 Advances in Microbiology, 2015, 5, 425-432 Published Online June 2015 in SciRes. http://www.scirp.org/journal/aim http://dx.doi.org/10.4236/aim.2015.56043 How to cite this paper: Ben Romdhane, I., Saidi, N., Deshaware, S. and Shamekh, S. (2015) Sanitizing Method Effects on Depending-Culture Microorganisms in Tuber aestivium. Advances in Microbiology, 5, 425-432. http://dx.doi.org/10.4236/aim.2015.56043 Sanitizing Method Effects on Depending-Culture Microorganisms in Tuber aestivium Ilef Ben Romdhane1, Neila Saidi1*, Shweta Deshaware2, Salem Shamekh3,4 1Laboratory of Treatment and Water Recycling Centre of Research and Water Technologies (CERTE) Technopark of Borj-Cedria, Soliman, Tunisia 2Food Engineering and Technology Department, Institute of Chemical Technology, Mumbai, India 3Department of Biotechnology and Chemical Technology, School of Chemical Technology, Aalto University, Aalto, Finland 4Juva Truffle Center, Ju va, Finland Email: *neila_saidi@yahoo.fr Received 15 May 2015; accep t ed 15 June 2015; published 19 June 2015 Copyright © 2015 by authors and Scientific Research Publishing Inc. This work is licensed under the Creative Commons Attribution International License (CC BY). http://creativecommons.org/licenses/by/4.0/ Abstract Tuber aestivum/uncinatum has been widely used as food, food additives, and traditional medicine. Truffles are extremely perishable with a short postharvest life quality requiring a special handling for marketing in order to delay its deterioration. This study aimed to assess the effects of different sanitizing methods on superficial Tuber aestivium quality ascocarp. The results showed that the best treatment was obtained by immersing the Truffle ascocarps in boiling water for 1 or 2 min where counts of total mesophilic microoraganisms (TMM) were respectively 81 and 7 CFU per g of dry Truffle ascocarps biomass, respectively. However, the highest TMM was obtained after rinsing Truffle ascocarps in 2% NaOH where recovery was 108 CFU per g of dry Truffle ascocarps biomass. Treatments applied to disinfect Truffle ascocarps were classified by increasing degree of efficien- cy as follows to reduce the microbial load expressed in CFU/g: Dipping in boiling water (2 min) 7 ± 3.41; dipping in boiling water (1 min) 81 ± 25.8, rinsing with alcohol 2.102 ± 13; rinsing with tap water 6.103 ± 36; rinsing with H2O2 6.104 ± 2; brushing 2.105 ± 28 and rinsing with NaOH 108 ± 15. Keywords Tuber aestivium Ascocarp s, Total Count Mesophilic Bacteria, Molds and Yeast Decontamination *  I. Ben Romdhane et al. 1. Introduction Truffles are the most highly edible valued mycorrhizae fungi in gastronomic and economic terms. Some species of T r uffl e s uc h a s Tuber magnatum, Tuber melanosporum and Tuber aestivum/uncinatum are highly praised due to their aroma and organoleptic propreties [1]. Truffles are extremely perishable with a short postharvest shelf life which req uires specia l han dling for mar ketin g in or der to dela y its deterior ation. T he d eterio ratio n of T ruffle organolep tic quality is caused by the mold mycelia growth on the surface of Truffle and loss of water, whic h af- fects their visual appearance and flavours. Tirmenia and Te rfezia species are usually eaten cooked whereas Tu- ber species are sometimes eaten uncooked. Several studies reported high microbial loads in the range of 105 cfu/g t o 108 cfu/g i n the gle ba of Tuber bor- chii, Tuber magnatum, T. melanosporum and T. aestivum species [2]-[4]. High microbial loads and the presence of potential pathogens such as Listeria and Salmonella species make Truffle spoilage easier [5]. It is essential for develop ing disinfection techniques to ex te nd Truffle storage and to i mprove sanita ry q uality. Early decontamination treatment can reduce the initial microbial loads thus increases the storage period. Sev- eral techniques have been used to reduce microorganisms on whole and cut fresh fruit, and their effectiveness depends on the product type, mitigation protocol, and other parameters. Contamination prevention seems to be the best way to eliminate pathogens from products even this is not always possible. The necessity of washing and sanitizing remains of paramount importance to prevent product spoiling outbreaks. It is well known that washing and sanitizing methods can’t totally eliminate pathogens. Therefore, sanitizing methods are usually used to reduce the total flora existing. Some berries are sensitive to washing due to their susceptibility to mold proliferation. So s imila r fruit are often package d in t he field wit h a light post har vest handling and wa shing. The increase in demand for T. aestivium leads food technologists to develop appropriate methods for its long and short term storage in order to retain its quality and reduce the threat of scarcity. After harvesting, T. aesti- vium is ra pidly altered at room temperature and at 4˚C due to na tural flor a existing in the Tr uffle ascocarps and to water content (76%). Moreover, Tr uffle ascocarps are sub terranean fruit bodies growing underground, and it is well known that soil serves as a natural habitat for various microbial communities growing along with asco- carps [6]. It has been shown that bacteria can promote the establishment of ectomycorrhizal symbiosis [7]-[9]. Several researchers analyzed the microbial load in different Truffle species. These microbial communities step in growing, physiology and preservation of Truffle ascocarps [10] [11]. The microbial consortium could have an additive or antagonistic effect on Truffle preservation. Different decontamination methods such as ionizing radiation, ultrasound, electron beam radiation, gamma rays and refrigeration at 4˚C have been applied to minimize the microbial load of fresh Truffle [11] [12]. How- ever, storage at a lower temperature is not an absolutely reliable method since many molds are able to grow at low temperatures. High concentration of chlorine (500 ppm) has been tried for decontamination but with unsa- tisfactory results due to high content o f organic matter in Truf fl e and production of potential c arcinogens such as trehalo-methanes [13]. Chemical fungicides have also been applied for preventing fungal decay; however, con- sumers demand minimum use of chemical preservatives. As a biological control, Lactic acid bacteria (LAB) have been reported to produce organic acids, bacteriocins, hydrogen peroxide, and low molecular weight anti- microbial agents which act as an anti-fungal agent [14]. Sorrentino et al. (2013) [15] reported that strains of Lactobacillus plantarum can be successful ly used to preve nt the growth of molds belongin g to penicill ium genus during storage of black Truffle at refrigeration temperature. The goal of this study is to find an appropriate decontamination method that will reduce the microbial load of fresh Truffle in order to increase its shelf-life . 2. Material and Methods 2.1. T. aestivum Ascocarps Samples The ascocarps of T. aestivum were collected in the experimental Truffle field of Juva Truffle Center (JT C), J uva, Finland, with the help of hunter Truffle dogs. The field soil was sandy with pH 7.2. The main host trees of T. aestivum were Quercus peduncularis, Quercus robur L and Corylus avellana L. To avoid cross conta mination, the Truffle ascocarps were individually wrapped in pla stic bags a nd transpo rted to the laborato ry in an insulated boxes for microbial analysis. The nu mber of Truffle ascocarps used for this study was about 11 weighing 20 - 25 grams.  I. Ben Romdhane et al. 2.2. Soil Sample The adhered soil to the Truffle ascoscarps is considered as a separate sample to enumerate the natural associated flora and it was obtained by ascocarps brushing under laminar flow. 2.3. Quantitative Microbiological Analysis Eight different decontamination treatments were applied to Truffle ascocarps: Brushing pending wholly extrac- tion of adhered surface ascocarp s soil, r insing with municip al d rinki ng wate r (prelimina ry ana lysis sho wed tha t the tap water is exempt of bacteria and was used this water to be near the habits of Finish people when they clean the T ruffle b y brush or by water), rinsing with ethanol [70%], dipping in boiled water during one or two min, rinsi ng with NaOH [2% ] and rinsi ng with H2O2 [30%]. 2.4. Truffle Ascocarp and Extract Preparation Bacteria colonize both the external (peridium) and internal part (gleba) of Truffle [16]. For this, we not only considered the surface ascocarps bacterial extraction but also the total ascocarp body. Briefly, in a laminar hood and under sterile conditions, each piece of Truffle ascocarp was treated and then cut into 2 - 4-mm length pieces. Te n g treated T ruffle sampl es wit h moisture content o f 76% (dry weight) was suspende d in 90 ml of sterile sa- line solution [0.9% w/v NaCl]. The mixture was submitted to a mechanical agitation for 1 hour at 240 rpm to allow bacteria extraction [17]. 2.5. Enumeration of Bacteria Enumeration of viable aerobic heterotrophic bacteria was performed by standard plate count procedures, in Plate Count Agar (PCA), Malt Extract Agar (MEA) and Yeast Extract Agar (YEA) (Difco, Germany). Plates were incubated in Biolog incubator at 25˚C, and the number of Colony Forming Units (CFU) were determined after 48 h and the average number of microorganisms per gram of each sample was then calculated and reported as g of dried Truffle ascocarp or soil. Plate Count Agar medium were adjusted to a final pH = 7 ± 0.2. This medium is specific for total count bacteria. Malt Extract Agar medium Sigma-Aldrich 70145 appropriate for molds and yeast present in gram per liter of purified water Malt extract 30.0; Mycological peptone 5.0 Agar 15.0 fina l pH = 3.5 +/− 0.2 was adjusted by 10% of citric acid instead of 5% of tartric acid. The typical for mula of Yeast Extract Agar medium Oxoid B O0635M per liter of distilled water included 3 g of Yeast Extract, 5 g of peptone and 15 g of Agar, final pH ad j usted to 7 ± 0.4. This medium is suitable for both P. aeruginosa and E. coli. 2.6. Statistical Analysis Results related to each treatment were repeated three times and analyzed by the SPSS statistical program (SPSS for Windows, SPSS Inc.). Values mentioned in Table 1 are the average of three replicates and means were se- parated by the least significant difference according to the StudenNewman-Keuls Test. Pearson correlation de- terminations were performed using SPSS statistical analysis. 3. Results and Discussion 3.1. Microbial Enumeration in T. aestivium Asc ocarps The results showed that the more effective treatment was obtained when Truffle ascocarps were immersed in boiling water for 1 or 2 min with a total mesophilic microorganisms (TMM) count respectively 81 and 7 CFU per g of dry Truffle ascocarps biomass. However, the highest TMM was obtained after rinsing Truffle ascocarps in 2% NaOH where the number saved was 108 CFU per g of dry Truffle ascocarps biomass. Applied treatments were classified with degree efficiency expressed in CFU/g: Dipping in boiled water (2 mi n) 7 ± 3.41; dipping in boiled water (1min) 81 ± 25.8, rinsing with ethanol (70%) 2. 102 ± 13; rinsing with tap water 6.103 ± 36; rinsing wit h H2O2 6 104 ± 20, brushing 2. 105 ± 28; rinsing with NaOH 108 ± 1 5. The same order of classification was obtained for total cou nt bacter ia, molds-yeast and P. aeruginosa-E. coli. H oweve r , 108 of TMM number present after NaOH and H2O2 was relatively high. In fact, freshly collected Truffles are associated with significant mi- 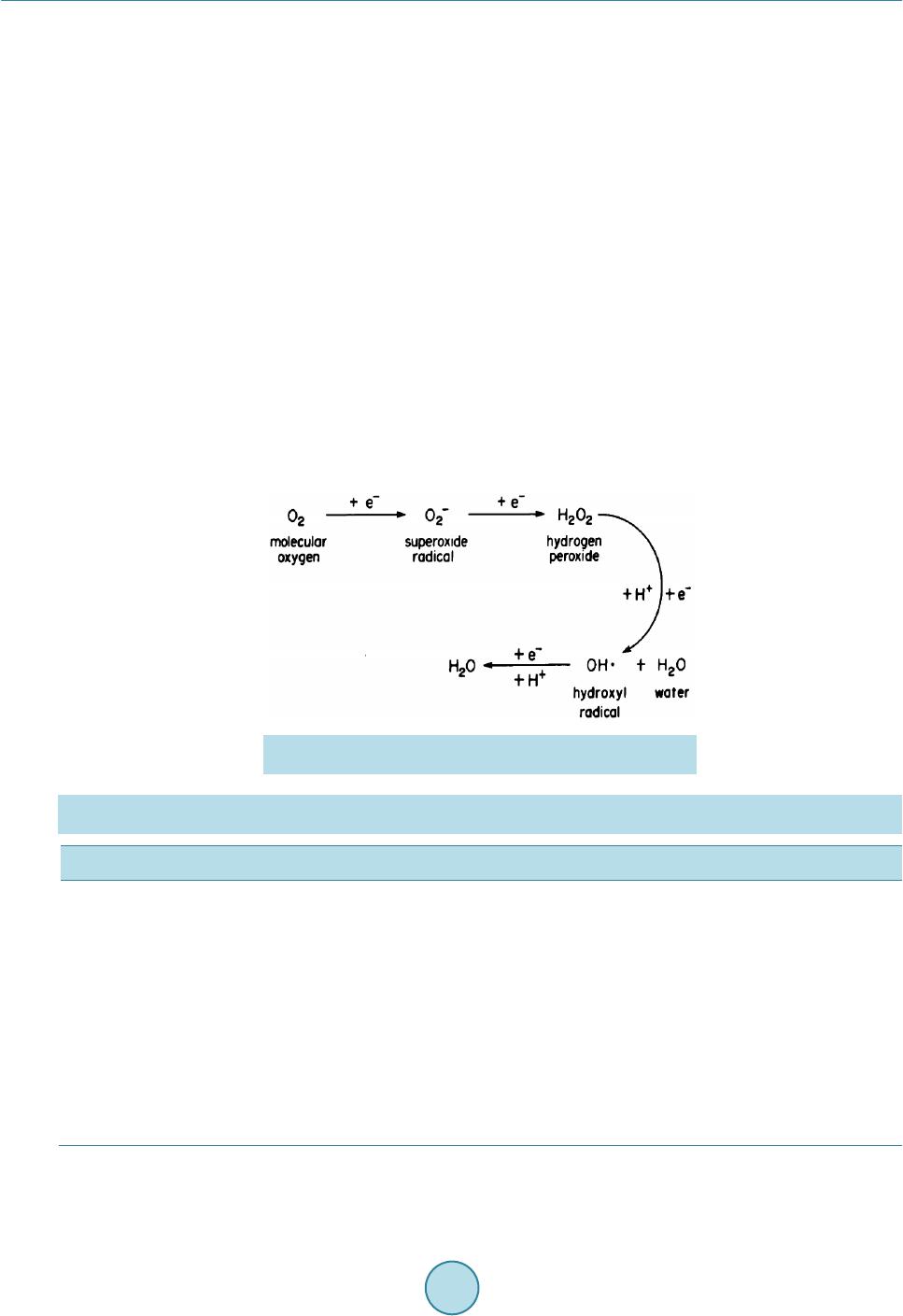 I. Ben Romdhane et al. crobial populations. Studies assessing the total microbial load of entire ascocarps obtained different results. Nazzaro et al. , (2007) [10] detected a total microbial load in ascocarps of T. aestivium near to107 CFU/g. How- ever, Reale et al., (2009) [11] reported only 4.0 CFU/g. These differences may be due to different procedures used to remove ascocarps surrounding soil. The development of superficial mold growth is one of the main problems in the postharvest phase of ascocarps affecting their vi sual quality, aroma and taste [18]. The significant difference in CFU number per gram of ascocarps between treatments generates four main group s. T he firs t gro up incl uded boil ing trea tment s for 1 or 2 min. ri nsin g with e thano l tr eatme nt rep resent s the second group, the third group contains rinsing with tap water and brushing; treatments with H2O2 and NaOH constitute the last group. Results showed in Table 1 reveal that the microbial population in T. aestivium is responsive to the type of treatment applied. This suggests that the treatment application may induce the number of microorganisms ex- tracted. In fact, actively growing bacteria were effectively expressed by the decontamination method. The high- est number of micro flora was obtained after NaOH or H2O2 treatment. This resul t pre sumed that NaOH or H 2O2 induced release of microrganisms and positively affected extraction the yield of bacteria. Based on H2O2 effect, only bacteria producing catalase may survive in presence of H2O2 [19]. The catalyzed H2O2 molecule may pro- vide o xyge n gas to the med iu m as indicated in Figure 1. This reaction may also induce the aerobic bacteria de- velopment. The whole and high number of bacteria 108 CFU/g per dry matter expressed following H2O2 treat- ment may suggest the presence of mainly aerobic bacteria the catalase enzyme is absent i n nearly all anaerobic bacteria and may thereby be suppressed by H2O2. It is well kno wn that some bacteria are ab le to produce H2O2 and could be used as a substrate for its growing. In fact, related to work of Eschenbach et al., (1989) [20], Figure 1. Intermediates in the Univalent Pathway of Oxygen Reduction (Archbald and Fridovichi; 1983) [34]. Table 1. Micro bial load expressed in CFU/g per dry matter of T. aestivium ascocarps in different applied treatments and in its adhered soil. Trea tmen ts PCA (1) MEA (2) YEA (3) Brushing 2. 105 ± 28 (a) 9. 103 ± 71 (a) 2. 104 ± 74 (a) Rinsing with tap water 6. 103 ± 36 (b) 8. 103 ± 11 (a) 2.103 ± 26 (b) Rinsing with etha nol (70%) 2.102 ± 13 (c) 2.102 ± 40 (b) 5.102 ± 11 (c) Dipping in boiled water (1 min) 81 ± 25.8 (d) 3 ± 1.8 (d) 102 ± 17 (c ) Dipping in boiled water (2 min) 7 ± 3.41 (d) 1.4 ± 0.73 (d) 1.2 ± 2 (d) Rinsing with NaOH 108 ± 15 (e) 6. 107 ± 23 (e) 9. 107 ± 19 (e) Rinsing with H2O2 6. 104 ± 20 (f) 108 ± 44 (f) 5. 107 ± 19 ( e) Adhered soil 2.109 ± 16 (g) 5.106 ± 27 (g) 107 ± 10 (e) (1) P late Count Agar , (2) Malt Extra ct Agar, (3) Ye ast Extra ct Aga r. Eth an ol [7 0 %, v/v], dipp in g in boi led water during one and two minutes, rinsing with H2O2 [30%, v/v]. (a, b, c, d, e, f, g) means number followed by the same number were not significantly different at p = 0.05 comparison estab- lished between lines using SPSS statistical program (SPSS for Windows, SPSS Inc.). Values mentioned are the average of three replicates.  I. Ben Romdhane et al. some Lactobacillus are kno wn to produc e hydroge n per oxide inducin g an incre ase in the number of Lactobacil- lus. Decontamination method using boiled water during one minute keeps 2Ulog of P. aeruginosa and E. coli higher than total b acteria and fungi (molds and yeast) numb er. It is well known that E. co li and other pathogens can be removed by a simple pasteurization. The presence of this high load of E. coli may be related to a highly resistant natural flora of the ascocarps. In fact, during its life cycle, T. aestivium generates a radial stress zone producing volatile substances able to affect the bacteria community around the ascocarps and likel y ind uce bac- teria resistance to diverse stresses [21] [22]. The induced alkaline environment is another type of ascocarps stress. In fact, when ascocarps were cleaned with 2% NaOH, total bacteria, fungi and P. aeruginosa-E. coli count increased. The large number of microor- ganisms achieved after NaOH application can be a result of specific flora extraction with a shift of the initial pH of 5 of Truffle ascocarps to neutral pH. Balanced pH may promote the development of some bacteria and inhibi- tion of fungi growing [23]. Unlike, another study showed that various high pH cleaners containing sodium hy- droxide, potassium hydroxide, sodium bicarbonate, and/or sodium orthophenylphenate (with or without surfac- tants) reduced populations of E. coli on orange surfaces [24]. The same study revealed that high pH waxes used on fresh market citr us pro vided substant ial inacti vatio n of E. co li on the surfaces oranges fruit [25]. The hig h p H of typical alkaline wash solutions and concerns about environmental discharge of phosphates may be limiting factors for use of certain alkaline compounds on produce. Furthermore, Somers et al. , (1994) [26] in a lab oratory study of suspended and attached cells of various foodborne pathogens on non-food surfaces showed that E. coli O157:H7 populations were reduced by 5 and 6 CFU after a 30-s decontamination method with 1% tri-sodium phosphate. Besides, H wang and Beuchat, (1995) [27] working in chic ken skin disinfectio n revealed that 0.05% NaOH significantl y reduce d the Salmonella spp. population but had no effect on L. monocytogenes. The d iffer- ence in effectiveness degree to remove bacteria with alkaline solution may be the result of many parameters in- teraction such as pH, water content in Truffle ascocarp and its own composition (hydrocarbons, proteins, fatty acids contents). Likewise, in the present study, ethanol may be considered as a potential disinfectant of Truffle ascocarps since the number of total microflora decreased 3 CFU compared to brushing. It is well known that ethanol is used i n ma ny fie lds suc h as cl inic al a nd fo od , it possess a large specter effect on bacteria discharge. Ethanol has pleiotropic effects on bacteria, membra ne damage and inhib ition o f macro molec ular bi osynthe sis being t he ma- jor mechanisms. However, many bacteria may escaped the ethanol effect when the load of bacteria is very im- portant or when the bacteria is not present at surface or covered by organic matter. Zhang et al. (2009) [28] sug- gested dipping in 80% ethanol for 10 seconds combined with mercuric chloride as the most effective surface disinfec t ion method for inactivating Escherichia coli O157:H7 on lettuce leaves and roots. As reported by Rivera et al., (2011b) [6], after gamma irradiation treatments, two species of yeasts survived and became the dominant microbial populations; this selective effect of disinfectant and resistance of one group rather other s sug gest t he ne cessi ty to c omb ine tr eatme nt in ord er to enhance deco ntamina tio n. For e xampl e, Ri- vera et al. , (2011b) [6] showed that 70 % ethanol combined with ultrasound has a greater efficacy to reduce mi- crobial load. 3.2. Microbial Presence in the Adhered Soil of T. aestivium The layer of soil obtained after brushing Truffle ascocarps may be considered as the source of specific flora present in T ruffle ascocarps. Wherefore, the adhered soil microflora of Tuber. aestivium ascocarps has been hig- hlight ed in the present wo rk. The number of CFU per gram of the adhered soil was respectively about 2.8 × 108 CFU/g of TMM bacteria, 4.7 × 106 CFU/g of both mold and yeast and about 1.8 × 106 CFU/g of both P. aeruginosa and E. c oli. T he high number of microflora in the adhered soil of T. aestivium substantiates the result of Rougieux (1963) [29]. Re- sults showed that desert truffle can excrete substances able to stimulate the development of these microorgan- isms. The abundance of TMM bacteria, mold, yeas t, P. aeruginosa and E. co li has been lo cated in T. borchii soil [4]. This study showed obviously that TMM bacteria were about 102 CFU/g d r y matt er hi gher t ha n fu n gi o r bo t h E. coli and P. aeruginosa. A similar study carried out by Dib -Bellahouel and Fortas (2014) [30] showed an es- timated total count about 8.3 to 8.6 × 10 7 CFU/g of dry soil, while fu ngal ge nera p re sent onl y 8.6 × 104 to 4.5 × 105 CFU/g. Thus, it is possible to suggest that the number of bacteria and fungi in adhered soil is balanced by  I. Ben Romdhane et al. the Truffle presence, this suggestion need to be more demonstrated in other studies. The soil microflora of Truffle ascocarps has been severally studied; most of them characterized types and ab- undance of bacteria such as Actinomycetes, yeasts and filamentous fungi [3] [31] [32]. Although, specific bac- terial communitie s of Tuber species were analyzed, a core micro-biome composed of α-Proteobacteria from the family of Bradyrhizobiaceae seems to be common to all Tuber species studied [3] [4] [16]. Moreover, many studies have been conducted on bacterial diversity soil horizon containing Truffle. The ho- rizo n 0 - 20 cm depth sho wed the highest micro bial divers ity [33]. These authors enumerated the number of mi- croorganisms respectively in s oil adhered to Terfezia boudieri ascocarps and in 0 - 10 cm depth horizon, thus it was demonstrated that so me microbial communities were higher in soil adjacent to Terfezia boudieri ascocarps than the horizon of 10 cm depth. Furt her a nal ysis need to a dvice that an ide al disin fectio n treat ment should b e si mple and easy to use; it will b e efficient in Truffle for its sensory characteristics preservation such as, aroma, texture and taste. Rivera et al., (2011) [6] showed that in the last week of storage, the aroma of non-decontaminated T. aestivium samples was modified and associated to a strange odor of fungi. This loss of typical aroma influences the overall acceptability of the no n-decontaminated Truffle out of marketability on day 28. The typical aroma odor and quality in the de- contaminated summer Truffle samples moderately decreased in the first week of storage. Chemical disinfectants and chemical residues, depending on their concentrations render Truffles to be unfit for human consumption and pose a threat for human health. 4. Conclusion Microbial populations of Truffle were very complex and heterogeneous and were mostly mesophilic bacteria. Dipping Truffle ascocarps in boiled water for 1 - 2 minutes reduced microbial load and seems to be an efficient method recommended before T. aestivum storage. Acknowledgements This research was supported by Juva Truffle Center Finland. The financial support of Regional Council of Sout hern Savo , Finla nd is appreciated and thanked. Autho rs are gr ateful for Mr. Antt i kinnune n for ad ministra- tive service and Mrs. Heli Valtonen for her technical assistance. Authors are grateful to Dr. P. S ivakumar and Dr. A. M. Ibekwe for their kind comment and improvement of the manuscript. The authors are also grateful to Pr Kremmer R. for improving English expression and s tyle. References [1] Culleré, L. , Ferreira, V., Ch evr et, B., Venturini , M.E. and Sánchez-Gimeno, A.C. (2010) Characterisation of Aroma Active Co mpou nds in Black Truffle (Tuber melanosporum) and Summer Truffle (Tuber aestivum) b y Gas Chromato- graphy-Olfatometry. Food Chemistry, 122, 300-306. http://dx.doi.org/10.1016/j.foodchem.2010.02.024 [2] Gazzanelli, G., Malatesta, M., P ian etti, A., Baffone, W., Stocchi, V. and Citterio, B. (1999) Bacteria Associated to Truffle Ascocarp Bodies of the Ecto-Mycorrhizal Fungus Tuber borchii Vittad. Symbiosis, 26, 211-219. [3] Barbieri, E., Bertini, L. , Ro ssi, I., Ceccaroli, P., Saltarelli, R., Guidi, C., Zambonelli, A. and Stocchi, V. (2005) New Evidence for Bacterial Diversity in the Ascoma of t he Ecto mycorrhizal Fungus Tuber borchii Vittad. FE MS Microbi- ology Letters, 247, 23-35. http://dx.doi.org/10.1016/j.femsle.2005.04.027 [4] Barbieri, E., Guidi, C., Ber taux, J. , Frey-Klett, P., Garbaye , J., Ceccaroli, P., Saltarelli, R., Zambonelli, A. and Stocchi, V. (2007) Ocurrence and Diversity of Bacterial Communities in Tuber magnatum During Truffle Maturation. Environmental Microbiology, 9, 2234-2246. http://dx.doi.org/10.1111/j.1462-2920.2007.01338.x [5] Rivera, C.S., Blanco, D., Marco, P., Oria, R. and Venturini, M.E. (2011) Selection of a Deco nt aminati on Treatmen t for Fresh Tuber aestivum and Tuber melanosporum Truffle Packaged in Modified Atmospheres. Food Control, 22, 626- 632. http://dx.doi.org/10.1016/j.foodcont.2010.10.015 [6] Rivera, C.S, Blanco, D., Marco, P., Oria, R. and Venturini, M.E. (2011) Effects of Electron-Beam Irradiation on the Shelf Life, Microbial Populations and Sensory Characteristics of Summer Truffle (Tuber aestivum) Packaged under Modified Atmospheres. Food Microbiology, 28, 141-148. http://dx.doi.org/10.1016/j.fm.2010.09.008 [7] Frey-Klett, P., Pierrat, J.C. and Garba ye, J. (1997) Location and Survival of Mycorrhi za Helper P. f luorescens during Establishment of Ectomycorrhizal Symbiosis between Laccaria bicolor and Douglas Fir. Applied and Environmental Microbiology, 63, 139-144.  I. Ben Romdhane et al. [8] Frey-Klett, P., Chavat te, M., Clausse, M.L., Courrier, S., Le Roux, C., Raaijmakers, J., et al. (2005) Ectomycorrhizal Symbiosis A ffects Functional Diversity of Rhizosphe r e Fl uore s c ent Pse udom ona ds. New Phytologist, 165, 317-328. http://dx.doi.org/10.1111/j.1469-8137.2004.01212.x [9] Aspray, T.J., Frey-Klett, P., Jones, J.E., Whipps, J. M., Garb aye, J. and Bending, G.D. (2006) Mycorrhization Helper Bacteria: A Cas e of Specificity for Alterin g Ectomycor rhiza Arch itect ur e But No t Ect omycorrhiza Fo rmation. Myco r r- hiza, 16, 533-541. http://dx.doi.org/10.1007/s00572-006-0068-3 [10] Nazzaro, F., Fratianni, F., Picariello, G., Coppola, R., Reale, A. and Di Luccia, A. (2007) Evaluation of Gamma Rays Influence on Some Biochemical and Micr obiological Aspects in Black Truffle. Food Chemistry, 103, 344-354. http://dx.doi.org/10.1016/j.foodchem.2006.07.067 [11] Reale, A., Sorrentino, E., Lacumin, L., Tremonte, P., Man zano, M., Ma iuro, L., Comi, G., Coppola, R. and Succi, M. (2009) Irradiation Treat ments to Improve the Sh elf Life of Fresh Black Truf fle (Truf fle P r eservati on by Gamma-Rays). Journal of Food Science, 74, M196-M200. http://dx.doi.org/10.1111/j.1750-3841.2009.01142.x [12] Wang, S. and Marcone, M.F. (2011) The Biochemistry and Biological Properties of the World’s Most Expensive Un- derground Edible Mushroom: Truffle. F ood Re s e ar ch International, 44, 2567-2581. http://dx.doi.org/10.1016/j.foodres.2011.06.008 [13] Fawell, J. (2000) Risk Assessment Case Study—Chloroform and Related Substances. Food and Chemical Toxicology, 38, S91-S95. http://dx.doi.org/10.1016/S0278-6915(99)00129-5 [14] P rema, P., Smila, D., Pal avesam, A. an d Immanuel, G. (2010) Production and Characterization of an Antifungal Com- pound (3-Phenyllactic Acid) Produced by Lactobacillus plantarum Strain. Food and Bioprocess Technology, 3, 379- 386. http://dx.doi.org/10.1007/s11947-008-0127-1 [15] Sorrentino, E., Reale, A., Tremonte, P., Maiuro, L., Succi, M., Tipaldi, L., Renzo, T., Pannella, G. and Coppola, R. (2013) Lactobacillus plantarum 29 Inhibits Penicillium spp. Involved in the Spoilage of Black Truffle (Tuber aesti- vum). Journal o f F ood Sci ence, 78, M1188-M1194. [16] Antony-Babu, S., Deveau, A., Van Nostrand, J.D., Zhou, J., Le Tacon, F., Robin, C., et al. (2013) Black Truffle-Associated Bacterial Communities during the Development and Maturation of Tuber Melanosporum Ascocarps and Putative Functional Roles. Env i r onm e n t al Micr obi ology, 16, 2831-2847. [17] Girard, H. and Rougieux, R. (1967) Technique de microbiologie agricole. Ed. Dunod, Paris, 216. [18] Massanti ni, R., Brunno, M., Salcini, C., Bellicontro, A. and Mencarelli, F. (2002) Conservazione in film plastico del tartufo fresco (Tuber aestivum). Indus t r ie A li m e ntari, 41, 1204-1207. [19] Whittenbury, R. (1963) Hydrogen Peroxide Formation and Catalase Activity in the Lactic Acid Bacteria. Journal of General Microbiology, 35, 16-26. [20] Eschenbach, D.A., Davick, P.R., Williams, B.L., Klebanoff, S.J., Smith, K.Y., Critchlow, C.M. and Holmes, K.K. (1989) Prevalence of Hydrogen Peroxide-Producing Lactobacillus Species in Normal Women and Women with Bac- terial Vaginosis. Journal of Clinical Microbiology, 27, 251-256. [21] Mello, A., Murat, C. and Bonfante, P. (2006) Truffle: Much More than a Prized and Local Fungal Delicacy. FEMS Microbiology Letters, 260, 1-8. http://dx.doi.org/10.1111/j.1574-6968.2006.00252.x [22] Splivallo, R., Deveau , A., Valdez, N., Kirchhoff, N., Frey-Klett, P. and Karlovsky, P . (2014 ) Bacteria Associated with Truffle-Fuiting Bodies Contribute to Truffle Aroma. Environmental Microbiology, Published Online. [23] Morris, J.G. (2000) The Effect of Redox Potential. In: Lund, B.L., Baird-Parker, T.C., Gould, G.W., Eds., The Micro- biologi cal Safe t y an d Qu ali ty of Food , Volume 1, Aspen, Gaithersburg, 235-250. [24] Pao, S., Davis, C.L. and Kelsey, D.F. (2000) Efficacy of Alkaline Washing for the Decontamination of Orange Fruit Surfaces In oculated with Escherichia coli. Journal of Food Protection, 63, 961-964. [25] Pao, S., Davis, C.L., Kelse y, D.F. and Petracek, P.D. (1999 ) Sanitizing E ffects of Truffle Ascocarp Waxes at High pH and Temper ature on Orange Surfaces Inoculat ed with Escherichia coli. Journal of Food Science, 64, 359-362. http://dx.doi.org/10.1111/j.1365-2621.1999.tb15900.x [26] Somers, E.B., Schoeni, J.L. and Wong, A.C.L. (1994) Effect of Trisodium Phosphate on Biofilm and Planktonic Cells of Campylobacter jejuni, Escherichia coli O157: H7, Listeria monocytogenes and Salmonella typhimurium. Interna- tional Journal of Food Microbiology, 22, 269-276. http://dx.doi.org/10.1016/0168-1605(94)90178-3 [27] Hwang, C.A. and Beuchat, L.R. (1995) Efficacy of Selected Chemicals for Killing Patho genic and S poilage Microo r- ganisms on Chicken Skin. International Association for Food Protection, 1, 19-23. [28] Zhang, G., Ma, L., Beuchat, L.R., Erickson, M.C., Phelan, V.H. and Doyle, M.P. (2009) Evaluation of Treatments for Elimination of Foodborne Pathogens on the Surface of Leaves and Roots of Lettuce (Lactuca sativa L.). Journal of Food Protection, 72, 228-234. [29] Rougieux, R. (1963) Actions antibiotiques et stimulantes de la truffe du désert (Terfezia boudieri Chatin). Annales de  I. Ben Romdhane et al. l’Institute Pasteur, 105, 315-318. [30] Dib-Bellahouel, S. and Fortas, Z. (2014) Activity of the Desert Truffle Terfezia boudieri Chatin, against Associated Soil Microflora. African Journal of Microbiology Research, 8, 3008-3016. [31] Buzzini , P., Gasparet ti, C., Turchett i, B., Cramaros sa, M .R. , Vau ghan-Martini, A., Martini, A., et al. (2005) Production of Volatile Organic Compounds (VOCs) by Yeasts Isolated from the Ascocarps of Black (Tuber melanosporum Vitt.) and White (Tuber magnatum Pico) Truffle. Archives of Microbiology, 184, 187-193. http://dx.doi.org/10.1007/s00203-005-0043-y [32] Pacion i, G., Leonardi, M., Aimola, P., Ragnelli, A.M., Rubini, A. and Paolocci, F. (2007) Isolation and Characteriza- tion of Some Mycelia Inhabiting Tuber as c om ata. Mycological Research, 111, 1450-1460. http://dx.doi.org/10.1016/j.mycres.2007.08.016 [33] Zacchi, L., Vaughan-Martini, A. and Angelini, P. (2003) Yeast Distribution in a Truffle Field Ecosystem. Annals of Microbiology, 53, 275-282. [34] Archbald, F.S. and Fridovichi, I. (1983) Oxygen Radicals, Oxygen Toxicity and the Life of Microoragnisms. Acta Médica Portuguesa, 4, 101-112.
|