Paper Menu >>
Journal Menu >>
 Open Journal of Stomatology, 2011, 1, 18-24 OJST doi:10.4236/ojst 2011.12004 Published Online June 2011 (http://www.SciRP.org/journal/OJST/). Published Online June 2011 in SciRes. http://www.scirp.org/journal/OJST The prevalence of virulent clonal strains of mutans streptococci in vivo and co-culture succession of the strains in vitro —Virulence potential of mutans streptococci Mingyu Li*, Guang-yun Lai, Jun Wang Shanghai Key Laboratory of Stomatology, Shanghai Research Institute of Stomatology, Ninth People’s Hospital, Medical College, Shanghai Jiao Tong University, Shanghai, China. E-mail: *lmy1691@hotmail.com Received 8 March 2011; revised 20 April 2011, accepted 3 May 2011. ABSTRACT Purpose:To examine selected putative virulent prop- erties of mutans streptococci in two groups with dif- ferent caries activity and to examine co-culture hy- bridization of the strains in vitro. Methods: A set of strains from caries-free subjects (115) and another set from caries-active subjects (165) were isolated. Each strain was characterized for three virulence determi- nants. The clinical bacteria were then cocultured by three strains exhibiting the highest levels of virulence. Isolate colonies of last filial generation bacteria were enrichment-incubated and estimated for virulence again. RAPD-PCR and MLEE analyses were proc- essed for parental bacteria and last filial generation one. Results: No difference associated with caries ac- tivity of the subjects from whom the isolates originated. Virulent properties of a filial generation strains was not different in the same generation, but was very different from their parent strains. Conclusion: The coexist properties of virulent polyclonal strain of MS may hold in a very general conditional sense in a dental plaque ecosystem in vivo, however, one of the co-culture strains may became dominant and displa- ced the others as the result of continuous ecological succession in vitro. Keywords: Ecological Phenomenon; Mutans Streptococci; Co-Culture; Virulent; in Vitr o 1. INTRODUCTION Dental caries is a transmissible and poly-microbial eco- logical disease, in which mutans streptococci (MS), mainly Streptococcus mutans and Streptococcus sobri- nus play the major role. The main virulent factors of MS include adhesion, acidogenicity and aciduricity [1-3]. Primary mechanism for adherence of S. mutans is the production of glucan from sucrose via glucosyltrans- ferases (GTFs) [4,5]. Acid production and aciduricity are other important caries-inducing virulent factors [6-8]. MS is generally considered to be the principal aetiologi- cal agent of dental caries [9,10]; however, they are widely distributed not only in populations with moderate or high caries prevalence [11,12] but also in populations having no or low caries experience [13,14]. Caries can also de- velop in the absence of MS species [15]. So Takahashi and Nyvad present a new view of caries, the Extended Caries Etiological Hypothesis, that it is no longer just a consideration of which specific bacteria are present, but rather what those bacteria are doing. They are behaving as acidogenic/aciduric bacteria contributing to the dental caries disease process [16]. The virulence properties of MS have the coexist properties of a poly- clonal epidemic [17]. The three highest virulent charac- ters (GTFs activity, acid production and aciduricity) were not assembled together on a same strain [18]. Kuramitsu and Wang suggested that S. gordonii, and perhaps addi- tional nonmutans streptococci, can attenuate some of the virulence properties of S. mutans [19]. Despite its importance, we have only a limited under- standing of the biological nature of MS virulent strains colonization. How the prevalence of virulent factors of MS from strain to strain in a dental plaque ecosystem in vivo. How do polyclonal strains replace one another in ecological succession in vitro? To attempt to determine what happens to ecological sites where more than one S. mutans strain is present, co-culture of this study was performed by the 3 clinical strains which exhibited the highest level of virulence properties in glycosyltrans- ferase activity, acid production, and acid tolerance, re-  M. Y. Li et al. / Open Journal of Stomatology 1 (2011) 18-24 Copyright © 2011 SciRes. OJST 19 spectively. The last filial generation bacteria on an agar plate were picked up and estimated for virulence proper- ties again. Genetic studies were processed to understand how parent bacteria and last filial one variations. 2. MATERIALS AND METHODS 2.1. Subjects The experimental procedures were approved by the Eth- ics Committee of Ninth People’s Hospital, Medical Col- lege, Shanghai Jiao Tong University. The study consisted of 100 subjects aged between 5 and 25 years (13.5 ± 4.7). Groups of caries-free and car- ies-active groups were 50 and 50, with the DMF (de- cayed, missing, filled) in caries-free (dmft = 0) and car- ies-active (dmft > 4), respectively. A total of 280 MS (both sobrinus and mutans species) was obtained from dental biofilm of the subjects. MS form Groups of car- ies-free and caries-active groups were 115 and 165. 2.2. Bacterial Isolates and Species Confirmation Plaque biofilm samples were plated on Mitis Salivarius Bacitracin (MSB) agar plates (Difco Laboratories, De- troit, MI). They were cultured in an Anaerobic Work Station (BUG BOX DUAL, Ruskinn, England) with 85% of N2, 15% of CO2 at 37˚C for 2 days. In order to detect MS, preliminary identification was done by Gram’s staining method. Further classification was done to ex- amine their sensitivity to antibiotics and fermentation of carbohydrates etc, by using a biochemical appraisal and verification system, API 20 Strep test kits according to the manufacturer’s instructions (bioMerieux Vitek Inc.). S. mutans UA159 and S. sobrinus 6715 were used as standard laboratory strains. 2.3. GTFs Activity, Acid Production and Aciduricity Assay Identified MS were incubated anaerobically in Trypti- case soy broth (TSB) in an incubator at 37˚C for 18 h. The samples of bacterial supernatants were obtained by means of centrifugation. Extracellular GTFs activity was determined by means of direct enzyme assay [20]. Each supernatant was exposed to 14C glucosyl-sucrose (Shang- hai Institute of Nuclear Research of Chinese Academy of Science, Shanghai, China, final concentration = 100 mmol/L sucrose, 40 μmol/L dextran 9,000 in buffered- KCl, pH 6.5) for 4 h at 37˚C, to form glucans. GTFs ac- tivity by direct enzyme assay was expressed as micromol glucans formed per milliliter supernatant. The acid sensitivity (tolerance) was assayed by using broth micro-dilution method on 96-microwell plates. Bacteria were incubated anaerobically in 5 ml of WIL- KINS-CHALGREN anaerobe broth (Oxoid, England) in the incubator at 37˚C for 24 hours to a density equal to that of a no. 1 McFarland standard. For the evaluation of acid sensitivity, strains were sub-cultured into fresh an- aerobe medium at pH of 5.5, 5.0, 4.5, 4.0, 3.5, 3.0 and 2.5 (medium pH was adjusted with HCl). The control was treated in the same way except the medium at pH 7.5. After 24 hours incubation, bacterial growth was observed by a stereoscope and recorded. At the same time, from every microwell, 50 μl of suspension was removed and smeared on an agar plate. Bacterial colony was counted correspondingly after 48 hours incubation. The pH value at which there was no bacterial growth was considered as acid sensitivity. For the evaluation of acid production, the strains were cultured in the anaerobe broth with glucose in microwell plate at pH of 7.5. After 24 hours incubation in an an- aerobic chamber, acid production of bacterial cultures was measured by an Orion pH meter (Model 501, Orion Research, Inc., Cambridge, MA). Un-inoculated broth (control) was also cultured under the same conditions. The experiments of virulent assay were performed in quadruplicate and the result was presented by means. 2.4. Co-Culture in Vitro Hybridization co-culture was performed by 3 strains with ratio of 1:1:1 (v/v/v) anaerobically in Trypticase soy broth (TSB) in an incubator at 37˚C, which exhibited the highest levels of aciduricity, acidogenicity and GTFs activity, respectively. Each respective strain was cultured alone under the same condition as control. After one week of co-culture, during which the growth medium was replenished daily, the cultures was plated out, indi- vidual colonies were enrichment incubated and assayed for aciduricity, acidogenicity and GTFs activity again. 2.5. RAPD-PCR Analysis A total of 26 of 96 strain colonies from last filial genera- tion were next subjected to RAPD-PCR analysis. The protocol for DNA amplification was based on Pereira [21], with a little modification. For DNA extraction, cells were washed in 0.1 M Tris buffer, lysed with ly- sozyme + 10% SDS and extracted with phenol-chloro- form. DNA was precipitated with sodium acetate and ethanol, and its concentration was calculated by mea- suring A260. Quality was estimated from A260/A280 ratio. RAPD working primers [21] were OPA-3 (5’-AGTCA- GCCAC-3’), OPA-5 (5’-AGGGGTCTTG-3’), and OPA- 18 (5’-AGGTGACCGT-3’) from Shanghai Sangon Bio- logical Engineering Technology & Services Co., Ltd (Shanghai, China). The RAPD reaction was performed in a total volume of 50 μl consisting of 5 μl template DNA, 10 × PCR buffer (750 mM Tris-HCl, 200 mM (NH4)2SO4, 0.1% Tween 20), 3.5 mM MgCl2, 200 μM deoxynucleoside triphosphates, 1.25 U of Taq DNA po- 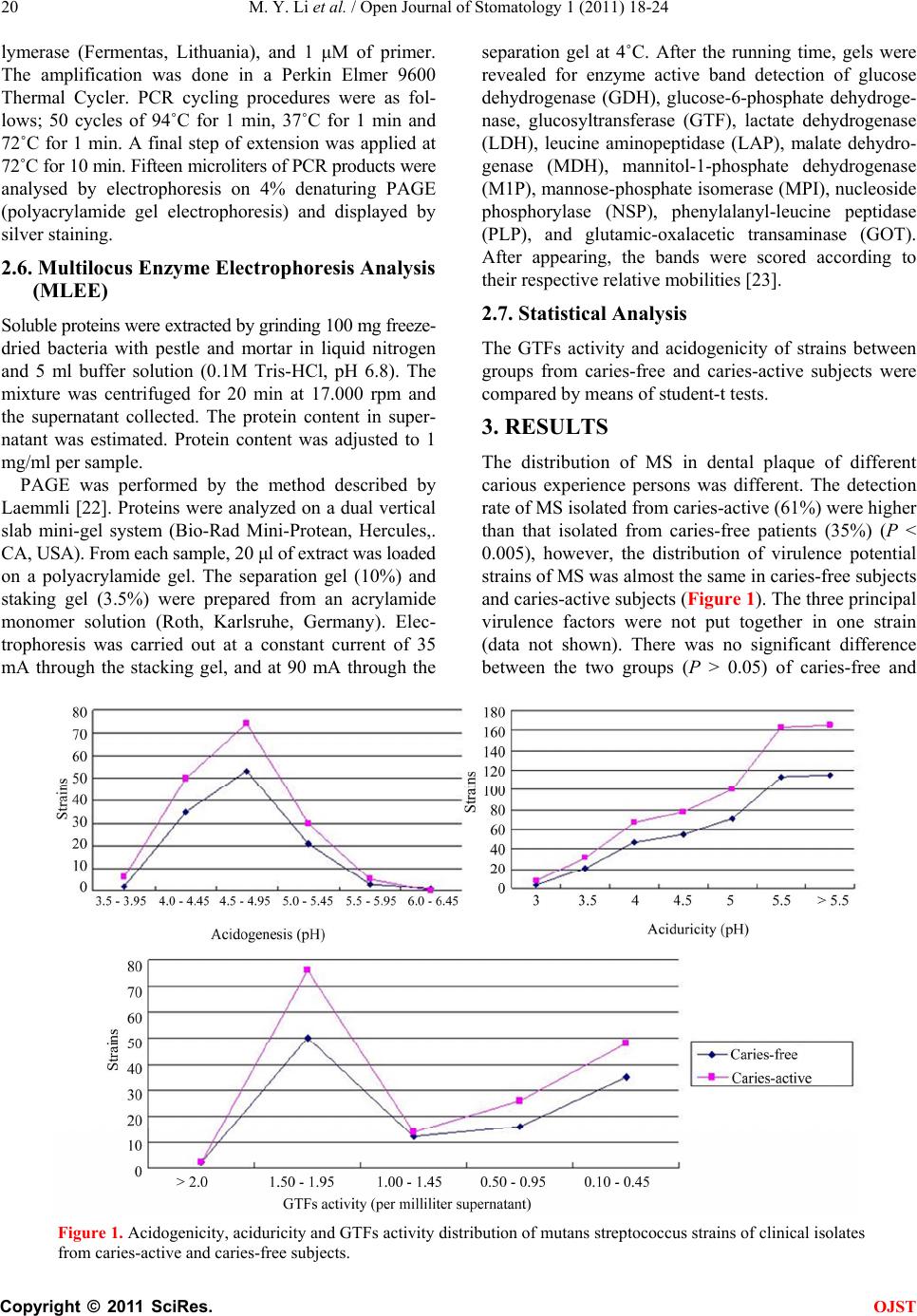 M. Y. Li et al. / Open Journal of Stomatology 1 (2011) 18-24 Copyright © 2011 SciRes. OJST 20 lymerase (Fermentas, Lithuania), and 1 μM of primer. The amplification was done in a Perkin Elmer 9600 Thermal Cycler. PCR cycling procedures were as fol- lows; 50 cycles of 94˚C for 1 min, 37˚C for 1 min and 72˚C for 1 min. A final step of extension was applied at 72˚C for 10 min. Fifteen microliters of PCR products were analysed by electrophoresis on 4% denaturing PAGE (polyacrylamide gel electrophoresis) and displayed by silver staining. 2.6. Multilocus Enzyme Electrophoresis Analysis (MLEE) Soluble proteins were extracted by grinding 100 mg freeze- dried bacteria with pestle and mortar in liquid nitrogen and 5 ml buffer solution (0.1M Tris-HCl, pH 6.8). The mixture was centrifuged for 20 min at 17.000 rpm and the supernatant collected. The protein content in super- natant was estimated. Protein content was adjusted to 1 mg/ml per sample. PAGE was performed by the method described by Laemmli [22]. Proteins were analyzed on a dual vertical slab mini-gel system (Bio-Rad Mini-Protean, Hercules,. CA, USA). From each sample, 20 μl of extract was loaded on a polyacrylamide gel. The separation gel (10%) and staking gel (3.5%) were prepared from an acrylamide monomer solution (Roth, Karlsruhe, Germany). Elec- trophoresis was carried out at a constant current of 35 mA through the stacking gel, and at 90 mA through the separation gel at 4˚C. After the running time, gels were revealed for enzyme active band detection of glucose dehydrogenase (GDH), glucose-6-phosphate dehydroge- nase, glucosyltransferase (GTF), lactate dehydrogenase (LDH), leucine aminopeptidase (LAP), malate dehydro- genase (MDH), mannitol-1-phosphate dehydrogenase (M1P), mannose-phosphate isomerase (MPI), nucleoside phosphorylase (NSP), phenylalanyl-leucine peptidase (PLP), and glutamic-oxalacetic transaminase (GOT). After appearing, the bands were scored according to their respective relative mobilities [23]. 2.7. Statistical Analysis The GTFs activity and acidogenicity of strains between groups from caries-free and caries-active subjects were compared by means of student-t tests. 3. RESULTS The distribution of MS in dental plaque of different carious experience persons was different. The detection rate of MS isolated from caries-active (61%) were higher than that isolated from caries-free patients (35%) (P < 0.005), however, the distribution of virulence potential strains of MS was almost the same in caries-free subjects and caries-active subjects (Figure 1). The three principal virulence factors were not put together in one strain (data not shown). There was no significant difference between the two groups (P > 0.05) of caries-free and Figure 1. Acidogenicity, aciduricity and GTFs activity distribution of mutans streptococcus strains of clinical isolates from caries-active and caries-free subjects.  M. Y. Li et al. / Open Journal of Stomatology 1 (2011) 18-24 Copyright © 2011 SciRes. OJST 21 caries-active in GTFs activity and acidogenicity (Table 1). In the test of co-culture, a total of 96 individual colo- nies were isolated and characterized biochemically. Virulent properties of a filial generation strains was not different in the same generation, but was very different from their parent strains (Table 2). The virulent poten- tial of the control strains did not change after one week culture alone (data not shown). A total of 26 of 96 strain colonies from last filial generation were next subjected to RAPD-PCR analysis. RAPD-PCR data showed that the band profiles of filial generation bacteria were not significantly different from each other but different from any ones of the parent strains (Figure 2). It may suggest that one strain (strain A) of last filial generation may become dominant and displaced the others (Table 2, Figures 2 and 3). In the results of MLEE, only GOT, NSP, PLP, MPI, LAP as well as M1P showed activity for S. mutans strain (Figure 3). The results of MLEE were similar to that of RAPD-PCR. RAPD was proved to be more discriminatory than MLEE. 4. DISCUSSION This work described an analysis of acidogenicity, acidu- ricity and GTFs activity amongst 280 clinical strains of MS. It was found that the distribution of virulence po- tential of MS was nearly the same in strains from caries- free subjects and caries-active subjects. Three highest virulent characters (GTFs activity, aciduricity, and aci- dogenicity) were not fixed together on a same strain. The result is consistent with the study by Guo [18]. So we may proposed a hypothesis that MS strains with varying degree of virulence potential may play different func- tions in vivo (for example one strain plays a function of GTFs activity, other strain plays a function of acid pro- duction and another strain plays a function of aciduric- ity), which makes them a good collective group to obtain the best potential of cariogenic. Co-culture of MS strains was performed by the 3 clinical strains which exhibited the highest level of dif- ferent virulent potential. The results of filial generation obtained neither a combination group of MS strains nor one strain with the highest virulence potential. Virulent properties of a filial generation strains was not different in the same generation, but was very different from their parent strains. RAPD-PCR and MLEE analysis revealed that the band profiles of filial generation bacteria were not significantly different from each other but different from any ones of the parent strains. In the micro-eco- logical environmental condition in vitro, one strain may become dominant and displace the others. This cannot exclude simple adaptation; however, the data indicated the different ecological succession outcomes of poly- clonal virulent strains between in vitro and in vivo. The niche concept was defined by G. E. Hutchinson. A species can only live within a certain environment, or within a certain environment a species can live. Accord- ing to the competitive exclusion principle, no two spe- cies can occupy the same niche in the same environment for a long time [24]. Different strains of the same species are more likely to occupy the same ecological niche, and hence undergo more intense competition than different species [25]. In the study of Rukayadi, if two different bacterial strains occupy the same niche on the leaf sur- face, then the antagonism between them can occur only when they use the same nutrient source and occupy the same niche [26]. The nontoxigenic strains applied to soil occupy the same niches as the natural occurring toxi- Table 1. GTFs activity and acidogenicity of clinical isolates of mutans streptococci. Caries-free (115 Strains)Caries-active (165 Strains)P-value (Student-t test) GTFs activity (per milliliter supernatant) 0.9611 ± 0.6630 1.2218 ± 0.6799 0.0790 Acidogenicity (pH) 4.8624 ± 0.5857 4.5657 ± 0.4543 0.0893 The result of aciduricity is not an absolute term and state at Figure 1. Table 2. The actual virulence properties of each strain of last filial generation and parental bacteria of 3 strains which exhibited the highest levels of aciduricity, acidogenicity and GTF activity. GTFs activity (per milliliter supernatant) Aciduricity pH Acidogenicity pH Strain of the most potential acidogenic capacity 0.27 5.0 3.9 Strain of the most potential aciduricity capacity 1.67 3.0 5.59 Parental bacteria Strain of the most potential GTFs activity capacity2.2 4.5 4.28 Strain colonies of last filial generation (mean ± SD, n = 96) 1.36 ± 0.08 4.0 ± 0.04 4.2 ± 0.3  M. Y. Li et al. / Open Journal of Stomatology 1 (2011) 18-24 Copyright © 2011 SciRes. OJST 22 Figure 2. RAPD-PCR analysis of the gene transformation of three parental generation and last filial generation mutans streptococci strains. M: marker, A, B and C: parental generation strains, A: acidogenicity strain, B: aciduricity strain, C: GTFs activity strain. 1 - 26: mutans streptococci strains of last filial generation. Bands spectrum from filial generation mu- tans streptococci were almost same but different from their three parental generation strains. Figure 3. Multilocus enzyme electrophoresis analysis of three parental generation and filial generation mutans strep- tococcus strains. A, B and C: parental generation strains, A: acidogenicity strain, B: aciduricity strain, C: GTFs activ- ity strain. 1 - 7: mutans streptococci strains of last filial generation. GOT: glutamic-oxalacetic transaminase, NSP: nu- cleoside phosphorylase, PLP: phenylalanyl-leucine peptidase, MPI: mannose-phosphate isomerase, LAP: leucine aminopeptidase, M1P: mannitol-1-phosphate dehydrogenase. Enzyme loci from filial generation mutans streptococci were almost same. genic strains. They are capable of competing and dis- placing toxigenic strains [27]. These data are more in agreement with our results which indicate that different strains of the same species undergo more intense compe- tition if they need protection from the surrounding harsh ecological niche. A group of different strains of the same specie can not occupy the same ecological niche in vitro. In conclusion, the coexist properties of virulent poly- clonal strain of MS may hold in a very general condi- tional sense in a dental plaque ecosystem in vivo, how-  M. Y. Li et al. / Open Journal of Stomatology 1 (2011) 18-24 Copyright © 2011 SciRes. OJST 23 ever, one of the co-culture strains may became dominant and displaced the others as the result of continuous eco- logical succession in vitro. 5. ACKNOWLEDGEMENTS This work was supported by Science and Technology Commission of Shanghai (08DZ2271100) and Shanghai Leading Academic Discipline Project (Project Number: S30206). Authors of this manuscript do not have any financial conflicts of interest and the funder (s) has/have not played any decision-making role in the research. REFERENCES [1] Qi, F. and Kreth, J. (2010) Characterization of anti- competitor activities produced by oral bacteria. Methods in Molecular Biology, 666, 151-166. doi:10.1007/978-1-60761-820-1_11 [2] Hamilton, I.R. (2000) Ecological basis for dental caries. In: Kuramitsu, H.K. and Ellen, R.P. Eds., Oral bacterial ecology: The molecular basis, Horizon Scientific Press, Norfolk, pp. 219-274. [3] Mattos-Graner, R.O., Smith, D.J., King, W.F. and Mayer, M.P. (2000) Water-insoluble glucan synthesis by mutans streptococcal strains correlates with caries incidence in 12 to 30-month-old children. Journal of Dental Research, 79, 1371-1377. doi:10.1177/00220345000790060401 [4] Yamashita, Y., Bowen, W.H., Burne, R.A. and Kura- mitsu, H.K., (1993) Role of the Streptococcus mutans gtf genes in caries induction in the specific- pathogen-free rat model. Infection and Immunity, 61, 3811-3817. [5] Kuramitsu, H.K. (1993) Virulence factors of mutans strepto-cocci: Role of molecular genetics. Critical Reviews in Oral Biology & Medicine, 4, 159-176. [6] Song, J.H., Kim, S.K., Chang, K.W., Han, S.K., Yi, H.K. and Jeon, J.G., (2006) In vitro inhibitory effects of Polygonum cuspidatum on bacterial viability and viru- lence factors of Streptococcus mutans and Strepto- coccus sobrinus. Archives of Oral Biology, 51, 1131- 1140. doi:10.1016/j.archoralbio.2006.06.011 [7] Yang, D.Q., Liu, T.J. and Li, S. (2006) A study of genetic diversity in lactate dehydrogenase of strepto- coccus mutans from clinical isolates. Journal of Sichuan University (Medicine Science Edition), 37, 781-784. [8] Marcelle, M.N., José, A.C.L., Jacqueline, A., Reginaldo, B.G. and Robert, A.B. (2004) Adaptive acid tolerance response of Streptococcus sobrinus. The Journal of Bacteriology, 186, 6383-6390. doi:10.1128/JB.186.19.6383-6390.2004 [9] Hamada, S. and Slade, H.D. (1980) Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiological Reviews, 44, 331-384. [10] Loesche, W.J. (1986) Role of Streptococcus mutans in human dental decay. Microbiological Reviews, 50, 353- 380. [11] Beighton, D., Rippon, H.R. and Thomas, H.E.C. (1987) The distribution of Streptococcus mutans serotypes and dental caries in a group of 5- to 8-year-old Hampshire schoolchildren. British Dental Journal, 162, 103-106. doi:10.1038/sj.bdj.4806033 [12] Alaluusua, S., Kleemola-Kujala, E. and Nyström, M. (1987) Caries in the primary teeth and salivary Strep- tococcus mutans and lactobacillus levels as indi- cators of caries in permanent teeth. Pediatric Dentistry, 9, 126-130. [13] Carlsson, P., Olsson, B. and Bratthall, D., (1985) The relationship between the bacterium Streptococcus mutans in the saliva and dental caries in children in Mozambique. Archives of Oral Biology, 30, 265-268. doi:10.1016/0003-9969(85)90043-3 [14] Matee, M.I.N., Mikx, F.H.M., de Soet, J.S., Maselle, S.Y., de Graff, J., van Palenstein and Helderman, W.H. (1993) Mutans streptococci in caries-active and caries- free infants in Tanzania. Oral Microbiology and Immu- nology, 8, 322-324. doi:10.1111/j.1399-302X.1993.tb00582.x [15] Aas, J.A., Griffen, A.L. and Dardis, S.R., (2008) Bacteria of dental caries in primary and permanent teeth in children and young adults. Journal of Clinical Micro- biology, 46, 1407-1417. doi:10.1128/JCM.01410-07 [16] Takahashi, N. and Nyvad, B. (2008) Caries ecology revisited: Microbial dynamics and the caries process. Caries Research, 42, 409-418. doi:10.1159/000159604 [17] Nakano, K., Lapirattanakul, J., Nomura, R., Nemoto, H., Alaluusua, S., Grönroos, L., Vaara, M., Hamada, S., Ooshima, T. and Nakagawa, I. (2007) Streptococcus mu- tans clonal variation revealed by multilocus sequence typing. Journal of Clinical Microbiology, 45, 2616-2625. doi:10.1128/JCM.02343-06 [18] Guo, L.H., Shi, J.N., Zhang, Y., Liu, X.D., Duan, J. and Wei, S. (2006) Identification of genetic differences be- tween two clinical isolates of Streptococcus mutans by suppression subtractive hybridization. Oral Microbiol Immunol, 21, 372-380. doi:10.1111/j.1399-302X.2006.00306.x [19] Kuramitsu, H.K. and Wang, B.Y. (2006) Virulence properties of cariogenic bacteria. BMC Oral Health, 6, S11. doi:10.1186/1472-6831-6-S1-S11 [20] Schilling, K.M. and Bowen, W.H. (1988) The activity of glucosyltransferases adsorbed onto saliva-coated hydro- xyapatite. Journal of Dental Research, 67, 2-8. doi:10.1177/00220345880670010201 [21] Pereira, M.S., Leal, N.C., Sobreira, M., Almeida, A.M.P., Siqueira-Júnior, J.P.G. and Takak, M. (2002) Typing hu- man bovine Sthaphylococcus aureus by RAPD-PCR and ribotyping-PCR. Letters in Applied Microbiology, 35, 32-36. doi:10.1046/j.1472-765X.2002.01127.x [22] Laemmli, U.K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680-685. doi:10.1038/227680a0 [23] Selander, R.K., Caugant, D.A., Ochman, H., Musser, J.M., Gilmour, M.N. and Whittam, T.S. (1986) Methods of multilocus enzyme electrophoresis for bacterial po- pulation genetics and systematics. Applied and Environ- mental Microbiology, 51, 873-884. [24] Hardin, G. (1960) The competitive exclusion principle. Science, 131, 1292-1297. doi:10.1126/science.131.3409.1292 [25] Massey, R.C., Buckling, A., R. ffrench-Constant, (2004) Interference competition and parasite virulence. Pro- ceedings of the Royal Society - Biological Sciences 22, 785-788. doi:10.1098/rspb.2004.2676 [26] Rukayadi, Y., Suwanto, A., Tjahjono, B., Harling, R., (2000) Survival and epiphytic fitness of a nonpathogenic mutant of Xanthomonas campestris pv. Glycines.  M. Y. Li et al. / Open Journal of Stomatology 1 (2011) 18-24 Copyright © 2011 SciRes. OJST 24 Applied and Environmental Microbiology, 66, 1183- 1189. doi:10.1128/AEM.66.3.1183-1189.2000 [27] Yin, Y., Yan, L., Jiang, J. and Ma, Z. (2008) Biological control of aflatoxin contamination of crops. Journal of Zhejiang University - Science B, 9, 787-792. ABBREVIATIONS GTF: glucosyltransferases; RAPD-PCR: Random amplified polymorphic DNA - Polymerase Chain Reaction. |

