Journal of Materials Science and Chemical Engineering
Vol.03 No.06(2015), Article ID:57703,6 pages
10.4236/msce.2015.36008
Carbohydrate-Derived Organocatalysts for the Reduction of Imines with Trichlorosilane
Xin Ge1, Chao Qian2*, Xinzhi Chen2
1School of Chemical and Material Engineering, Jiangnan University, Wuxi, China
2Key Laboratory of Biomass Chemical Engineering of Ministry of Education, College of Chemical and Biological Engineering, Zhejiang University, Hangzhou, China
Email: *qianchao@zju.edu.cn


Received April 2015

ABSTRACT
The backbone of D-glucosamine hydrochloride was fine-tuned and modified by protecting the hydroxyl groups. In order to reduce imines with trichlorosilane, the carbohydrate-derived organocatalysts were prepared and screened. Methyl-4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyr anoside was found as the best catalyst. The reduction was proceeded under CHCl3 as solvent at 40˚C, affording 68% - 94% yield.
Keywords:
Carbohydrate, Organocatalyst, Reduction, Imine

1. Introduction
The reduction of imines is an attractive approach for preparing amines, which have a wide application in the pharmaceuticals, agricultural chemicals and bioactive compounds [1]. In addition to the reductive amination catalyzed by the transition metals [2], boranes [3] and borohydrides [4], an organocatalytic approach is a promising method to obtain amine compounds [1]. In the organocatalytic approach, trichlorosilane and Hantzsch dihydropyridine were separately used as reducing agents for the reduction of imines in the presence of organocatalyst. Moreover, organocatalysis has received hot attention to catalyze the reaction. In spite of the rapid development of organocatalysts, it is important to continue exploiting and developing more organocatalysts.
Until now, carbohydrates have been developed as organocatalysts for application in organic synthesis [5]. In 2007, Becker et al. [6] reported enantioselective Streck and Mannich reactions catalyzed by D glucosa-mine- derived bifunctional urea schiff base organocatalysts. Subsequently, Becker et al. [7] synthesized carbohydrate- derived bifunctional primary amine-thiourea catalysts to catalyze Michael addition of aromatic ketones with nitroolefins. In 2003, Dekamin et al. [8] used the chitosan as recoverable and reused catalyst for the expeditious synthesis of α-amino nitriles and imines under mild conditions. In our preliminary work, we developed the carbohydrate-derived amino alcohols [9] and novel carbohydrate-derived prolinamide [10] to catalyze asymmeteric aldol reaction. As a part of our continued interests in carbohydrates [9]-[16], herein we reported carbohydrate- derived organocatalysts for the reduction of imines with trichlorosilane.
2. Experimental Details
2.1. General Methods
Melting points were determined on an X4-Data microscopic melting point apparatus and were uncorrected. Optical rotation values were measured on a PerkinElmer P241 polarimeter operating at 589 nm. Nuclear magnetic resonance (NMR) spectra were measured at 400 MHz (1H) or at 100 MHz (13C) on a Bruker Avance DRX- 400 spectrometer. All reactions were monitored by analytical thin-layer chromatography (TLC) from Merck with detection by spraying with 5% (w/v) phosphomolybdic acid in ethanol and subsequent heating or UV. All reagents and solvents were general reagent grade unless otherwise stated.
2.2. The Synthesis of Carbohydrate Derived Organocatalysts 4-5
The carbohydrate derived organocatalysts 4-5 were prepared by previously described methods. [9] [10] [17] [18]
2.3. Methyl-4,6-O-Benzylidene-2-Amino-2-Deoxy-α-D-Glucopyranoside 5a
White solide. M.p. 166˚C - 167˚C; [α]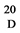 = +103.1 (c = 0.905, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.47-7.41 (m, 2H), 7.41 - 7.35 (m, 3H), 5.61 (s, 1H), 4.62 (d, J = 3.6 Hz, 1H), 4.18 (dd, J = 9.9, 4.8 Hz, 1H), 3.76 3.56 (m, 3H), 3.48 (t, J = 9.2 Hz, 1H), 3.29 (s, 3H), 2.81 (dd, J = 9.7, 3.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ142.99, 134.09, 133.24, 131.63, 106.13, 103.97, 87.27, 73.27, 72.63, 67.68, 59.96, 59.35. Anal. Calcd. (%) for C14H19NO5: C, 59.78; H, 6.81; N, 4.98; O, 28.44. Found: C, 59.764; H, 6.75; N, 4.81.
= +103.1 (c = 0.905, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.47-7.41 (m, 2H), 7.41 - 7.35 (m, 3H), 5.61 (s, 1H), 4.62 (d, J = 3.6 Hz, 1H), 4.18 (dd, J = 9.9, 4.8 Hz, 1H), 3.76 3.56 (m, 3H), 3.48 (t, J = 9.2 Hz, 1H), 3.29 (s, 3H), 2.81 (dd, J = 9.7, 3.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ142.99, 134.09, 133.24, 131.63, 106.13, 103.97, 87.27, 73.27, 72.63, 67.68, 59.96, 59.35. Anal. Calcd. (%) for C14H19NO5: C, 59.78; H, 6.81; N, 4.98; O, 28.44. Found: C, 59.764; H, 6.75; N, 4.81.
2.4. Benzyl-4,6-O-Benzylidene-2-Amino-2-Deoxy-α-D-Glucopyranoside 5b
White solide. Mp 172.4˚C - 173.8˚C. [α]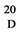 = +59.7 (c = 1.05, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.80 - 6.91 (m, 10H), 5.53 (s, 1H), 4.90 (d, J = 3.6 Hz, 1H), 4.75 (d, J = 11.7 Hz, 1H), 4.52 (d, J = 11.8 Hz, 1H), 4.24 (dd, J = 10.1, 4.8 Hz, 1H), 3.88 (td, J = 9.9, 4.8 Hz, 1H), 3.83 - 3.66 (m, 2H), 3.49 (t, J = 9.3 Hz, 1H), 2.81 (dd, J = 9.7, 3.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 137.86, 137.67, 128.97, 128.42, 128.06, 127.89, 127.76, 126.62, 101.47, 99.58, 82.08, 71.70, 69.33, 68.74, 63.30, 57.06. Anal. Calcd. (%) for C20H23NO5: C, 67.21; H, 6.49; N, 3.92; O, 22.38. Found: C, 67.12; H, 6.32; N, 3.81.
= +59.7 (c = 1.05, CHCl3). 1H NMR (400 MHz, CDCl3) δ 7.80 - 6.91 (m, 10H), 5.53 (s, 1H), 4.90 (d, J = 3.6 Hz, 1H), 4.75 (d, J = 11.7 Hz, 1H), 4.52 (d, J = 11.8 Hz, 1H), 4.24 (dd, J = 10.1, 4.8 Hz, 1H), 3.88 (td, J = 9.9, 4.8 Hz, 1H), 3.83 - 3.66 (m, 2H), 3.49 (t, J = 9.3 Hz, 1H), 2.81 (dd, J = 9.7, 3.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ 137.86, 137.67, 128.97, 128.42, 128.06, 127.89, 127.76, 126.62, 101.47, 99.58, 82.08, 71.70, 69.33, 68.74, 63.30, 57.06. Anal. Calcd. (%) for C20H23NO5: C, 67.21; H, 6.49; N, 3.92; O, 22.38. Found: C, 67.12; H, 6.32; N, 3.81.
2.5. Methyl-4,6-O-Benzylidene-2-Acetylamino-2-Deoxy-α-D-Glucopyranoside 4a
White solide. M.p. 250˚C - 252˚C; [α] = +90 (c = 0.11, MeOH); 1H NMR (400 MHz, DMSO) δ 7.90 (d, J = 8.4 Hz, 1H), 7.46 (dd, J = 6.6, 3.2 Hz, 2H), 7.41 - 7.35 (m, 3H), 5.61 (s, 1H), 4.62 (d, J = 3.6 Hz, 1H), 4.18 (dd, J = 9.9, 4.8 Hz, 1H), 3.89 - 3.80 (m, 1H), 3.74 (t, J = 10.1 Hz, 1H), 3.69 - 3.63 (m, 1H), 3.63 - 3.56 (m, 1H), 3.48 (t, J = 9.2 Hz, 1H), 3.29 (s, 3H), 1.85 (s, 3H). 13C NMR (100 MHz, DMSO) δ 169.43, 137.74, 128.84, 127.99, 126.37, 100.87, 98.71, 82.01, 68.02, 67.37, 62.43, 54.71, 54.10, 22.57. Anal. Calcd. (%) for C16H21NO6: C, 59.43; H, 6.55; N, 4.33; O, 29.69 Found: C, 59.67; H, 6.72; N, 4.21.
= +90 (c = 0.11, MeOH); 1H NMR (400 MHz, DMSO) δ 7.90 (d, J = 8.4 Hz, 1H), 7.46 (dd, J = 6.6, 3.2 Hz, 2H), 7.41 - 7.35 (m, 3H), 5.61 (s, 1H), 4.62 (d, J = 3.6 Hz, 1H), 4.18 (dd, J = 9.9, 4.8 Hz, 1H), 3.89 - 3.80 (m, 1H), 3.74 (t, J = 10.1 Hz, 1H), 3.69 - 3.63 (m, 1H), 3.63 - 3.56 (m, 1H), 3.48 (t, J = 9.2 Hz, 1H), 3.29 (s, 3H), 1.85 (s, 3H). 13C NMR (100 MHz, DMSO) δ 169.43, 137.74, 128.84, 127.99, 126.37, 100.87, 98.71, 82.01, 68.02, 67.37, 62.43, 54.71, 54.10, 22.57. Anal. Calcd. (%) for C16H21NO6: C, 59.43; H, 6.55; N, 4.33; O, 29.69 Found: C, 59.67; H, 6.72; N, 4.21.
2.6. Benzyl-4,6-O-Benzylidene-2-Acetylamino-2-Deoxy-α-D-Glucopyranoside 4b
White solide. M.p. 189˚C - 192˚C; [α]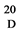 = +56 (c = 0.21, MeOH); 1H NMR (400 MHz, DMSO) δ 8.00 (d, J = 8.2 Hz, 1H), 7.49 - 7.43 (m, 2H), 7.42 - 7.33 (m, 7H), 7.30 (ddd, J = 8.4, 3.6, 1.8 Hz, 1H), 5.62 (s, 1H), 5.19 (d, J = 5.8 Hz, 1H), 4.80 (d, J = 3.6 Hz, 1H), 4.70 (d, J = 12.6 Hz, 1H), 4.49 (d, J = 12.6 Hz, 1H), 4.18 - 4.11 (m, 1H), 3.86 (ddd, J = 10.6, 8.3, 3.7 Hz, 1H), 3.72 (ddd, J = 21.9, 12.6, 7.9 Hz, 3H), 3.51 (t, J = 9.0 Hz, 1H), 1.84 (d, J = 9.8 Hz, 3H). 13C NMR (100 MHz, DMSO) δ 169.93, 138.19 (d, J = 3.3 Hz), 129.34, 128.7, 128.49, 128.07 (d, J = 7.3 Hz), 126.86, 101.34, 97.43, 69.06, 68.48, 67.73, 63.33 54.67, 23.00. Anal. Calcd. (%) for C22H25NO6: C, 66.15; H, 6.31; N, 3.51; O, 24.03. Found: C, 66.04; H, 6.15; N, 3.48.
= +56 (c = 0.21, MeOH); 1H NMR (400 MHz, DMSO) δ 8.00 (d, J = 8.2 Hz, 1H), 7.49 - 7.43 (m, 2H), 7.42 - 7.33 (m, 7H), 7.30 (ddd, J = 8.4, 3.6, 1.8 Hz, 1H), 5.62 (s, 1H), 5.19 (d, J = 5.8 Hz, 1H), 4.80 (d, J = 3.6 Hz, 1H), 4.70 (d, J = 12.6 Hz, 1H), 4.49 (d, J = 12.6 Hz, 1H), 4.18 - 4.11 (m, 1H), 3.86 (ddd, J = 10.6, 8.3, 3.7 Hz, 1H), 3.72 (ddd, J = 21.9, 12.6, 7.9 Hz, 3H), 3.51 (t, J = 9.0 Hz, 1H), 1.84 (d, J = 9.8 Hz, 3H). 13C NMR (100 MHz, DMSO) δ 169.93, 138.19 (d, J = 3.3 Hz), 129.34, 128.7, 128.49, 128.07 (d, J = 7.3 Hz), 126.86, 101.34, 97.43, 69.06, 68.48, 67.73, 63.33 54.67, 23.00. Anal. Calcd. (%) for C22H25NO6: C, 66.15; H, 6.31; N, 3.51; O, 24.03. Found: C, 66.04; H, 6.15; N, 3.48.
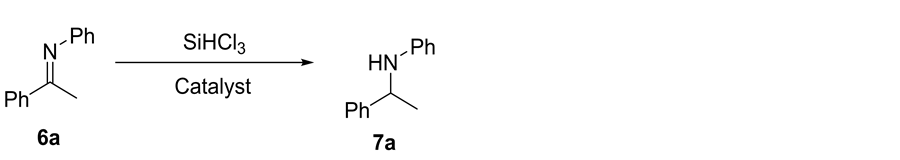
2.7. General Experimental Procedure for the Reduction of Imines with Trichlorosilane Catalyzed by 5a
To a stirred solution of imine 6 (0.5 mmol) and catalyst 5a (25 mg, 0.05 mmol) in dry CHCl3 (2 mL) was added the trichlorosilane (0.1 ml, 1 mmol) at 0˚C and the reaction mixture was stirred at 0˚C for 24 h. Then, saturated NaHCO3 (2 ml) was added and extracted with CHCl3 (3 × 5 ml). The combined organic phases were washed with saturated brine, dried over MgSO4, and concentrated in vacuo. Then the crude product was purified by column chromatography through silica gel, eluting with 1:99 ethyl acetate/petroleum ether solvent mixture, to give the pure 7.
N-Phenyl-1-phenylethylamine 7a Yield 91%. Yellow oil. 1H NMR (400 MHz, CDCl3) δ 7.32 - 7.18 (m, 4H), 7.14 (t, J = 7.1 Hz, 1H), 7.01 (t, J = 7.6 Hz, 2H), 6.56 (t, J = 7.2 Hz, 1H), 6.43 (d, J = 7.6 Hz, 2H), 4.41 (q, J = 6.6 Hz, 1H), 1.43 (d, J = 6.7 Hz, 3H). 13C NMR (1010 MHz, CDCl3) δ 146.26, 144.20, 128.07, 127.60, 125.83, 124.82, 116.21, 112.29, 52.43, 23.98.
3. Results and Discussion
First we attempted to synthesize the carbohydrate-derived organocatalysts (Scheme 1). The amino group in the position C-2 of D-glucosamine hydrochloride 1 was first protected by acetylation. The hydroxyl group in the position C-1 was modified by glycoside and benzyl glycoside. Then the hydroxyl groups in the position C-4 and C-6 were protected by benzylidene acetal. Finally, the acetyl group in the position C-2 was removed by alkaline alcohol solution. The carbohydrate-derived alcohols 5 were obtained.
In order to screen the catalysts (Figure 1), the reduction of imine 5a with trichlorosilane was investigated as a model reaction. The results of the catalysts screening and condition optimizations are summarized in Table 1. In our initial practice, we attempted to use carbohydrate-derived amino alcohols 5a and 5b as catalysts at room temperature, affording 74% yield and 63% yield separately (Table 1, entries 1-2). Then, the carbohydrate-derived
Scheme 1. Synthesis of carbohydrate-derived alcohols.
Figure 1. Structures of carbohydrate-derived organocatalysts evaluated in this study.
Table 1. Asymmetric reduction of imine 6aa.
aThe reactions were carried out with 10 mol % catalyst and 2.0 equiv of SiHCl3 on a 0.5 mmol scale in 2.0 mL of solvent for 24 h. bIsolated yield based on the imine.
acetamide alcohols 4a and 4b also could catalyze the reduction of imine 6a (Table 1, entries 3-4). The 67% yield and 61% yield were obtained separately. Thus, the catalyst effect of carbohydrate-derived amino alcohols 5a was best. Then the optimization of reaction conditions was studied. The effect of solvent was firstly investigated (Table 1, entries 1, 5-7). We found that trichloromethane was the best solvent affording the product with 81% yield (Table 1, entry 6). Therefor, the reaction temperature was further studied (Table 1, entries 1, 8-9). The best result was obtained at 40˚C, affording 91% yield (Table 1, entry 9). Thus, we selected 40˚C as the best temperature in this reaction.
Encouraged by these results, the substrate scope of the reduction of imines with trichlorosilane was further studied under the optimized conditions. The results were summarized in Table 2. For aromatic N-Ph imines 6b-6g with electron-withdrawing groups, only 68-77% yields were obtained (Table 2, entries 2-4). When aromatic N-Ph imines 6c-6e with electron-donating groups were reduced, the yields were increased to 94-95% (Table 2, entries 5-6). The benzyl N-Ph imine 6h could afford the 91% yield (Table 2, entry 7). Phenyl N-aryl imines 6i-6k with electron-withdrawing groups could be reduced in 78-82 yields (Table 2, entries 9-11). When the Phenyl N-aryl imine 6l with electron-donating group, the yield was also increased (Table 2, entry 12). N-aryl propiophenone imines were similar to N-Ph acetophenone imines, affording the 78% - 89% yields (Table 2, entries 13-15).
4. Conclusion
In sum, we have described carbohydrate-derived organocatalyst for the reduction of imines with trichlorosilane. The backbone of D-glucosamine hydrochloride was fine-tuned and modified. The amino group in the position C-2 was first protected by acetylation. The hydroxyl group in the position C-1 was modified by glycoside and benzyl glycoside. Then the hydroxyl groups in the position C-4 and C-6 were protected by benzylidene acetal. Finally, the acetyl group in the position C-2 was removed by alkaline alcohol solution. The carbohydrate-de- rived organocatalysts were screened. Methyl-4,6-O-benzylidene-2-amino-2-deoxy-α-D-glucopyranoside was selected as the best catalyst. This reduction reaction of imines with trichlorosilane could be carried out in CHCl3 at 40˚C, affording 68% - 94% yield.
Table 2. Asymmetric reduction of imine 6 with catalyst 1a.
aThe reactions were carried out with 10 mol % catalyst and 2.0 equiv of SiHCl3 on a 0.5 mmol scale in 2.0 mL of CHCl3 at 40 oC for 24 h. bIsolated yield based on the imine.
*Corresponding author.
Acknowledgements
The authors thank for the financial support from the Natural Science Foundation of China (21376213) and the Research Fund for the Doctoral Program of Higher Education of China (20120101110062).
Cite this paper
Xin Ge,Chao Qian,Xinzhi Chen, (2015) Carbohydrate-Derived Organocatalysts for the Reduction of Imines with Trichlorosilane. Journal of Materials Science and Chemical Engineering,03,48-53. doi: 10.4236/msce.2015.36008
References
- 1. Tripathi, R.P., Verma, S.S., Pandey, J. and Tiwari, V.K. (2008) Recent Development on Catalytic Reductive Amination and Applications. Current Organic Chemistry, 12, 1093-1115. http://dx.doi.org/10.2174/138527208785740283
- 2. Crabtree, R. (1979) Iridium Compounds in Catalysis. Accounts of Chemical Research, 12, 331-338. http://dx.doi.org/10.1021/ar50141a005
- 3. Connolly, T.J., Constantinescu, A., Lane, T.S., Matchett, M., Mcgarry, P. and Paperna, M. (2005) Assessment of a Reductive Amination Route to Methyl(3-nitrobenzyl)amine Hydrochloride. Organic Process Research & Development, 9, 837-842. http://dx.doi.org/10.1021/op050120x
- 4. Heydari, A., Khaksar, S., Akbari, J., Esfandyari, M., Pourayoubi, M. and Tajbakhsh, M. (2007) Direct Reductive Amination and Selective 1,2-Reduction of alpha,beta-Unsaturated Aldehydes and Ketones by NaBH4 Using H3PW12O40 as Catalyst. Tetrahedron Letters, 48, 1135-1138. http://dx.doi.org/10.1016/j.tetlet.2006.12.069
- 5. Shen, C. and Zhang, P.F. (2013) D-Glucosamine-Derived Chiral Catalysts for Asymmetric Reactions. Current Organic Chemistry, 17, 1507-1524. http://dx.doi.org/10.2174/1385272811317140007
- 6. Becker, C., Hoben, C. and Kunz, H. (2007) Enantioselective Organocatalysis of Strecker and Mannich Reactions Based on Carbohydrates. Advanced Synthesis & Catalysis, 349, 417-424. http://dx.doi.org/10.1002/adsc.200600370
- 7. Liu, K., Cui, H.F., Nie, J., Dong, K.Y., Li, X.J. and Ma, J.A. (2007) Highly Enantioselective Michael Addition of Aromatic Ketones to Nitroolefins Promoted by Chiral Bifunctional Primary Amine-Thiourea Catalysts Based on Saccharides. Organic Letters, 9, 923-925. http://dx.doi.org/10.1021/ol0701666
- 8. Dekamin, M.G., Azimoshan, M. and Ramezani, L. (2013) Chitosan: A Highly Efficient Renewable and Recoverable Bio-Polymer Catalyst for the Expeditious Synthesis of Alpha-Amino Nitriles and Imines under Mild Conditions. Green Chemistry, 15, 811-820. http://dx.doi.org/10.1039/c3gc36901c
- 9. Shen, C., Shen, F.Y., Xia, H.J., Zhang, P.F. and Chen, X.Z. (2011) Carbohydrate-Derived Alcohols as Organocatalysts in Enantioselective Aldol Reactions of Isatins with Ketones. Tetrahedron: Asymmetry, 22, 708-712. http://dx.doi.org/10.1016/j.tetasy.2011.04.007
- 10. Shen, C., Shen, F., Zhou, G., Xia, H., Chen, X., Liu, X. and Zhang, P. (2012) Novel Carbohydrate-Derived Prolinamide as a Highly Efficient, Recoverable Catalyst for Direct Aldol Reactions in Water. Catalysis Communications, 26, 6-10.
- 11. Ji, L., Zhang, D.F., Zhao, Q., Hu, S.M., Qian, C. and Chen, X.Z. (2013) Diastereospecific Epoxidation and Highly Regioselective Ring-Opening of (+)-Valienamine: Practical Synthesis of (+)-Valiolamine. Tetrahedron, 69, 7031-7037. http://dx.doi.org/10.1016/j.tet.2013.06.046
- 12. Xia, H.J., Yan, H., Shen, C., Shen, F.Y. and Zhang, P.F. (2011) Syn-thesis of Novel Carbohydrate-Based Chiral P, N Ligands and Their Applications in Cu-Catalyzed Enantioselective 1, 4-Conjugate Additions. Catalysis Communications, 16, 155-158. http://dx.doi.org/10.1016/j.catcom.2011.09.021
- 13. Shen, C., Xia, H.J., Yan, H., Chen, X.Z., Ranjit, S., Xie, X.J., Tan, D., Lee, R., Yang, Y.M., Xing, B.G., Huang, K.W., Zhang, P.F. and Liu, X.G. (2012) A Concise, Efficient Synthesis of Sugar-Based Benzothiazoles through Chemoselective Intramolecular C-S Coupling. Chemical Science, 3, 2388-2393. http://dx.doi.org/10.1039/c2sc20248d
- 14. Ge, X., Qian, C., Chen, Y.B. and Chen, X.Z. (2014) Novel Carbohydrate-Derived Pyridinecarboxylic Organocatalysts for the Enantioselective Reduction of Imines with Trichlo-rosilane. Tetrahedron: Asymmetry, 25, 596-601. http://dx.doi.org/10.1016/j.tetasy.2014.03.015
- 15. Ji, L., Zhou, G.Q., Qian, C. and Chen, X.Z. (2014) Synthesis of 1,2,3-Triazoles from Azide-Derivatised Aminocyclitols by Catalytic Diazo Transfer and CuAAC Click Chemistry. European Journal of Organic Chemistry, 2014, 3622- 3636. http://dx.doi.org/10.1002/ejoc.201301874
- 16. Ge, X., Qian, C. and Chen, X.Z. (2014) Synthesis of Novel Carbohydrate-Based Valine-Derived Formamide Organocatalysts by CuAAC Click Chemistry and Their Application in Asymmetric Reduction of Imines with Trichlorosilane. Tetrahedron: Asymmetry, 25, 1450-1455. http://dx.doi.org/10.1016/j.tetasy.2014.10.003
- 17. Emmerson, D.P.G., Hems, W.P. and Davis, B.G. (2006) Carbohydrate-Derived Amino-Alcohol Ligands for Asymmetric Alkynylation of Aldehydes. Organic Letters, 8, 207-210. http://dx.doi.org/10.1021/ol052503l
- 18. Shen, C., Xia, H.J., Zheng, H., Zhang, P.F. and Chen, X.Z. (2010) Synthesis of Novel Carbohydrate-Based Iminophosphinite Ligands in Pd-Catalyzed Asymmetric Allylic Alkylations. Tetrahedron: Asymmetry, 21, 1936-1941. http://dx.doi.org/10.1016/j.tetasy.2010.06.037
NOTES

*Corresponding author.




