 America n Journal of Analy tic al Chemistry, 2011, 2, 376-382 doi:10.4236/ajac.2011.23046 Published Online July 2011 (http://www.scirp.org/journal/ajac) Copyright © 2011 SciRes. AJAC Imprinted Polymer Inclusion Membrane Based Potenti om et ric S en sor fo r Determinat i on an d Quantification of Diethyl Chlorophosphate in Natural Waters Varada Vishnuvardhan1, Yakka la Kalyan1, Krishn apillai Padmajaku mar i Prathish2, Battala Gangadhar1, Yadamari Tharakeswar1, Talasila Prasada Rao2, Gurijala Ramakrishna Naidu1* 1Department of Environmental Sciences, Sri Venkateswara University, Tirupati, India 2National Institute for Interdisciplinary Science & Technology, CSIR, Trivandrum, India E-mail: naidugrk@yahoo.com Received January 3, 2011; revised Janua ry 26, 2011; accepted May 6, 2011 Abstract Biomimetic potentiometric sensor for the determination of diethyl chlorophosphate was developed using im- pr int ed pol ymer inclusion membrane strategy. Semi-covalent i mprinted and non-imprinted polymer particles were synthesized and found that non-imprinted polymer inclusion membrane was unstable in contrast to im- printed polymer inclusion membrane in determination and quantification of diethyl chlorophosphate. Im- printed polymer inclusion membrane based sensor found to be pH dependant with a 5 min equilibrium re- sponse time at pH = 10.5 and linearly responds to diethyl chlorophosphate in the concentration range of 1 × 10–9 to 1 × 10–4 a nd 1 × 10–4 to 1 × 10–2 mol·L–1 with a detection limit of 1 × 10–9 mol·L–1 (0.17 ppb). It was found that diethyl chlorophosphate response was selective against various selected interferents like pinacolyl methylphosphonate, dimethyl methyl phosphonate, methylphosphonic acid, Phorate and 2, 4-D. The devel- oped sensor was found to be stable for 3 months and can be reusable more than 30 times without loosing sensitivity. The developed sensor was successfully applied for the determination of diethyl chlorophosphate in natural waters. Keywords: Sensor, Imprinted Polymer Inclusion Membrane, Potentiometry, Diethyl Chlorophosphate, Natural Waters 1. Introduction Growing concerns with regard to determine the trace amounts of chemical warfare agents (CWAs) in envi- ronment, necessitate to the continuous development of simple analytical methods that can be employed as long term monitoring aids, used primarily as alarms. Nerve agents, in particular, are among the most lethal CWAs. The uses of nerve agents by terrorist organizations or even states are significant as they can be readily synthe- sized by simple chemical reactions and often have an extremely high toxicity. Highly toxic nerve agents such as G series agents-(GA) Tabun, GB (Sarin), GD (So- man), GF and V series agents-VE, VG, VM and VX are pow- erful inhibitors of acetyl cholinesterase, which is critical in nerve function [1]. The use of nerve agents in 1988 that killed thousands of Kurdish villagers and 1991 Gulf war further emphasized the threat of chemical war- fare [2]. Two Sarin gas attacks in Matsumotoa and Tokyo, Japan in 1994-1995 and events in the United States in 2001 have confirmed this horrible reality [3]. Due to the lethality of these agents, detection and moni- tor ing of ner ve age nts a re of prime importance in overall safety and security of humans, animals and plants. Therefore, it is necessary to develop detection systems that are port- able, inexpensive, simple, rapid, selective and sensitive for analyzing environmental security threats. The molecular imprinting technique [4] continues to be a fascinating field of analytical chemistry offering strategies for creating molecule-specific recognition ma- trices with recognition capabilities analogous to those o f  V. V I SHN UVAR D HAN ET AL. Copyright © 2011 SciRes. AJAC biological receptors [5]. The shape, size and p ositions of the functional groups in the recognition sites generated are complementary to thos e of the o rigina l anal yte . Thus, molecularly imprinted polymers (MIPs) rebind their original analytes in preference to related molecules. MIPs have considerable potential for applications in the areas of clinical analysis, medical diagnostics, environ- mental monitoring and drug delivery. Imprinted polymer materials possess several other virtues viz. physical and chemical stability, storage endurance and imprint mem- ory which is esse ntial for preparation of recognition me- mbranes in a robust and reusable sensing device. More- over, MIPs are usually cheaper and more accessible high affinity rec ognition material s in contrast to many biolo g- ical entities [6]. Because of the high toxicity of CWAs, less toxic structural analogs that directly mimic or imitate the ac- tual CWAs of simulant diethyl chlorophosphate (DCP) detection is very important task for verification studies since the corresponding simulant can be used to indicate the presence of the nerve agents produced or used. There have been many innovations for the detection of this species including colorimetric detection methods [7,8] surface acoustic wave devices [9,10], enzymatic assays [11], interferometry [12] and fluo re sce nt sensor s [1 3 -15]. However, all are plagued by at least one limitation or other such as slow response, lack of selectivity, poor sensitivity, operational complexities or non-portability. Even though sensitivities are high, most of the fluores- cent detectors performances have been demonstrated in non-aqueous media, which makes them unsuitable for real time analysis, which may require tedious extraction procedures. Pote ntiometric sensor s, a subgroup of chemi - cal sensors, are attractive for practical applications, as they are associated with small size, portability and low energy consumption and cost compared to other group of sensors. The development of MIP based sensors with potentio metric transduct ion do es not require the te mplate or print molecule to be extracted from the membrane to create membrane potential and does not have to diffuse through the membra ne, so that there is no size restriction on the template molecule, the main achilles heel of MIP’s until recently [16-18]. Zhou, et al. [19] reported for the first time MIP based potentiometric sensor for methylphosphonic acid, an ultimate degradation product of nerve age nts b y c oupl ing sur face i mp rin ting t ec hnique with a nanoscale transducer, indium tinoxide. The litera- ture reveals that the prepared imprinted polymer mem- branes can be effectively used for the detection of nerve agents by fabricating them into potentiometric sensors [19-21]. These sensing devices are essentially based on use of polymer materials prepared via non-covalent strategy. However, due to non-persistent nature of nerve agents, in non-covalent strategy, the decomposition pro- ducts of nerve agents lead to a variety of binding sites depending on the nature of decomposition products. The previous studies [22] had succeeded in construction of semi-covalent strategy based in-situ monolithic polymer membrane based sensor for DCP via single pot synthesis. The extractio n of all possible degrad ation pro ducts o f the chemical warfare agents using molecularly imprinted polymers were developed by various research groups [20,23,24]. The semi-covalent strategy has been suc- cess fully d emonstra ted in t he pr esent stud y for the fabr i- cation of imprinted polymer inclusion membrane (IPIM) based sensor for the detection and quantification of DCP , a si mulant of so man pre sent in sp iked na tural wat ers. As this technology does not require large instrumentation, it has feasibility for routine monitoring studies for the de- termination of chemical warfare agents or their simu- lants, where samplin g is dif ficult and t his met hod ca n be extensively applied for the determination of trace amou nts o f c ont a mi na nt s i n e nvir o n ment , p ha rmaceutical and food processing industries and in biomedical appli- cations 2. Experimental 2.1. Reagents and Electrochemical Equipment Diethyl chlorophosphate (DCP), 4-vinyl aniline (VA), dimethyl methyl phosphonate (DMMP), pinacolyl meth- ylphosphonate (PMP), methylphosphonic acid (MPA) were obtained form Aldrich (Milwauke, WI, USA). Phorate, 2,4-D were obtained from SUPELCO, Penn- sylvania, USA. 2-hydroxyethyl methacrylate (HEMA), ethylene glycol dimethacrylate (EGDMA), 2,2’-azobis isobutyronitrile, 2-nitrophenyl octyl ether (NPOE), di-n- octyl phthalate (DOP), bis-(2-ethylhexyl) sebacate (BE HS), tris-(2-eth ylhexyl ) phosp hate (T EHP) a nd high mo- lecular mass poly (vinyl chlor ide) (PVC) were purchased from Aldrich (Milwauke, WI, USA). All other chemicals were of analytical grade reagents. Stock standard solu- tion of (0.1 mol·L–1) DCP was prepared by dissolving 1.725 g of DCP in 100 mL of deionized water. The solu- tions of 1.0 × 10–2 to 1.0 × 10–11 mo l·L–1 were prepared by aqueous dilution of a definite volume from the stock standard solution. Deionized water was used throughout the experiment. Potentiometric response characteristics were studied with an ELICO makes Ion analyzer, Model No. LI 126 (ELICO, Hyderabad, India). 2.2. Synthesis of Semi-Covalent Imprinted and Non-Imprinted Po ly mer Parti cles Diethyl chlorophosphate imprinted particles were syn-  V. V I SHN UVAR D HAN ET AL. Copyright © 2011 SciRes. AJAC thesized as follows, 1 mmol of DCP, 1 mmol of VA to form covalent bond with DCP thus resulting in covalent interactions, 8 mmol HEMA, 32 mmol of EGDMA, 0.03 g of AIBN and 5 mL of methanol (porogen) were taken in a 50 mL round bottom flask. The mixture was purged with N2 for 5 min. and the flask was sealed under inert atmosphere. Then it was kept for stirring in an oil bath maintained at a temperature of 60˚C. Af ter 2 h, the mate- rial obtained was ground and sieved and the particles with sizes between 50 - 105 m were collected. The re- sulting imprinted particles obtained were washed with metha nol a nd the n leac hed with 0. 1 mol·L–1 HCl for 1 h. During leaching (hydrolysis) the template DCP is str ip - ped off as the diethylphosphonic acid. So the cavity formed in the polymer matrix on leaching presumably corresponds to that of diethylphosphonic acid. During the rebinding process, the analyte DCP when added to the sample solution containing tris buffer will undergo hydrolysis to form diethylphosphonic acid [25], which is same a s that o f the leached template. Therefore, it can be construed that template rebinding takes place by non- covalent interactions. The covalent imprinting and sub- sequent rebinding via non-covalent interactions will be termed as semi-covalent strategy. The non-i mprinted polymer particles were synthesized, washed and treated analogous to the imprinted polymer in the absence of template, i.e. DCP during syn thesis. 2.3. Casting of Semi-Covalent Imprinted and Non-Imprinted Po ly mer I nclus ion Mem- branes The polymer inclusion membranes were cast by the fol- lowing procedure mentioned below. DCP imprinted po- lymer particles (90 mg) synthesized via semi-covalent strategy were dispersed in 0.2 mL of NPOE and were mixed with tetrahydrofuran (THF) (2.5 mL) solution of PVC (90 mg). The sol utio ns were ho moge nize d and t hen poured into a Teflon mould having an internal diameter of 21 mm. The THF on evaporation at room temperature results in the formation of imprinted polymer inclusion membranes of thickness ~0.45 mm. In a similar manner non-imprinted polymer inclusion membranes were also casted. 2.4. Sensor Fabrication and EMF Measurement The imprinted and non-imprinted membranes cast via inclusion by semi-covalent strategy were glued to one end of a pyrex glass tube with Araldite. The tube was then filled with an internal filling solution of 10–3 mol ·L–1 DCP. A schematic diagram of membrane for ma- tion and fabrication of biomimetic potentiometric sensor is given in (Figure 1). The sensor was kept in air when not in use. Figure 1. A Schematic representation of membrane forma- tion and f abrication of IPIM ba sed sensor for DCP. Table 1. Effect of plasticizers on potential response of IPIM based sensor for each decade. DCP (m ol·L–1) Potential response (mV/decade) 1 × 10 to 1 × 10 40.0 21.0 21.0 24.0 Sensors were conditioned in 10–5 mol ·L–1 DCP solu- tion +0.1 mo l·L–1 tris buffer (adjusted to pH 10.5) for 24 h and the n s ti rr ed i n tr is b uf fer for 1/2 h to remo ve b ound DCP ions after which the membranes would generate stable potentials. The test solution whose pH was main- tained at 10.5 after the addition of 5 mL of 1 mo l·L–1 tris buffer was taken and response of the sensor was exam- ined by measuring the electromotive force (EMF) of the following electrochemical cell. Ag-AgCl10–3 mol·L–1 DCP| DCP membrane║test solution| Hg-HgCl2·KCl (sa- turated). The potential responses of the sample solu- tions containing different concentrations (1.0 × 10–11 to 1.0 × 10–2 mol·L–1) of DCP in 50 mL of 0.1 mol·L–1 Tris buffer (pH 10.5) was measured. The EMF was plotted as a func t ion of DCP concent ration. 2.5. Analysis of Natural Water Samples The river water or ground water samples (~45 mL) were adjusted to pH = 10.5 after the addition of 5 mL of 1 mol ·L–1 tris buffer using HCl or NaOH. The samples were analyzed using the above fabricated IPIM based potentiometric sensor by following the analytical proce- dure mentioned in section 2.4. 3. Results and Discussions The optimal design of the membrane enables the per- 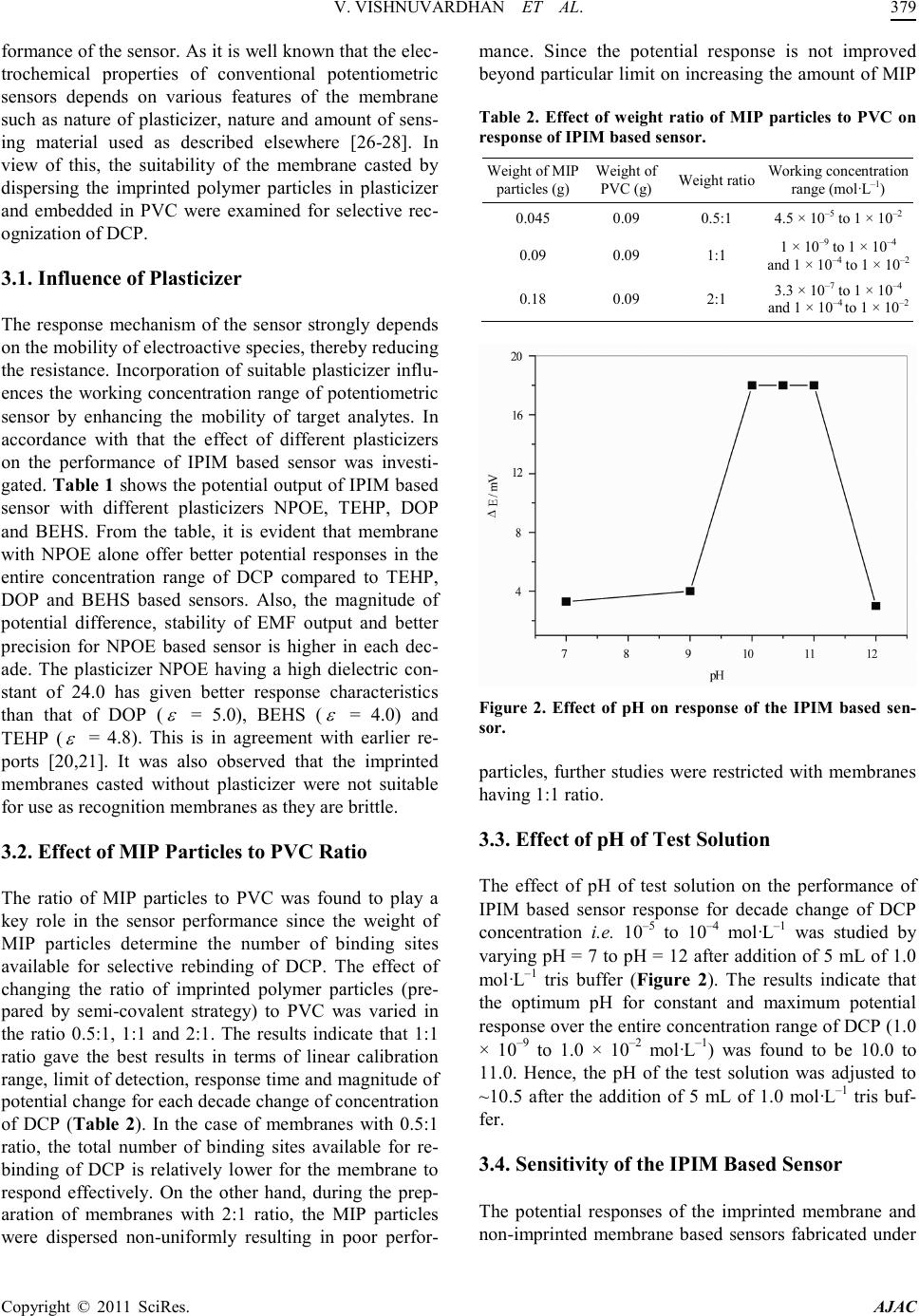 V. V I SHN UVAR D HAN ET AL. Copyright © 2011 SciRes. AJAC formance of the sensor . As it is well known that the elec- trochemical properties of conventional potentiometric sensors depends on various features of the membrane such as nature of plasticizer, nature and amount of sens- ing material used as described elsewhere [26-28]. In view of this, the suitability of the membrane casted by dispersing the imprinted polymer particles in plasticizer and embedded in PVC were examined for selective rec- ognization of DCP . 3.1. Influen ce of Plast ic iz er The response mechanism of the sensor strongly depends on the mobilit y of electr oacti ve species, thereby reducing the resistance. Incorporation of suitable plasticizer influ- ences the working concentration range of potentiometric sensor by enhancing the mobility of target analytes. In accordance with that the effect of different plasticizers on the performance of IPIM based sensor was investi- gated. Ta ble 1 shows the po tential output of IPIM b ased sensor with different plasticizers NPOE, TEHP, DOP and BEHS. From the table, it is evident that membrane with NPOE alone offer better potential responses in the entire concentration range of DCP compared to TEHP, DOP and BEHS based sensors. Also, the magnitude of potential difference, stability of EMF output and better precision for NPOE based sensor is higher in each dec- ade. The plasticizer NPOE having a high dielectric con- stant of 24.0 has given better response characteristics than that of DOP ( = 5.0), BEHS ( = 4.0) and TEHP ( = 4.8). This is in agreement with earlier re- ports [20,21]. It was also observed that the imprinted membranes casted without plasticizer were not suitable for use as recognition membranes as they are brittle. 3.2. Effect o f MIP Particles to PVC Ratio The ratio of MIP particles to PVC was found to play a key role in the sensor performance since the weight of MIP particles determine the number of binding sites available for selective rebinding of DCP. The effect of changing the ratio of imprinted polymer particles (pre- pared by semi-covalent strategy) to PVC was varied in the ratio 0.5:1, 1:1 and 2:1. The results indicate that 1:1 ratio gave the best results in terms of linear calibration range, limit of detection, response time and magnitude of potential change for each decade change of concentration of DCP (Table 2). In the case of membranes with 0.5:1 ratio, the total number of binding sites available for re- binding of DCP is relatively lower for the membrane to respond effectively. On the other hand, during the prep- aration of membranes with 2:1 ratio, the MIP particles were dispersed non-uniformly resulting in poor perfor- mance. Since the potential response is not improved beyond par ticular limit on inc reasing the amount o f MIP Table 2. Effect of weight ratio of MIP particles to PVC on response of IPIM base d sensor. Weight of MIP particl es (g) Weight of PVC (g) Weight ratio Working concentration range (mol·L–1) 0.045 0.09 0.5:1 4.5 × 10–5 to 1 × 10–2 0.09 0.09 1:1 1 × 10–9 to 1 × 10–4 and 1 × 10–4 to 1 × 10–2 0.18 0.09 2:1 3.3 × 10–7 to 1 × 10–4 and 1 × 10–4 to 1 × 10–2 Figure 2. Effect of pH on response of the IPIM based sen- sor. particles, further studies were restricted with membranes having 1:1 ratio. 3.3. Effect of pH of Test Solution The effect of pH of test solution on the performance of IPIM based sensor response for decade change of DCP concentration i.e. 10–5 to 10–4 mo l·L–1 was studied by var yin g pH = 7 to pH = 12 after addition of 5 mL of 1.0 mol ·L–1 tris buffer (Figure 2). The results indicate that the optimum pH for constant and maximum potential response over the entire concentration range of DCP (1.0 × 10–9 to 1.0 × 10–2 mo l·L–1) was found to be 10.0 to 11.0. Hence, the pH of the test solution was adjusted to ~10.5 after the addition of 5 mL of 1.0 mol·L–1 tris buf- fer. 3.4. Sensitivity of the IPIM Based Sensor The potential responses of the imprinted membrane and non-imprinted membrane based sensors fabricated under  V. V I SHN UVAR D HAN ET AL. Copyright © 2011 SciRes. AJAC the optimal conditions arrived above was studied and the results obtained are shown in (Figure 3). It can be no- ticed from the figure that the plot obtained for the IPIM based sensor gave a linear calibration curve in the con Figure 3. Potential response of the IPIM and NIPM based sensors with respect to DCP concentration. Figure 4 . Potentiometric resp onse of the IPIM based sensor to DCP and other selected interferents for each decade change of concentrati on from 1 × 10–9 to 1 × 10–2 mol L–1. centration range 1 × 10–9 to 1 × 10–4 and 1 × 10–4 to 1 × 10–2 mo l·L–1 of DCP. On the other hand, the non-im printed polymer inclusion membrane (NIPIM) based sensor gave linear response for DCP in the concentration range 1 × 10–4 to 1 × 10–2 mol·L–1. It was observed that the absolute potentials obtained from NIPIM based sen- sor were unstable which is due to nonspecific binding o f analyte in contrast to the specific site selective binding in the case of IPIM based sensor. In addition, the LOD for IPIM and NIPIM based sensors calculated based on IUPAC recommendation [29] were found to be 1 × 10–9 mol ·L–1 and 1 × 10–4 mol·L–1, respectively. Whereas the higher value for IPIM based sensor over NIPIM based sensor in the entire concentration range is attri- buted to significant imprinting effect. The equilibrium Table 3. Co mpariso n of experi mental sel ectivi ty coef ficient s of DCP against various selected interferents using NIPIM and IPIM based sensors. Inter ferents = Potentiometric se lectivity coefficient ; A = DCP; B = Interferent. response time was found to be 5 min. for the IPIM based sensors employing particles prepared by semi-covalent strategy. 3.5. Selectivity of the IPIM Based Sensor Selectivity refers to the extent of suitability of the de- veloped IPIM based sensors to determine particular ana- lyte in mixtures or matrices without interferences from other components. In environmental applications, the concentrations of the analytes are quite low and thus, high selectivity is essential for an effective monitoring. Hence, the selectivity of the developed IPIM based sen- sor with various common simulants (PMP and DMMP) and degradation product (MPA) of CWAs, pesticides like phorate and 2,4-D which may co-exist in real sam- ples were tested. The response profiles of DCP and se- lected coexisting interferents obtained with IPIM based sensor fabricated with particles prepared by semi-covale nt strate gy are shown in Fig ure 4. T he high- er selectivity noticed in the case of IPIM based sensor can be attributed to the more rigid polymeric structure leading to more stabilized cavities. Similar imprinting effec t can also be visualized from Table 3 a nd compares the selectivity coefficients of DCP over selected interfe- rents o btained by IPIM based sensor with corresponding NIPIM based sensor by employing IUPAC method [30] as described elsewhere. 3.6. Stability and Reus a bil ity Another important criteria for any sensing device in ad- dition to sensitivity and selectivity is stability and reusa- bility. The developed IPIM based sensors prepared by employing semi-covalent strategy were found to be sta- ble with deviatio ns less than 0 .5 mV for 1 × 10–4 mol·L–1  V. V I SHN UVAR D HAN ET AL. Copyright © 2011 SciRes. AJAC DCP for 3 months and can be reused for more than 30 times without loosing sensing abilit y. 3.7. Analytical Application to Natu ral Water Samples It was succe ss fully ap plied to natural water samples, as it Table 4. Analysi s of natural water samples. Sample selected interferents in mixture spiked to nat ura l wa ters –1 a DCP added (× 10–8 mol· L–1) DCP fou ndb (× 10–8 mol· L–1) Reco very (%) Ground water Ri v er water aMixture of PMP, DMMP, MPA, Phorate and 2,4-D; bAverage of three determinations. is clear from the selectivity studies that several interfe- rents co-exist in real samples do not have any deleterious effect on IPIM based sensor performance. Ground and river water samples were analyzed by spiking known amounts of DCP and varying concentrations of interfe- rent mixtures. The results thus obtained are shown in Table 4. T he recovery obtained in the range 98% - 101% shows that the developed IPIM based sensor can reliably be used for monitoring the natural waters, which, if found, can alert the authorities for appropriate control measures. 4. Conclusions Semi-co va l ent i mprinted and non-imprinted polymer par- ticles were synthesized and found that non-imprinted polymer inclusion membrane (NIPIM) was unstable in contrast to imprinted polymer inclusion membrane (IPIM) in determination and quantification of DCP. In addition, the sensor performance of the IPIM based sen- sor is remarkable with a detection limit of 1 × 10–9 mol ·L–1 (0.17 ppb) compared to corresponding NIPIM based sensor. The interferents co-exist in real sa mples do not have any deleterious effect on IPIM based sensor performance. The recovery studies of DCP from ground and river waters indicated the possibility of using the sensor investigated in the present work for nature water samples. The developed technology found to be a effec- tive analytical tool as it requires simple sample prepara- tion procedures and does not require large instrumenta- tion as it is a miniat ure device that respo nds to a particu- lar analyte in a selective way and can be effectively used for the determination of pollutants in the environmental and biological samples [20,23]. Further studies are in progress to integrate other transducers with MIP mate- rials for a chosen template. 5. Reference [1] S. Chauhan, R. D. Cruz, S. Faruqi, K. K. Singh, S. Var- ma, M. Singh and V. Karthik, “Chemical Warfare Agent,” Environmental Toxicology and Pharmacology, Vol. 26, No. 2, 2008, pp. 113-122. doi:10.1016/j.etap.2008.03.003 [2] A. B. Kanu, P. E. Haigh and H. H. Hill, “Surface Detec- tion of Chemical Warfare Agents Simulants and Degra- dation Products,” Analytica Chimica Acta, Vol. 553, No. 1-2, 2005, pp. 148-159. doi:10.1016/j.aca.2005.08.012 [3] Y. Seto, M. Kanamori-Kataoka, K. Tsuge, I. Ohsawa, K. Matsushita, H. Sekiguchi, T. Itoi, K. Iura, Y. Sano and S. Yamashiro, “Sensing Technology for Chemical Warfare Agents and its Evaluation using Authentic Agents,” Sen- sors and Actuators B, Vol. 108, No. 1-2, 2005, pp. 193- 197. doi:10.1016/j.snb.2004.12.084 [4] G. Wulff, “Molecular Imprinting in Cross-Linked Mate- rials with the Aid of Molecular Templates-A Way to- wards Artificial Antibodies,” Angewandte Chemie Intrnational Edition in English, Vol. 34, No. 17, 1995, pp. 1812 -1832. doi:10.1002/anie.199518121 [5] G. Vlatkis, L. I. Andersson, R. Muller and K. Mosbach, “Drug Assa y using Antibody Mimics Made by Molecular Imprinting,” Nature, Vol. 361, 1993, pp. 645-647. doi:10.1038/361645a0 [6] N. Lavignac, C. J. Allender and K. R. Brain, “Current Status of Molecularly Imprinted Polymers as Alternatives to Antibodies in Sorbent Assays,” Ana lytica Chimica Ac- ta, Vol. 510, No. 2, 2004, pp. 139-145. doi:10.1016/j.aca.2003.12.066 [7] H. O. Michel, E. C. Gorder and J. Epstein, “Det ection and Estimation of Isopropyl Methylphosphonofluoridate and O-Ethyl S-Diisopropylaminoethylme Thylphosphonoth- ioate in Sea Water in Partsper-Trillion Level,” Environ- mental Science and Technology, Vol. 7, No. 11, 1973, pp. 1045-1049. doi:10.1021/es60083a010 [8] V. Pavlov, Y. Xiao and I. Willner, “Inhibition of the Acetycholine Esterase Stimulated Growth of Au Nano- particles: Nanotechnology-Based Sensing of Nerve gas- es,” Nano Lette r s , Vol. 5, No. 4, 2005, pp . 649-653. doi:10.1021/nl050054c [9] Y. Yang, H. F. Ji and T. Thundat, “Nerve agents detec- tion using a Cu2+/l-cysteine bilayer-coated microcanti- lever,” Journal of American Chemical Society, Vol. 125, No. 5, 2003, pp. 1124-1125. doi:10.1021/ja028181n [10] C. Hartmann-Thompson, J. Hu, S. N. Kaganove, S. E. Keinath, D. L. Keeley and P. R. Dvornic, “Hydrogen- Bond Acidic Hyperbranched Polymers for Surface Acoustic Wave (SAW) Sensors,” Chemistry o f Material s, Vol. 16, No. 25, 2004, pp. 5357-5364. doi:10.1021/cm040346z [11] K. E. LeJeune, J. R. Wild and A. J. Russell, “Nerve  V. V I SHN UVAR D HAN ET AL. Copyright © 2011 SciRes. AJAC Agents Degraded by Enzymatic Foams,” Nature, Vol. 395, 1998, pp. 27-28. doi:10.1038/25634 [12] H. Sohn, S. Letant, M. J. Sailor and W. C. Trogler, “De- tection of Fluorophosphonate Chemi cal Warfare Agents by Catalytic Hydrolysis with a Porous Silicon Interfero- meter,” Journal of American Chemical Society, Vol. 122, No. 22, 20 00 , pp. 5399-5400. doi:10.1021/ja0006200 [13] S. W. Zhang and T. M. Swager, “Fluorescent Detection of Chemical Warfare Agents: Functional Group Specific Ratiometric Chemosensors,” Journal of American Chem- ical Society, Vol . 125, No. 12, 2003, pp. 3420-3421. doi:10.1021/ja029265z [14] S. B. Nagale, T. Sternfeld and D. R. Walt, “Microbead Chemical Switches: An Approach to Detection of Reac- tive Organophosphate Chemical Warfare Agent Va- pours,” Journal of American Chemical Society, Vol. 1 28, No. 15, 20 06 , pp. 5041-5048. doi:10.1021/ja057057b [15] T. J. Dale and R. Rebek, “Fluorescent Sensors for Orga- nophosphorus Nerve Agent Mimics,” Journal of Ame- rican Ch emica l Soci ety, Vol. 128, No. 14, 2006, pp. 4500- 4501. doi:10.1021/ja057449i [16] T. Prasada Rao, K. Prasad, R. Kala and J. M. Gladis, “Biomimetic Sensors for Toxic Pesticides and Inorganics Based on Op to electronic/ Electrochemical Transducers —An Overview,” Critical Reviews in Analytical Chemi- stry, Vol. 37, No. 3, 2007, pp. 191-210. [17] M. C. Blaco-hopez, M. J. Lobo-castanon, A. J. Miranda- ordieres and P. Tunon-Blanco, “Electrochemical Sensors Based on Molecularly Imprinted Polymers,” Trends in Analytical Chemistry, Vol . 23, No. 1, 2004, pp. 36-48. doi:10.1016/S0165-9936(04)00102-5 [18] S. A. Piletsky and A. P. F. Turner, “Electrochemical Sensors Based on Molecularly Imprinted Polymers,” Electroanalysis, Vol. 14, No. 5, 2002, pp. 317-323. doi:10.1002/1521-4109(200203)14:5<317::AID-ELAN31 7>3.0.CO;2-5 [19] Y. Zhou, B. Yu, E. Shiu and K. Levon, “Potentiometric Sensing of Chemical Warfare Agents: Surface Imprinted Polyme r Integrat ed with an Indium Tin Oxide Electrode,” Analytical Chemistry, Vol. 76, No. 10, 2004, pp. 2689- 2693. doi:10.1021/ac035072y [20] K. P. Prathish, K. Prasad, T. Prasada Rao and M. V. S. Suryanarayana, “Molecularly Imprinted Polymer-Based Potentiometric Sensor for Degradation Product of Chem- ical Warfare Agents Part.I. Methylphosphonic Acid,” Talanta, Vol. 71, No. 5, 2007 , pp. 1976-1980. doi:10.1016/j.talanta.2006.09.002 [21] V. Vishnuvardhan, K. P. Prathish, G. R. K. Naidu and T. Prasad a Rao, “Fabri cation and Topographical Analysis o f Non-Covalently Imprinted Polymer Inclusion Membranes for the Selective Sensing of Pincolyl Methylphosphonate —A Simulant of Soman,” Electrochimica Acta, Vol. 52, No. 24, 20 07 , pp. 6922-6928. doi:10.1016/j.electacta.2007.05.005 [22] K. P. Prathish, V. Vishnuvardhan and T. Prasada Rao, “Rational Design of in Situ Monolithic Imprinted Poly- mer Membranes for the Potentiometric Sensing of Die- thyl Chlorophosphate—A Chemical Warfare Agent Sti- mulant,” Electroana lysis, Vol. 21, No. 9, 2009 , pp. 1048 - 1056. doi:10.1002/elan.200804515 [23] Z.-H. Meng and Q. Liu, “Determination of Degradation Products of Nerve Agents in Human Serum by Solid Phase Extraction using Molecularly Imprinted Polymer,” Analytica Chimica Acta, Vol. 435, No. 1, 2001, pp. 121- 127. doi:10.1016/S0003-2670(01)00858-3 [24] Sophie Le Moullec, Arlette Begos, Valerie Pichan and Bruno Bellier, “Selective Extraction of Organophospho- rus Nerve Agent Degradation Products by Molecularly Imprinted Solid-Phase Extraction,” Journal of Chroma- togra phy A, Vol. 1108, No. 1, 2006, pp. 7-13. doi:10.1016/j.chroma.2005.12.105 [25] G. A. Sega, B. A. Tomkins and W. H. Griest, “Analysis of Methylphophonic Acid, Ethyl Methylphosphonic Acid and Isopropyl Methylphosphonic Acid at Low Micro- gram per Litre Levels in Ground Water,” Journal of Chromatography A, Vol. 790, No. 1-2, 1997, pp. 143-152. doi:10.1016/S0021-9673(97)00747-4 [26] G. J. Moody, J. M. Slater and J. D. R. Thomas, “Poly (vinyl chloride) Matrix Membrane Uranyl Ion-Selective Electrodes Based on Organophosphorus sensors,” Ana- lyst, Vol. 113, No. 5, 1988, pp. 699-703. doi:10.1039/an9881300699
|