical processes and inhibited lipid peroxidation in a concentration and time-dependent manner [38]. On the other hand, N. sativa seeds contain glutamic acid and methionine [39]; this may explain its antioxidant effect. The beneficial effects of the use of the N. sativa seeds and thymoquinone (one of its constituent) might be related to their cytoprotective and antioxidant actions, and to their effect on some mediators of inflam- mation [3]. Cisplatin administration caused hypocalcaemia [24, 40]. Calcium released from intracellular calcium storage in the early phase of nephrotoxicity caused oxidative stress in renal tubular epithelial cells [41]. The N. sativa seeds are a source of calcium, iron, and potassium [42]. This can explain the improvement in cisplatin-induced hypocalcaemia in groups received N. sativa oil with cis- platin. Zinc pre-treatment caused significant protection against cisplatin enhanced mortality in rats, and reduc- tion in lipid peroxidation and NO [43]. However, zinc content had an inverse correlation with platinum incor- poration owing to a positive linkage with glutathione (GSH), a zinc-dependent detoxification factor. The com- bined treatment with cisplatin and Zn(II)-chelator in- creased platinum uptake with a concomitant reduction of intracellular GSH [44]. Zinc was effective factor for pro- tection of weight loss and increased MDA levels in cis- platin hepatotoxicity [45]. N. sativa and M. chamomilla has been used as tea from long time indicate their safety and minor side effects. In addition, their nephroprotective effect may encourage physicians to prescribe them after and inbetween chemotherapy. 5. Acknowledgements The authors thank Professor Hanaa M. Sayed, Pharma- cognosy Department, Faculty of Pharmacy, Assiut Uni- versity, Egypt for her advice and help during extractions. REFERENCES [1] X. Yao, K. Panichpisal, N. Kurtzman and K. Nugent, “Cisplatin Nephrotoxicity: A Review,” The American Journal of Medical Sciences, Vol. 334, No. 2, 2007, pp. 115-124. doi:10.1097/MAJ.0b013e31812dfe1e [2] B. Shaikh and J.Hatcher, “Complementary and Alterna- tive Medicine in Pakistan: Prospects and Limitations,” Evidence-Based Complementary Alternative Medicine, Vol. 2, No. 2, 2005, pp. 139-142. doi:10.1093/ecam/neh088  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury 194 [3] B. H. Ali and G. Blunden, “Pharmacological and Toxico- logical Properties of Nigella sativa,” Phytother Research, Vol. 17, No. 4, 2003, pp. 299-305. doi:10.1002/ptr.1309 [4] M. R. Heidari, Z. Dadollahi, M. Mehrabani, H. Mehrabi, M. Pourzadeh-Hosseini, E. Behravan and L. Etemad, “Study of Antiseizure Effects of Matricaria recutita Ex- tract in Mice,” Annals of the New York Academy of Sci- ence, Vol. 1171, 2009, pp. 300-304. doi:10.1111/j.1749-6632.2009.04917.x [5] D. L McKay and J. B Blumberg, “A Review of the Bio- activity and Potential Health Benefits of Chamomile Tea (Matricaria recutita L.),” Phytother Research, Vol. 20, No. 7, 2006, pp. 519-530. [6] V. M. Chandrashekhar, V. L Ranpariya, A. Parashar, A. A. Muchandi and S. Ganapaty, “Neuroprotective Activity of Matricaria recutita Linn against Global Model of Ischemia in Rats,” Journal of Ethnopharmacology, Vol. 127, No. 3, 2010, pp. 645-651. [7] I. Barene, I. Daberte, L. Zvirgzdina and V. Iriste, “The Complex Technology on Products of German Chamo- mile” Medicina, Vol. 39, No. 2, 2003, pp. 127-131. [8] E. S. El-Daly, “Protective Effect of Cysteine and Vitamin E, Crocus sativus and Nigella sativa Extracts on Cis- platin-Induced Toxicity in Rats,” Journal de Pharmcie de Belgique, Vol. 53, No. 20, 1998, pp. 87-95. [9] H. P. Lipp and J. T. Hartmann, “Platinum Compounds: Metabolism, Toxicity and Supportive Stratigies,” Praxis, Vol. 94, No. 6, 2005, pp. 187-198. [10] R. van Bezoijen, I. Que, A. G. Ederveen, H. J. Klooster- boer, S. E. Papapoulos and C. W. Lowik, “Plasma Nitrate and Nitrite Level are Regulated by Ovarian Steroids But do not Correlate with Trabecular Bone Mineral Density in Rats,” Journal of Endocrinology, Vol. 159, 1998, pp. 27- 34. doi:10.1677/joe.0.1590027 [11] W. Thayer, “Lipid Peroxide in Rats Treated Chronically with Adriamycin,” Biochemical Pharmacology, Vol. 33, No. 14, 1984, pp. 2259-2263. doi:10.1016/0006-2952(84)90664-6 [12] H. P. Misra and I. Fridovich, “The Role of Super Oxide ion in the Oxidation of the Epinephrine and a Simple As- say for Superoxide Dismutase,” The Journal of Biological Chemistry, Vol. 247, No. 10, 1972, pp. 3170-3187. [13] G. L. Ellman, “Tissue Sulfhydryl Groups,” Archives Bio- chemistry and Biophysics, Vol. 82, No. 1, 1959, pp. 70- 77. doi:10.1016/0003-9861(59)90090-6 [14] D. Maruhn, “Rapid Colorimetric Assay by -Galactosi- dase and N-Acetyl- -Glucosaminidase in Human Urine,” Clinica Chimica Acta, Vol. 73, No. 3, 1976, pp. 453-461. doi:10.1016/0009-8981(76)90147-9 [15] E. Beutler, O. Duron and B. Kelly, “Improved Method for Determination of Blood Glutathione,” Journal of Labo- ratory and Clinical Medicine, Vol. 61, 1963, pp. 882-888. [16] S. Kühn and M. deKock, “A Preliminary Study of Ele- vated Alkaline Phosphatase and Cathepsin in Bronchial Aspirates of Patients with Lung Cancer and Bronchitis,” CHEST, Vol. 38, No. 3, 1975, pp. 326-330. [17] C. E. Paradones, V. A. Illera, D. Peckham, L. L. Stunz and R. F. Ashman,” Regulation of Apoptosis in Vitro in Mature Spleen T Cells,” The Journal of Immunology, Vol. 151, No. 7, 1993, pp. 3521-3529. [18] G. Bancroft and L. Stevens, “A Theory and Practice of Histology Technique,” 2nd Edition, Churchili Livingston, Philadelphia, Vol. 5, 1982, pp. 433-516. [19] B. Bonevski, A. Wilson and D. A Henry, “An Analysis of News Media Coverage of Complementary and Alterna- tive Medicine,” PLoS One., Vol. 3, No. 6, 2008, p. 2406. [20] Z. Hawsawi, B. Ali and A. Bamosa, “Effect of Nigella sativa (Black Seed) and Thymoquinone on Blood Glu- cose in Albino Rats,” Annals of Saudi Medicine, Vol. 21 No. 3-4, 2001, pp. 242-244. [21] O. A. Ghosheh, A. A. Houdi and P. A. Crooks, “High Performance Liquid Chromatographic Analysis of the Pharmacologically Active Quinones and Related Com- pounds in the Oil of the Black Seed (Nigella sativa L.),” Journal of Pharmaceutical and Biomedical Analysis, Vol. 19, No. 5, 1999, pp. 757-762 doi:10.1016/S0731-7085(98)00300-8 [22] I. Ogata, T. Kawanai, E. Hashimoto, Y. Nishimura, Y. Oyama and H. Seo, “Bisabololoxide A, One of the Main Constituents in German Chamomile Extract, Induces Apoptosis in Rat Thymocytes,” Archives Toxicology, Vol. 84, No. 1, 2010, pp. 45-52. doi:10.1007/s00204-009-0472-5 [23] M. H. Hanigan, E. D. Lykissa, D. M. Townsend, et al., “Gamma Glutamyl Transpeptidase-Deficient Mice are Resistant to the Nephrotoxic Effects of Cisplatin,” Ame- rican Journal of Pathology, Vol. 159, No. 5, 2001, pp. 1889-1894. doi:10.1016/S0002-9440(10)63035-0 [24] S. Y. Saad, T. A. O. Najjar, M. H. Daba and A. C. Al-Rikabi, “Inhibition of Nitric Oxide Synthase Aggre- vates Cisplatin Induced Nephrotoxicity: Effect of 2-Ami- no-4-Methylpyridine,” Experimental Chemotherapy, Vol. 48, No. 6, 2002, pp. 309-315. doi:10.1159/000069714 [25] A. A. Al-Majed, A. R. A. Abd-Allah A. C. Al-Rikabi, O. A. Al-Shabanah and A. M. Mostafa, “Effect of Oral Ad- ministration of Arabic Gum on Cisplatin-Induced Neph- rotoxicity in Rats,” Journal of Biochemical and Molecu- lar Toxicology, Vol. 17, No. 3, 2003, pp. 146-153. doi:10.1002/jbt.10072 [26] K. J. Weichert-Jacobsen, A. Bannowski, F. Küpp.ers, T. Loch and M. Stöckle, “Direct Amifostine Effect on Renal Tubule Cells in Rat,” Cancer Research, Vol. 59, No.14, 1999, pp. 3451-3453. [27] Y. Kawai, S. Taniuchi, S. Okahara, M .Nakamura and M. Gemba, “Relationship between Cisplatin or Nedaplatin Induced Nephrotoxicity and Renal Accumulation,” Bio- logical and Pharmaceutical Bulletin, Vol. 28, No. 8, 2005, pp. 1385-1388. doi:10.1248/bpb.28.1385 [28] C. Bazzi, C. Petrini,V. Rizza, G. Arrigo, M. Paparella, P. Napodano, et al., “Urinary N-Acetyl-β-Glusominidase Excretion is a Marker of Tubular Cell Dysfunction and a Predictor of Outcome in Primary Glomerulonephritis,” Nephrology Dialysis Transplantation, Vol. 17, No. 11, Copyright © 2011 SciRes. IJCM  Nephroprotective Effect of Nigella sativa and Matricaria chamomilla in Cisplatin Induced Renal Injury Copyright © 2011 SciRes. IJCM 195 2002, pp. 1890-1896. doi:10.1093/ndt/17.11.1890 [29] S. Lee, W. Kim, S. Moon, M. Sung, D .Kim, K. Kang, et al., “Protective Role of L-2-Oxothiazolidine-4-Carboxylic Acid in Cisplatin-Induced Renal Injury,” Nephrology Di- alysis Transplantation, Vol. 21, No. 8, 2006, pp. 2096- 2105. doi:10.1093/ndt/gfl194 [30] N. Sheena, T. Ajith and K. Janardhanan, “Prevention of Nephrotoxicity Induced by the Anticancer Drug Cisplatin, Using a Medicinal Mushroom Occurring in South India,” Current Science, Vol. 85, No. 4, 2003, pp. 478-482. [31] L. M. G. Antunes, J. D. C. Darin, M. de L. P. Bianchi, “Protective Effects of Vitamin C against Cisplatin-Induced Nephrotoxicity and Lipid Peroxidation in Adult Rats: A Dose Dependent Study,” Pharmacology Research, Vol. 41, No. 4, 2000, pp. 405-411. doi:10.1006/phrs.1999.0600 [32] I. Hara, H. Miyake, K. Yamanaka, S. Hara and S. Kami- dono, “Serum Cathepsin D and Its Density in Men with Prostate Cancer as New Predictors of Disease Progres- sion,” Oncology Reports, Vol. 9, No. 6, 2002, pp. 1379- 1383. [33] A. Pompella, A. Corti, A. Paolicchi, C. Giommarelli and F. Zunino, “Gamma-Glutamyltransferase, Redox Regula- tion and Cancer Drug Resistance,” Current Opinion in Pharmacology, Vol. 7, No. 4, 2007, pp. 360-366. doi:10.1016/j.coph.2007.04.004 [34] M. W Lieberman, A. L Wiseman, Z. Shi, B. Z Carter, R. Barrios, C. Ou, et al., “Growth Retardation and Cysteine Deficiency in Gamma-Glutamyl Transpeptidase-Deficient Mice,” Proceedings of the National Academy of Sciences, Vol. 93, No. 15, 1996, pp. 7923-7926. doi:10.1073/pnas.93.15.7923 [35] D. M. Townsend, M. Deng, L. Zhang, M. Lapus and M. Hanigan, “Metabolism of Cisplatin to a Nephrotoxin in Proximal Tubule Cell,” Journal of the American Society of Nephrology, Vol. 14, No. 1, 2003, pp. 1-10. doi:10.1097/01.ASN.0000042803.28024.92 [36] D. M. Townsend and M. H. Hanigan, “Inhibition of Gamma-Glutamyl Transpeptidase or Cysteine S-Conju- gate Beta-Lyase Activity Blocks the Nephrotoxicity of Cisplatin in Mice,” The Journal of Pharmacology and Experimental Therapeutics, Vol. 300, No. 1, 2002, pp. 142-148. doi:10.1124/jpet.300.1.142 [37] H. Viola, C. Wasowski, M. Levi de Stein, et al., “Api- genin, a Component of Matricaria recutita Flowers, Is a Central Benzodiazepine Receptors-Ligand with Anxioly- tic Effects,” Planta Medica, Vol. 61, No. 3, 1995, pp. 213- 216. doi:10.1055/s-2006-958058 [38] E. A. Rekka, A. P. Kourounakis and P. N. Kourounakis, “Investigation of the Effect of Chamazulene on Lipid Per- oxidation and Free Radical Processes,” Search of Com- munications in Molecular Pathology and Pharmacology, Vol. 92, No. 3, 1996, pp. 361-364. [39] M. Boskabady, S. Kiani, P. Jan Daghi, T. Ziaei and A. Zarei, “Comparison of Antitussive Effect of Nigella sa- tiva with Codeine in Guinea Pig,” International Journal of Molecular Sciences, Vol. 28, No. 3, 2003, pp. 111-115. [40] M. Krych, “Acute Renal Failure,” Der Internist, Vol. 46, No. 1, 2005, pp. 30-38. doi:10.1007/s00108-004-1316-2 [41] Y. Kawai, T. Nakao, N. Kunimura, Y. Kohada and M. Gemba, “Relationship of Intracellular Calcium and Oxy- gen Radicals to Cisplatin Related Renal Cell Injury,” Journal of Pharmacological Sciences, Vol. 100, No. 1, 2006, pp. 65-72. doi:10.1254/jphs.FP0050661 [42] A. M. Al-Gaby, “Amino Acid Composition and Biologi- cal Effects of Supplementing Broad Bean and Corn Pro- teins with (Black Cumin) Cake Protein,” Die Nahrung, Vol. 42, No. 5, 1998, pp. 290-294. [43] S. Joshi, S. K. Hasan, R. Chandra, M. M. Husain and R. C. Srivastava, “Scavenging Action of Zinc and Green Tea Polyphenol on Cisplatin and Nickel Induced Nitric Oxide Generation and Lipid Peroxidation in Rats,” Biomedical and Environmental Sciences, Vol. 17, No. 4, 2004, pp. 402-409. [44] M. Shimura, A .Saito, S. Matsuyama, T. Sakuma, Y. Terui, K. Ueno, et al., “Element Array by Scanning X-Ray Fluorescence Microscopy after Cis-Diamminedi- chloro-Platinum (II) Treatment,” Cancer Research, Vol. 65, No. 12, 2005, pp. 4998-5002. doi:10.1158/0008-5472.CAN-05-0373 [45] Y. J. Liao, X. Q. Lu, C. W. Lu, G. X. Li, Y. P. Jin and H. Tang, “Selection of Agents for Prevention of Cisplatin- Induced Hepatotoxicity,” Pharmacology Research, Vol. 57, No. 2, 2008, pp. 125-131. doi:10.1016/j.phrs.2008.01.001
|