Paper Menu >>
Journal Menu >>
 Open Journal of Stomatology, 2011, 1, 13-17 OJST doi:10.4236/ojst 12003 Published Online June 2011 (http://www.SciRP.org/journal/OJST/). Published Online June 2011 in SciRes. http://www.scirp.org/journal/OJST Efficacy of IMOD in the treatment of oral lichen planus —Efficacy of IMOD in oral lichen planus Farzaneh Agha-Hosseini1, Iraj Mirzaii-Dizgah2, Mohammad Abdollahi3, Neda Akbari-Gillani1 1Department of Oral Medicine/Dental Research Center, Dentistry School, Tehran University of Medical Sciences, Tehran, Iran; 2Department of Physiology, School of Medicine, AJA University of Medical Sciences, Tehran, Iran; 3Pharmaceutical Sciences Research Center, Tehran University of Medical Sciences, Tehran, Iran. E-mail: aghahose@sina.tums.ac.ir; emirzaii@razi.tums.ac.ir; mohammad@t ums.ac .ir Received 17 March 2011; revised 26 April 2011, accepted 13 May 2011. ABSTRACT Objectives: Oral lichen planus (OLP) is a chronic, immunologically mediated, mucocutaneous disorder. A wide range of topical and systemic therapies have been used in the treatment of OLP. The efficacy of IMOD (an Iranian new immunomodulator drug, con- taining selenium, carotene, and flavonoids) in the management of oral lichen planus was evaluated. Study design: In a before-after clinical trial study, thirty patients (21 women and 9 men; age range 35 - 66 years with 112 lesions) with lichen planus were enrolled. The study covered a three-month period of therapy by IMOD (400 mg/day) and a three-month follow-up period after drug cessation. Outcome mea- sures include soreness relief based on the “nu-meric scale”, and clinical improvement of lesion size and score. Saliva levels of TNF-α was analysed at the baseline and after treatment by ELISA. Statistical analysis of Wilcoxon and paired student’s t-test were used. Results: Approximately 85% of patients showed partial to complete improvement and re-mained sym- ptom free after drug cessation. There was no signifi- cant difference in mean saliva TNF-α level before and after the treatment. Conclusion: These results suggest that IMOD seems to be an effective alternative treat- ment for OLP and TNF-α may not be a good indica- tor for monitoring therapeutic response of OLP. Keywords: Oral Lichen Planus; IMOD; TNF-α 1. INTRODUCTION Oral lichen planus (OLP) is a chronic, immunologically mediated, mucocutaneous disorder [1]. Prevalence rate reported for OLP varies between 0.5% and 2.2% in the literature, and the mean age at the time of diagnosis is approximately 55 years. Despite the self-limiting nature of cutaneous lesions, oral lesions are chronic and rarely undergo spontaneous remission, and are potentially pre- malignant. World Health Organization has categorized OLP as a pre-cancerous condition, which is “a general state associated with a significant increased risk of can- cer”. The pathogenesis of OLP is unclear. It was shown that OLP patients have more cellular lipid peroxidation [2]. Both antigen specific and non-specific mechanisms maybe involved. Antigen specific pathways include a cell mediated immunological response to an induced antigenic change in the oral mucosa [1], in which cyto- toxic CD8+ T cells trigger the apoptosis of epithelial cells following formation of autoreactive T lymphocytes [3]. A wide range of topical and systemic therapies have been used in the treatment of OLP. Since the etiology behind OLP is unknown, basic conditions are lacking for development of preventive therapies. Several topical drugs have been suggested, including steroids, calcineurin inhibitors (cyclosporine and tacrolimus), retinoid and ultraviolet phototherapy [3]. Among these, topical ster- oids are widely used and have been accepted as the first choice treatment [3], because they have anti- inflamma- tory and anti-immunologic effects at the same time. But their prolonged use should be avoided, because of the associated adverse effect such as candidiasis over infec- tion, hypothalamic-pituitary-adrenal axis suppression, skin fragility and telangectasis, and lesion recurrence after treatment cessation [4]. IMOD is an Iranian new immunomodulator drug (in- ternational publication number: WO 2007/087825A1) with herbal origin. It is produced from a combination of ethanolic Rosa sp Urtica Dicica and Tanacetum Vulgare extracts, contains selenium, carotene, flavonoids and urea, and having been exposed to a pulsed electromagnetic field. The immunomodulatory mechanisms of IMOD are not clearly understood, but it can increase CD4 + lym- phocyte count after a course of treatment of 1 to 3 months. Its effect is also preserved for a long period after cessa-  F. Agha-Hosseini et al. / Open Journal of Stomatology 1 (2011) 13-17 Copyright © 2011 SciRes. OJST 14 tion of treatment. It has passed 3 phases of clinical trial and there were no adverse effects found. Pregnancy and breast feeding is the only contra-indications of this drug. Considering the durability, safety and immunologic ef- fects, it could be used in treatment of immunologic dis- orders [5]. Serum or saliva level of TNF-α has been used in some studies for monitoring the therapeutic response of OLP [6,7]. TNF-α is a multi-functional cytokine that mediates inflammation, immune response, and apoptosis, and also has a significant role in normal development and homeo- stasis of several organs [8]. Positive statistical correla- tion exists between TNF-α level in serum and saliva. On the other hand, saliva collection is non-aggressive and more cost effective. The purpose of this study was to evaluate the efficacy of IMOD in the relief of symptoms and signs of OLP lesion. We have also measured the level of TNF-α in unstimulated whole saliva before and after treatment. 2. MATERIAL AND METHOD This was an open label before-after clinical trial study enrolling 30 patients (21 females and nine males). The study cases were selected from patients referred to the Oral Medicine Department, Faculty of Dentistry, Tehran University of Medical Sciences. Inclusion criteria were the presence of symmetric re- ticular or papular lesions with or without erosive/atro- phic components proven by biopsy. Histopathological di- agnosis of OLP (Presence of well-defined band like zones of inflammatory infiltration confined to the super- ficial part of the connective tissue, consisting mainly of mature lymphocytes—vacuolar alteration of the basal layer of the epithelium), was based on “a modified defi- nition of the World Health Organization” [9]. The pa- tients were required to sign an informed consent ap- proved by the ethics committee of Tehran University of Medical Sciences, and to be available for monthly ap- pointments up to 6 months. Exclusion criteria were his- tological sign of dysplasia, lichenoid drug reactions, and drug consumption in the past month, pregnancy, breast feeding or any kind of localized or systemic disease. Patients receiving immunosuppressive or immunomodu- latory treatments or any kind of systemic or local drugs were either eliminated or asked to discontinue their treat- ment for a minimum of 1 month before entering the in- vestigation. Patient who had lesions adjacent to amalgam filling were also excluded from the study sample. Data associated with demographic, medical history, symptoms and clinical score of the lesion at the baseline were documented by an oral medicine specialist. Each patient received IMOD in the form of a daily single dose capsule (400 mg) for 90 days. All participants were ex- amined at baseline, after 2 weeks and at months 1, 2, 3, 4, 5 and 6 in order to evaluate treatment efficacy. The clin- ical data or lesion score (LS) as an index of overall clin- ical improvement was scored as: 0 no lesion, 1 hyperk- eratotic lesion, 2 atrophic areas < 1 cm, 3 atrophic areas > 1 cm, 4 erosive areas < 1 cm, and 5 erosive areas >1 cm. A zero value equates to being pain free, 1 indicates low degree of pain, 2 moderate pain, 3 severe pain, and 4 very severe pain [10]. A scaled tongue blade was used to assess the size of lesions [11]. Patients were given an oral examination, their lesions photographed, and then assessed against the numeric scale. Symptomatic response (SR) for each pa- tient was calculated by subtracting the initial numeric scale from final score. Similarly, clinical response was estimated through subtraction LS values obtained in the first and last examination sessions. Positive and negative values were considered as improvement and deteriora- tion, respectively. The saliva level of TNF-α was measured at baseline, and at the end of treatment period. The unstimulated whole saliva (UWS) was collected between 9:00 a.m. and 12:00 a.m. The patients abstained from eating, drinking and smoking for at least 2 hours prior to the sampling. All subjects were requested to swallow first, tilt the head forward and then expectorate WUS into a sterile centrifugal tube without swallowing, for 5 - 15 minutes. The samples were then frozen until the time of analysis. TNF-α tests were performed by using ELISA kits (BioSource, Nevellis, Belgium). 3. STATISTICAL ANALYSIS Analysis and comparison of symptomatic and clinical responses before and after treatment were performed using the Wilcoxon test. Paired student’s t-test was used for comparison of TNF-α level before and after treat- ment. A P-value less than 0.05 was considered statisti- cally significant. Statistical package for social science software (SPSS), used to analyze the data. 4. RESULTS The study sample consisted of 21 women and 9 men with a mean age of 50.7 ± 7.5 years (range 35 - 66 years) and a total 112 lesions. The buccal mucosa was the most common site (47%) for OLP, followed by gingiva (24%), tongue (22%), labial mucosa (0.5%), and palate (0.2%). Approximately 85% of the lesions showed partial to complete clinical improvement at the end of the treat- ment period, and the results were also persistent in the three months follow-up period after drug therapy cessa- tion (Table 1). 14% of LSs had no change, and one per- son showed deterioration (Table 1). Analysis of Wil- coxon test showed that there was a significant difference 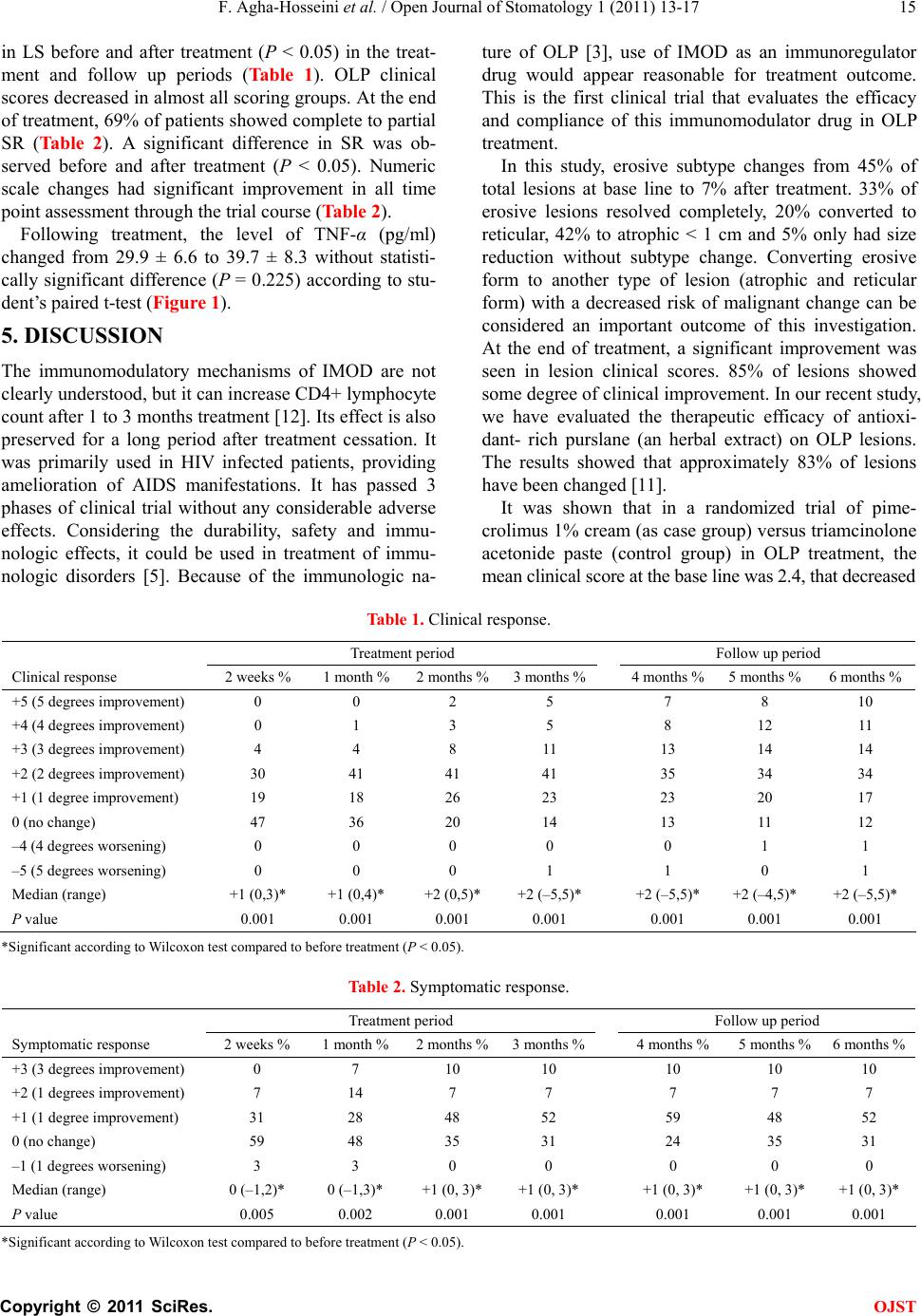 F. Agha-Hosseini et al. / Open Journal of Stomatology 1 (2011) 13-17 Copyright © 2011 SciRes. OJST 15 in LS before and after treatment (P < 0.05) in the treat- ment and follow up periods (Table 1). OLP clinical scores decreased in almost all scoring groups. At the end of treatment, 69% of patients showed complete to partial SR (Table 2). A significant difference in SR was ob- served before and after treatment (P < 0.05). Numeric scale changes had significant improvement in all time point assessment through the trial course (Table 2). Following treatment, the level of TNF-α (pg/ml) changed from 29.9 ± 6.6 to 39.7 ± 8.3 without statisti- cally significant difference (P = 0.225) according to stu- dent’s paired t-test (Figure 1). 5. DISCUSSION The immunomodulatory mechanisms of IMOD are not clearly understood, but it can increase CD4+ lymphocyte count after 1 to 3 months treatment [12]. Its effect is also preserved for a long period after treatment cessation. It was primarily used in HIV infected patients, providing amelioration of AIDS manifestations. It has passed 3 phases of clinical trial without any considerable adverse effects. Considering the durability, safety and immu- nologic effects, it could be used in treatment of immu- nologic disorders [5]. Because of the immunologic na- ture of OLP [3], use of IMOD as an immunoregulator drug would appear reasonable for treatment outcome. This is the first clinical trial that evaluates the efficacy and compliance of this immunomodulator drug in OLP treatment. In this study, erosive subtype changes from 45% of total lesions at base line to 7% after treatment. 33% of erosive lesions resolved completely, 20% converted to reticular, 42% to atrophic < 1 cm and 5% only had size reduction without subtype change. Converting erosive form to another type of lesion (atrophic and reticular form) with a decreased risk of malignant change can be considered an important outcome of this investigation. At the end of treatment, a significant improvement was seen in lesion clinical scores. 85% of lesions showed some degree of clinical improvement. In our recent study, we have evaluated the therapeutic efficacy of antioxi- dant- rich purslane (an herbal extract) on OLP lesions. The results showed that approximately 83% of lesions have been changed [11]. It was shown that in a randomized trial of pime- crolimus 1% cream (as case group) versus triamcinolone acetonide paste (control group) in OLP treatment, the mean clinical score at the base line was 2.4, that decreased Table 1. Clinical response. Treatment period Follow up period Clinical response 2 weeks % 1 month %2 months %3 months %4 months % 5 months % 6 months % +5 (5 degrees improvement) 0 0 2 5 7 8 10 +4 (4 degrees improvement) 0 1 3 5 8 12 11 +3 (3 degrees improvement) 4 4 8 11 13 14 14 +2 (2 degrees improvement) 30 41 41 41 35 34 34 +1 (1 degree improvement) 19 18 26 23 23 20 17 0 (no change) 47 36 20 14 13 11 12 –4 (4 degrees worsening) 0 0 0 0 0 1 1 –5 (5 degrees worsening) 0 0 0 1 1 0 1 Median (range) +1 (0,3)* +1 (0,4)* +2 (0,5)* +2 (–5,5)* +2 (–5,5)* +2 (–4,5)* +2 (–5,5)* P value 0.001 0.001 0.001 0.001 0.001 0.001 0.001 *Significant according to Wilcoxon test compared to before treatment (P < 0.05). Table 2. Symptomatic response. Treatment period Follow up period Symptomatic response 2 weeks % 1 month %2 months %3 months %4 months % 5 months % 6 months % +3 (3 degrees improvement) 0 7 10 10 10 10 10 +2 (1 degrees improvement) 7 14 7 7 7 7 7 +1 (1 degree improvement) 31 28 48 52 59 48 52 0 (no change) 59 48 35 31 24 35 31 –1 (1 degrees worsening) 3 3 0 0 0 0 0 Median (range) 0 (–1,2)* 0 (–1,3)* +1 (0, 3)* +1 (0, 3)* +1 (0, 3)* +1 (0, 3)* +1 (0, 3)* P value 0.005 0.002 0.001 0.001 0.001 0.001 0.001 *Significant according to Wilcoxon test compared to before treatment (P < 0.05).  F. Agha-Hosseini et al. / Open Journal of Stomatology 1 (2011) 13-17 Copyright © 2011 SciRes. OJST 16 Figure 1. The unstimulated whole saliva concentration of TNF-α (pg/ml) in baseline and end of the treatment with IM- OD in patients with OLP; P < 0.05 was considered statistically significant. to 1.6 after two months of treatment [4]. Mean clinical score before treatment in this study was 3.38, improving to 1.55 at the end of the three month treatment period, and 1.22 at the end of the follow-up period. It shows and emphasizes the highly durable therapeutic effect of IMOD after treatment cessation. It was suggested methylene blue-mediated photody- namic therapy may be as a possible alternative treatment for OLP [13]. Thirteen persons with 26 lesions took part in their study. At the end of treatment, despite 4 reticular lesions, no complete remission was found in other scor- ing groups. In this investigation 33% of erosive lesions had complete remission and 20% converted to reticular form. 57% of reticular lesions disappeared after the treatment period. This shows the higher effectiveness of IMOD rather than photodynamic therapy in management of OLP lesion. A downward shift of the numeric scale occurred in our patients at the end of the treatment period. The results were successfully preserved 3 months after treatment cessation in the follow up period. One study evaluated the efficacy of 0.1% dexamethasone oral rinse (3 times a day for 6 weeks) for treatment of erosive OLP. VAS (visual analog scale) value was decreased significantly at the end of the treatment period, but they did not assess the maintenance of treatment outcome in their study group [14]. However, it has been reported that one third of OLP patients treated with topical corticosteroids de- velop secondary candidiasis [15]. Until recent years, corticosteroids have been the mainstay of OLP treatment because of their an- ti-inflamma- tory and immunosuppressive effects. Ac- cording to some reports TNF-α may be a good index for monitoring treat- ment response of corticosteroids, but not in any other drug subtypes. The mechanism of cyto- kine regulation by immunomodulator drugs has not been identified. But they potently inhibit TNF-α production by peripheral blood mononuclear cells, and enhance se- cretion of IL-2 and INF-γ [16]. Despite significant im- provement in patient symptoms and sign, no statistical differences were found in TNF-α level before and after treatment in this study. This finding may be explained by the assumption that TNF-α may be involved in the im- munopathogenesis of OLP. Increasing differentiated T cell numbers after treatment by IMOD may be a possible explanation of the slight upward shift of TNF-α level. Despite the devastating effect of TNF-α, it has pri- mary beneficial effects by initiation of inflammatory response, promoting angiogenesis and reparative process as well [10]. Corticosteroid drugs suppress both aspects of this double-edged sword cytokine. But an immu- noregulator drug such as IMOD just regulates its action and promotes improvement in sign and symptom of OLP lesion, without deletion of the TNF-α beneficial effect that provides wound healing. It may be a possible ex- planation for the slight but statistically unimportant in- crease in TNF-α level after treatment with IMOD. 6. CONCLUSIONS IMOD May be an efficacious choice of OLP treatment, but further controlled studies required to elucidate its real efficacy. TNF-α may not be a worthwhile tool in the follow-up in this study. The relationship of TNF-α level and other type 1 and type 2 cytokines in immunopatho- genesis of OLP needs further study and may shed more light on its pathogenesis. REFERENCES [1] Ismail, S.B., Kumar, S.K. and Zain, R.B. (2007) Oral lichen planus and lichenoid reactions: Etiopathogenesis, diagnosis, management and malignant transformation. Journal of Oral Sciences, 49, 89-106. doi:10.2334/josnusd.49.89 [2] Agha-Hosseini, F., Mirzaii-Dizgah, I., Mikaili, S. and Abdollahi, M. (2009) Increased salivary lipid pero- xidation in human subjects with oral lichen planus. International Journal of Dental Hygiene, 7, 246-250. doi:10.1111/j.1601-5037.2009.00365.x [3] Greenberg S. (2008) Burket’s oral medicine. 11th Edition, BC Decker Inc, Hamilton, 89-94. [4] Gorouhi, F., Solhpour, A., Beitollahi, J.M., Afshar, S., Davari, P., Hashemi, P., Nassiri Kashani, M. and Firooz, A. (2007) Randomized trial of pimecrolimus cream versus triamcinolone acetonide paste in the treatment of oral lichen planus. Journal of the American Academy of Dermatology, 57, 806-813. doi:10.1016/j.jaad.2007.06.022 [5] Khairandish, P., Mohraz, M., Farzamfar, B., Abdollahi, M., Shahhosseiny, M.H. and Madani, H. (2009) Preclinical and phase 1 clinical safety of Setarud (IMOD™), a novel immunomodulator. 17, 148-156. [6] Zhang, Y., Lin, M., Zhang, S., Wang, Z., Jiang, L., Shen,  F. Agha-Hosseini et al. / Open Journal of Stomatology 1 (2011) 13-17 Copyright © 2011 SciRes. OJST 17 J., Bai, J., Gao, F., Zhou M. and Chen, Q. (2008) NF-kappaB-dependent cytokines in saliva and serum from patients with oral lichen planus: A study in an ethnic. Chinese population Cytokine, 41, 144-149. doi:10.1016/j.cyto.2007.11.004 [7] Pezelj-Ribaric S., Prso, I.B., Abram, M., Glazar, I., Brumini, G. and Simunovic-Soskic, M. (2004) Salivary levels of tumor necrosis factor-alpha in oral lichen planus. Mediators of Inflammation, 13, 131-133. doi:10.1080/09629350410001688530 [8] Banno, T., Gazel, A. and Blumenberg, M. (2004) Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional pro- filing. The Journal of Biological Chemistry, 279, 32633- 32642. doi:10.1074/jbc.M400642200 [9] van der Meij, E. H. and van der Waal, I. (2003) Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. Journal of Oral Pathology & Medicine, 32, 507-512. doi:10.1034/j.1600-0714.2003.00125.x [10] Thongprasom, K., Luangjarmekorn, L., Sererat T. and Taweesap, W. (1992) Relative efficacy of fluocinolone acetonide compared with triamcinolone acetonide in treatment of oral lichen planus. Journal of Oral Pathology & Medicine, 21, 456-458. doi:10.1111/j.1600-0714.1992.tb00974.x [11] Agha-Hosseini, F., Borhan-Mojabi, K., Monsef-Esfahani, H.R., Mirzaii-Dizgah, I., Etemad-Moghadam S. and Karagah, A. (2010) Efficacy of Purslane in the treatment of oral lichen planus. Phytotherapy Research, 24, 240-244. [12] Mohraz, M., Khairandish, P., Kazerooni, P.A., Da- varpanah, M.A., Shahhosseiny, M.H., Mahdavian, B., Vaziry, S., Shahriary, S., Kamali, K., Khorram Khorshid, H.R., Heshmat, R., Farhadi M. and Gharibdoust, F. (2009) A clinical trial on the efficacy of IMOD in AIDS patients. 17, 277-284. [13] Aghahosseini, F., Arbabi-Kalati, F., Fashtami, L.A., Djavid, G.E., Fateh M. and Beitollahi, J.M., (2006) Methylene blue-mediated photodynamic therapy: A possible alternative treatment for oral lichen planus. Lasers in Surgery and Medicine, 38, 33-38. doi:10.1002/lsm.20278 [14] Rhodus, N.L., Cheng, B., Bowles, W., Myers, S., Miller L. and Ondrey, F. (2006) Proinflammatory cytokine levels in saliva before and after treatment of (erosive) oral lichen planus with dexamethasone. Oral Diseases, 12, 112-116. doi:10.1111/j.1601-0825.2005.01165.x [15] Lodi, G., Tarozzi, M., Sardella, A., Demarosi, F., Cane- gallo, L., Di Benedetto, D. and Carrassi, A. (2007) Miconazole as adjuvant therapy for oral lichen planus: a double-blind randomized controlled trial. British Journal of Dermatology, 156, 1336-1341. doi:10.1111/j.1365-2133.2007.07883.x [16] Payvandi, F., Wu, L., Naziruddin, S.D., Haley, M., Parton, A., Schafer, P.H., Chen, R.S., Muller, G.W., Hughes, C.C. and Stirling, D.I. (2005) Immunomo- dulatory drugs (IMiDs) increase the production of IL-2 from stimulated T cells by increasing PKC-theta activation and enhancing the DNA-binding activity of AP-1 but not NF-kappaB, OCT-1, or NF-AT. Journal of Interferon & Cytokine Research, 25, 604-616. doi:10.1089/jir.2005.25.604 |

