 Journal of Power and Energy Engineering, 2015, 3, 373-377 Published Online April 2015 in SciRes. http://www.scirp.org/journal/jpee http://dx.doi.org/10.4236/jpee.2015.34050 How to cite this paper: Rosen, M.A. (2015) The Prospects for Renewable Energy through Hydrogen Energy Systems. Journal of Power and Energy Engineering, 3, 373-377. http://dx.doi.org/10.4236/jpee.2015.34050 The Prospects for Renewable Energy through Hydrogen Energy Systems Marc A. Rosen Faculty of Engineering and Applied Science, University of Ontario Institute of Technology, Oshawa, Ontario, Canada Email: Marc.Rosen@uoit.ca Received November 2014 Abstract The prospects for renewable energy are enhanced through the use of hydrogen energy systems in which hydrogen is an energy carrier. As easily accessible fossil fuel supplies become scarcer and environmental concerns increase, hydrogen is likely to become an increasingly important chemi- cal energy carrier. As the world’s energy sources become less fossil fuel-based, hydrogen and electricity are expected to be the two dominant energy carriers for the provision of end-use ser- vices, in a hydrogen economy. Thus, hydrogen energy systems allow greater use of renewable energy resources. In this paper, the role of hydrogen as an energy carrier and hydrogen energy systems, and their economics, are described and reviewed. Keywords Renewable Energy, Hyd rogen, Hydrogen Economy 1. Introduction Many energy sources exist, e.g., fossil fuels, uranium, renewable energy resources. Fossil fuels are the world’s main sources and are also energy carriers. Most renewable energy sources (solar energy, wind, falling water, tides, waves, etc.) and uranium must first be converted to an energy carrier (commonly electricity) before use. As fossil fuels become harder to obtain and environmental impacts (climate change, stratospheric ozone deple- tion, acid precipitation, smog, etc.) increase, renewable energy sources will likely be increasingly sought. But renewable energy sources cannot act as energy carriers and presently are mainly used to produce the energy car- rier electricity. This limits the prospects for renewable energy since societies cannot operate effectively with only electricity. They also need chemical fuels for processes such as transportation [1]-[3]. Thus, eventually it will be necessary to produce chemical fuel, either directly from non-hydrocarbon energy sources or from the electricity they can generate [4]. Many researchers feel that hydrogen is the most logical choice as a future chemical fuel. A “hydrogen economy,” with hydrogen and electricity acting as complementa- ry energy carriers, has been envisioned for decades [1] [4] [5]. Although some feel that renewable energy re- sources can supply all energy requirements, others believe the potential is limited due to their challenges, e.g., intermittency, but hydrogen energy systems appear capable of improving the prospects for renewable energy. 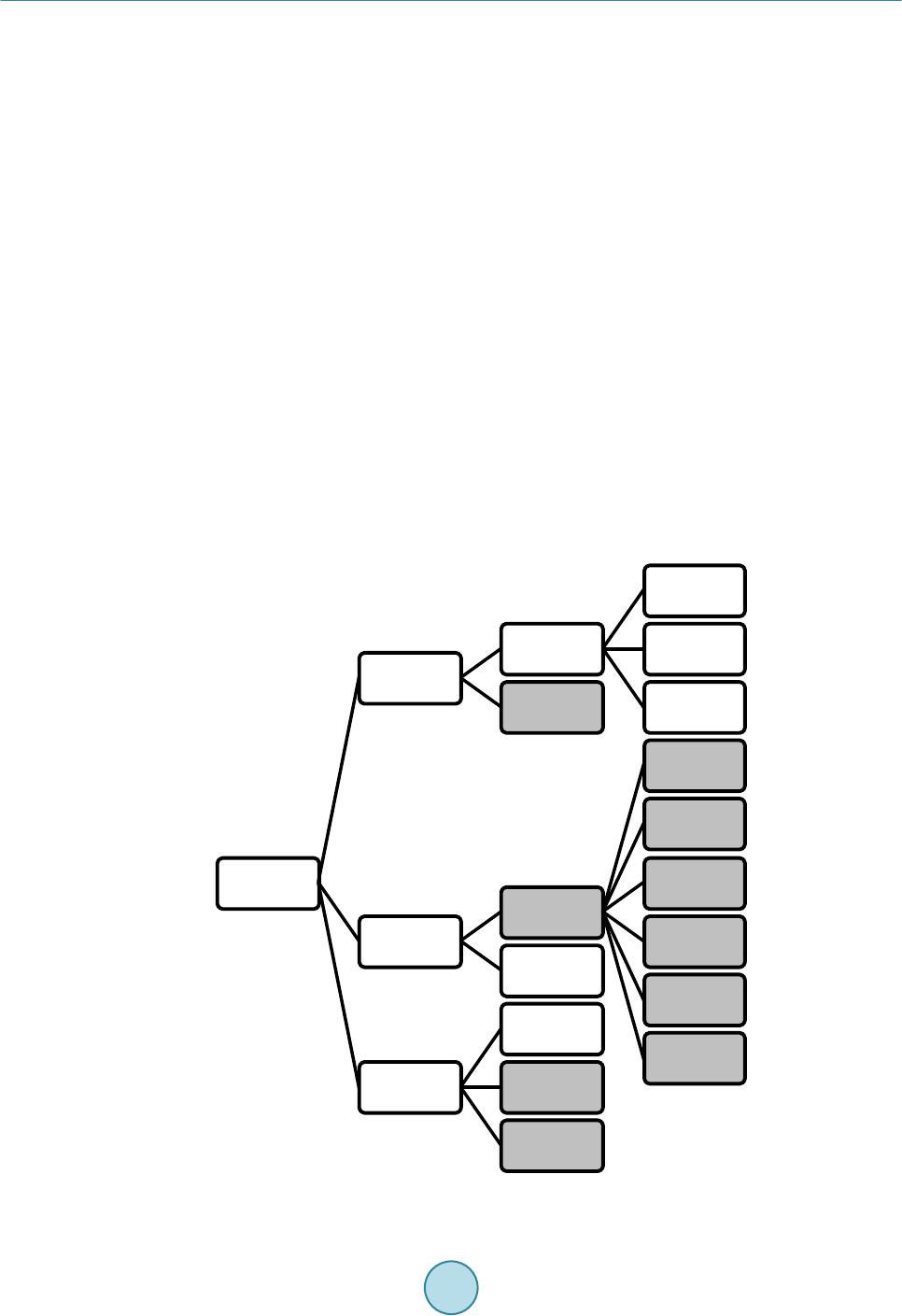 M. A. Rosen The objective of this paper is to describe the prospects for renewable energy provided by hydrogen energy systems, by reviewing hydrogen energy and hydrogen energy systems and their economics. The use of hydrogen as an energy carrier and hydrogen energy technologies, including those for hydrogen production, utilization, storage and distribution, are described. 2. Hydrogen as an Energy Carrier Hydrogen is a useful energy carrier for numerous reasons: • Hydrogen can be produced from fossil fuels, uranium and renewable energy sources. The hydrogen is ob- tained by splitting water in the last two cases. • Hydrogen can be used as a fuel (and feedstock) in industrial, transportation, residential and commercial ac- tivities, and for electricity generation in devices such as fuel cells. • Hydrogen can be stored in large quantities in a variety of forms, unlike electricity. • Hydrogen can be transported in many ways to (road, rail, ship, pipeline, etc.). • Hydrogen use is environmentally benign, the main output of its oxidation being water [6]. 3. Hydrogen Energy Systems and Technologies Hydrogen energy systems are mainly comprised of technologies for the production, utilization, storage and dis- tribution of hydrogen [4] [7]. 3.1. Production The main energy sources for hydrogen production are shown in Figure 1. The main processes for hydrogen production include fossil fuel-based processes (e.g., steam reforming of natural gas, catalytic decomposition of Figure 1. Main energy sources for hydrogen production, with renewables highlighted. Hydrogen Hydrocarbons Fossil fuels Coal Heavy oil Natural gas Biomass Electricity Renewable energy Solar PV Solar thermal Hydroele ctric W in d Geothermal Other renewables Nuclear Heat Nuclear Solar thermal Geothermal 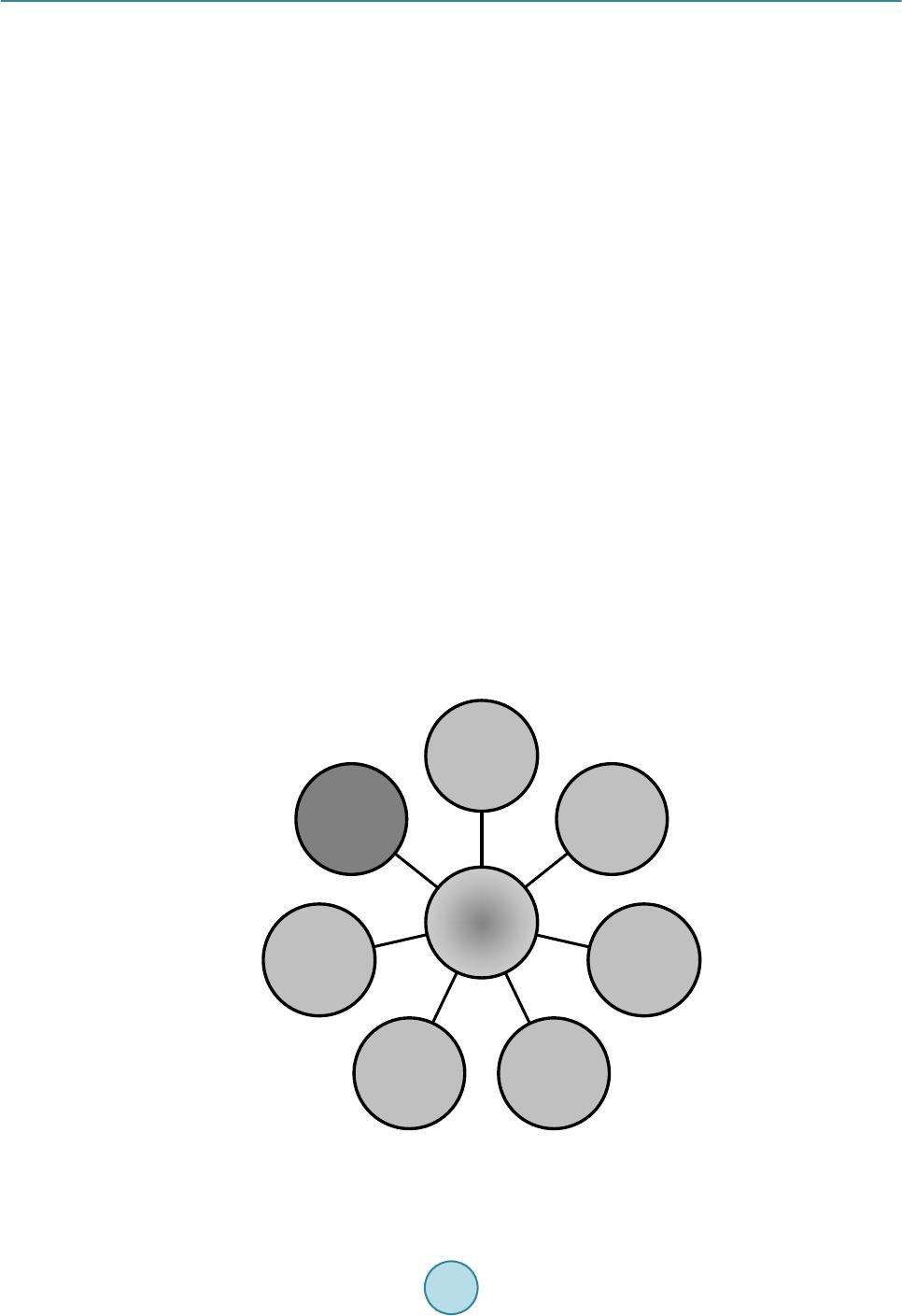 M. A. Rosen natural gas, partial oxidation of heavy oil, coal gasification), and water splitting technologies (e.g., water elec- trolysis, thermochemical water decomposition, photo-chemical, electrochemical and biological processes). The latter group can be driven by renewable energy. Most hydrogen is produced today by steam reforming of natural gas. For hydrogen production processes using hydrocarbons such as fossil fuels and biomass, the hydrogen is derived from the hydrogen in the hydrocarbon itself and water. The hydrogen is derived from the hydrogen in water for the hydrogen production processes driven by electrical and thermal energy, via the chemical reaction H2O → H2 + O2. Photochemical, photoelectrochemical and photobiological processes are at the early research stage, but water electrolysis and thermochemical water decomposition have greater potential: • Water electrolysis is a mature technology in which hydrogen and oxygen are produced by using electricity to split water. Water electrolysis is used at present on a small scale, often in specialized processes that require high purity hydrogen. Water electrolysis achieves a hydrogen concentration over 99.5% compared to 97% - 98% for fossil fuel-derived hydrogen. High-temperature electrolysis, which utilizes steam, is under devel- opment [4] [8]. The near-term option for hydrogen production from renewable energy is likely water elec- trolysis. • Thermochemical water decomposition (or splitting) consists of a sequence of chemical reactions for which the net reaction is water decomposition [9]-[12]. Many such cycles have been proposed, potentially driven by heat from nuclear power plants [13] or high temperature solar thermal facilities. Two examples are the copper-chlorine cycle, which requires heat at up to 550˚C, and the s ulp h ur-iodine cycle, which requires heat at about 900˚C. Hybrid thermochemical cycles that use electricity and heat to decompose water are also pos- sible. Thermochemical water decomposition is only at the research and development stage, but it is a sig- nificant future candidate for lar ge-scale hydrogen production. The main processes for hydrogen production are illustrated in Figure 2, where three categories presented: processes based on fossil fuels, processes not based on fossil fuels, and processes that integrate multiple hydro- gen production processes. 3.2. Storage One reason hydrogen complements electricity as an energy carrier is that it can be stored in bulk over long times, in many forms and using many technologies. For example, hydrogen can be stored as a cryogenic liquid (at 20.3 Figure 2. Main hydrogen production processes, including those based on fossil fuels (dark shading), non-fossil fuels (light shading) and in- tegrated processes (center gradient shading). Integrated processes Water electrolysis Th ermo- chemical water de compos- ition Pho to- chemical processes Pho to- electro- chemical processes Pho to- biological processes Biomass conversion Fossil fuel- based processes  M. A. Rosen K) in insulated dewers (tanks), using metal hydride technologies, which allow large quantities of gaseous hy- drogen to be thermally adsorbed and desorbed from the surface of certain metals, and as a compressed gas in cylinders or in underground reservoirs and caverns. 3.3. Distribution Several technologies for the distribution of hydrogen exist. Hydrogen can be transported in bulk by pipeline as a gas, or possibly, for short distances, as a liquid; truck or rail as a highly compressed gas in cylinders; and truck or rail or ship as a liquid. 3.4. Utilization Hydrogen can be used as an energy carrier in various ways: • a fue l for producing electricity in fuel cells [14], which combine hydrogen and oxygen electrochemically to produce electricity and water; • a supp le me nt to natural gas, which can be added directly in natural gas distribution networks; • a feedstock in the manufacturing of synthetic fuels, especially methane and ammonia; • a fuel for urban motor vehicles, locomotives, marine vessels and aircraft, using fuel cells and/or combustion engine technology [1] [15]; and • a fuel for space heating, in appropriate circumstances. At present, hydrogen is used extensively as a chemical feedstock in industry, mainly in refinery processes, and petrochemical and chemical production. But in a hydrogen economy, hydrogen will be used for energy services as the prime chemical energy carrier, with the sector most likely to rely on hydrogen being transportation [1] [3]. 4. Hydrogen Energy Systems Economics Economic assessments of hydrogen energy systems typically depend on many factors and assumptions. Such assessments must account for costs of producing, storing, distributing and utilizing hydrogen, all of which are significant in a hydrogen economy. Details on these follow: • Production: Costs for producing hydrogen via conventional and non-conventional methods depend on fac- tors such as feedstock, labour and capital costs, and are often a significant part of the costs of hydrogen en- ergy systems. • Transport and storage: Hydrogen transport and storage costs vary by method. Usually, hydrogen transport by pip eline as a compressed gas is often least expensive for large volumes and distances, while small quanti- ties of gaseous hydrogen are transported most economically over short distances in tube tanks by truck. The transport cost is much lower for liquid hydrogen than compressed gaseous hydrogen, but liquefaction in- creases the hydrogen cost by about 60%. Costs are normally significantly less for hydrogen transport and storage than for production. • Utilization: Several hydrogen uses are expected to be economic in the future, with a main focus likely to be in transportatio n. The earliest large-scale use of hydrogen from water electrolysis is expected to be in up- grading of refinery products, where hydrogen produced by steam-methane reforming can be substituted. Hy- drogen may also be used in biomass-to-methanol conversion. 5. Conclusions As accessible fossil fuel supplies become scarcer and environmental concerns increase, hydrogen is likely to become an important chemical energy carrier. Hydrogen energy systems can thereby enhance the prospects for renewable energy. There are many commercial processes for producing hydrogen from fossil fuel and non-fossil fuel sources (including renewables). Technologies for the storage and distribution of hydrogen exist. Technolo- gies are developing for utilizing hydrogen as an energy carrier, especially in transportation. The technologies needed for hydrogen energy systems are undergoing much research and development. Acknowledgements The Natural Sciences and Engineering Research Council of Canada provided support.  M. A. Rosen References [1] Scott, D.S. (20 07 ) Smelling Land: The Hydrogen Defense against Climate Catastrophe. Canadian Hydrogen Associa- tion, Ottawa, Canada. [2] Balat, M. (2008) Potential Importance of Hydrogen as a Future Solution to Environmental and Transportation Prob- lems. International Journal of Hydrogen Energy, 33, 4013-4029. http://dx.doi.org/10.1016/j.ijhydene.2008.05.047 [3] Turgut, E.T. and Rosen, M.A. (2010) Partial Substitution of Hydrogen for Conventional Fuel in an Aircraft by Utiliz- ing Unused Cargo Compartment Space. International Journal of Hydrogen Energy, 35, 1463-14 73 . http://dx.doi.org/10.1016/j.ijhydene.2009.11.047 [4] Muradov, N.Z. and Veziroglu, T.N. (2008) “Green” Path from Fossil-Based to Hydrogen Economy: An Overview of Carbon-Neutral Technologies. International Journal of Hydrogen Energy, 33, 6804-6839. http://dx.doi.org/10.1016/j.ijhydene.2008.08.054 [5] Gnanapragasam, N.V., Reddy, B.V. and Rosen, M.A. (2010) Feasibility of an Energy Conversion System in Canada Involving Large-Scale Integrated Hydrogen Production Using Solid Fuels. International Journal of Hydrogen Energy, 35, 4788-480 7. http://dx.doi.org/10.1016/j.ijhydene.2009.10.047 [6] Lubis, L.I., Dincer, I., Naterer, G.F. and Rosen, M.A. (2009) Utilizing Hydrogen Energy to Reduce Greenhouse Gas Emissions in Canada’s Residential Sector. International Journal of Hydrogen Energy, 34, 1631 -16 37. http://dx.doi.org/10.1016/j.ijhydene.2008.12.043 [7] Holladay, J.D., Hu, J., King, D.L. and Wang, Y. (2009) An Overview of Hydrogen Production Technologies. Catalysis Today, 139, 244-260. ht tp:/ /dx. doi. or g/10 .10 16/ j. catto d.2 008. 08 .039 [8] Turner, J., Sverdrup, G., Mann, M.K., Maness, P.-C., Kroposki, B., Ghirardi, M., Evans, R.J. and Blake, D. (2008) Renewable Hydrogen Production. International Journal of Energy Research, 32, 379-407. http://dx.doi.org/10.1002/er.1372 [9] Rosen, M.A. (2010) Advances in Hydrogen Production by Thermochemical Water Decomposition: A Revie w. En- ergy—The International Journal, 35, 1068-1076. http://dx.doi.org/10.1016/j.energy.2009.06.018 [10] Lewis, M.A., Masin, J.G. and O’Hare, P.A. (2009) Evaluation of Alternative Thermochemical Cycles—Part I: The Methodology. International Journal of Hydrogen Energy, 34, 4115-4124 . http://dx.doi.org/10.1016/j.ijhydene.2008.06.045 [11] Lewis, M.A. and Masin, J.G. (2009) Evaluation of Alternative Thermochemical Cycles—Part II: The Do wn -Selection Process. International Journal of Hydrogen Energy, 34, 4125-41 35. http://dx.doi.org/10.1016/j.ijhydene.2008.07.085 [12] Andress, R.J., Huang, X., Bequette, B.W. and Martin, L.L. (2009) A Systematic Methodology for the Evaluation of Alternative Thermochemical Cycles for Hydrogen Production. International Journal of Hydrogen Energy, 34, 4146- 4154. http://dx.doi.org/10.1016/j.ijhydene.2008.11.118 [13] Naterer, G.F., Suppiah, S., Stolberg, L., Lewis, M., Ahmed, S., Wang, Z., Rosen, M.A., Dincer, I., Gabriel, K., Secnik, E., Easton, E.B., Lvov, S.N., Papangelakis, V. and Odukoya, A. (2014) Progress of International Program on Hydrogen Production with the Copper-Chlorine Cycle. International Journal of Hydrogen Energy, 39, 2431-244 5 . http://dx.doi.org/10.1016/j.ijhydene.2013.11.073 [14] Thomas, C.E. (2009) Fuel Cell and Battery Electric Vehicles Compared. International Journal of Hydrogen Energy, 34, 6005-6020. http://dx.doi.org/10.1016/j.ijhydene.2009.06.003 [15] Lapeña-Rey, N., Mosquera, J., Bataller, E. and Ortí, F. (2010) First Fu el-Cell Manned Aircraft. Journal of Aircraft, 47, 1825-1835. http://dx.doi.org/10.2514/1.42234
|