 American Journal of Plant Sciences, 2011, 2, 245-254 doi:10.4236/ajps.2011.22026 Published Online June 2011 (http://www.SciRP.org/journal/ajps) Copyright © 2011 SciRes. AJPS Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against Sudden-Death Syndrome of Soybean Brenda Bertinetti1, Mercedes Scandiani2, Gabriela Cabrera1 1Organic Chemistry Dept and UMYMFOR (CONICET), Buenos Aires University, Ciudad Universitaria, Buenos Aires, Argentina; 2Agricultural Laboratory Río Paraná, San Pedro, Buenos Aires, Argentina. Email: gabyc@qo.fcen.uba.ar Received March 1st, 2011; revised April 28th, 2011; accepted May 8th, 2011. ABSTRACT Based on the precedent discovery of a weak antifungal indole isolated from Aporpium caryae, which increased its ac- tivity when changing the N-alkyl chain, nineteen N-alkyl indoles, with alkyl chains from one to ten carbons and one or two hydroxyls, one amine or bromine functional groups, were prepared and fully characterized by spectroscopic meth- ods. The aim of this study is the search for new synthetic agrochemical leads derived from natural products. The anti- fungal activity of the synthesized compounds against three fungal strains was measured in vitro. Six compounds pre- sented good activity against Fusarium virguliforme, the causal agent of sudden-death syndrome (SDS) in soybean, in a bioautography assay. Four of them were tested in a germination test and in a greenhouse experiment. All tested com- pounds, applied as seed treatment, showed antifungal properties being effective to control SDS when there was low level of fungal contamination. Results indicate that some of the tested compounds are acting as growth inhibitors and represent new leads for the treatment of SDS for which no specific treatment has been previously reported. Keywords: N-Alkyl Indole, Soybean Phytopathogen, Fusarium Virguliforme, Sudden-Death Syndrome 1. Introduction Fungal infections are one of the main limiting factors in the production of soybean and other crops, affecting yield, and the quality of seeds and byproducts. Approxi- mately 50 fungal diseases are known, although only some of them are harmful, and only under specific condi- tions. Among the important fungal pathogens of soy- beans are Sclerotinia sclerotiorum (Sclerotinia stem rot), Fusarium virguliforme and F. tucumaniae [1] (sudden- death syndrome, SDS), Cercospora kikuchii (purple seed stain), Colletotrichum truncatum (anthracnose), Macro- phomina phaseolina (charcoal rot) and Rhizoctonia so- lani (damping-off). Most of the commercial, extended-use fungicides used to control these diseases are simple synthetic compounds with disadvantages, such as their possible contamination of the environment and the introduction of risks to worker health [2-4]. The development of sustainable alternatives to syn- thetic fungicides has attracted growing interest as the use of natural products. Some examples of natural microbial agrochemicals are griseofulvin, isolated from Penicillium griseofulvum, cycloheximide, isolated from Streptomyces griseus, strobilurin A, isolated from Strobilurus tenacel- lus and oudemansin A, isolated from Oudemansiella mucida [5]. The use of microorganisms to produce agrochemicals has been limited [6], mainly because agrochemical use requires large amounts of product, and because of the comparative costs of producing simple synthetic com- pounds versus cultured natural products. Even though the low-cost production of natural agrochemicals by fermen- tation cannot be achieved in many cases, the use of these compounds as templates for the production of synthetic agrochemicals derived from natural products is a viable alternative, as in the case of strobilurins. Strobilurin A and oudemansin A were the lead compounds in the de- velopment of azoxystrobin from Zeneca and kresoxim- methyl from Basf, which are employed as fungicides for several crops [7]. In this context, the goal of this study was the discovery of new leads based on a weakly antifungal indole, iso-  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against 246 Sudden-Death Syndrome of Soybean lated from Aporpium caryae and designated compound 1 [8]. In this work, we describe the performance in antifun- gal activity tests of new synthetic analogs of 1. 2. Materials and Methods 2.1. General Procedures Optical rotation was recorded on a Perkin Elmer po- larimeter 343. FTIR spectra were recorded on a Nicolet Magna-IR 550. NMR spectra were recorded on a Bruker Avance II instrument at 500.13 MHz for 1H (referenced to TMS, δ = 0) and at 125.13 MHz for 13C NMR (refer- enced to the center line of CDCl3, δ 77.0). High per- formance liquid chromatography used a variable-wave- length UV detector coupled with a refractive index de- tector (RefractoMonitor IV, Thermo Separation Prod- ucts). Accurate ESI MS was carried out on a Bruker Mi- crOTOF-Q II, whereas EIMS employed a mass spec- trometer Trio-2 VG Masslab (Manchester, UK). All chemicals were purchased from standard commercial suppliers. Methyl 3-indole-carboxylate was purchased from Acros Organics and all the alkyl halides (including (R)-(-)-3-chloro-1,2-propanediol and (S)-(+)-3-chloro- 1,2-propanediol) were purchased from Sigma-Aldrich. 2.2. Bioassays 2.2.1. Bioautography on Silicagel Direct bioautography on TLC was employed as a method for detecting fungitoxic substances [9,10]. Fusarium virguliforme O’Donnell & T. Aoki NRRL 34551, Fusa- rium lateritium Nees ex Link (BAFC 759), Macro- phomina phaseolina (Tassi) Goid (BAFC 3428) and Bo- trytis cinerea Pers.: Fr. (BAFC 535) were employed as fungal targets. A concentration level of 50 μg/spot of each assayed compound was used. Benomyl and Maxim® XL (50 μg of total fludioxonil plus metalaxyl active ingredients), were used as test compounds. Benomyl was tested at a conc. level of 25 μg/spot. When big inhibitory halos were observed, minimum inhibitory concentrations (MIC) were measured by the same method [9]. The experiments were repeated 3 times or 5 times for the most active com- pounds. 2.2.2. See d T reatment Soybean untreated seeds (50 g) were treated with an aqueous suspension (Tween 80, 2 drops; water, 0.5 ml) of the test compound (100 mg) with agitation for 60 sec- onds. These treated (C) seeds were employed in the bio- assays. For a positive control, the abovementioned treatment was repeated employing Maxim XL (fludioxonyl + metalaxyl, 0.1 ml) instead of test compound. These treated (M) seeds were employed in the following bioas- says. 2.2.3. Germination Test in the Laboratory. Blotter Paper Technique 400 treated (C), treated (M) and untreated seeds (U) were used to estimate seed fungal incidence of pathogens [11] and compound efficacy. The seeds were plated on trays (16 × 20 × 5 cm, 50 seeds per tray) and incubated at 25˚C ± 1˚C under alter- nating periods of 12 h fluorescent cool daylight (Osram 18 W/765) for 7 days [12]. The seeds were then exam- ined under stereomicroscope at 40x magnification and the identification of the fungi was based on the presence of conidiophores and conidia of the pathogen at 20 – 40x [13]. The experiment was repeated three times. Data from this experiment were subjected to analysis of variance (ANOVA). Treatment means were compared by least significant differences at P = 0.05. 2.2.4. Greenhouse Experiment Ninety eight disinfected untreated seeds and ninety eight treated (C) seeds were added to pots containing field soil and covered with another 2 cm of soil. Half of them were inoculated with Fusarium virguliforme NRRL 34551 [1]. Pots were then placed on a greenhouse bench and grown under natural photoperiod at 25˚C ± 2˚C for 4 - 5 weeks. Soil was watered to saturation after planting and main- tained at near field capacity throughout the study. Plants were rated for incidence of SDS-like symptoms on the foliage, plant height and shoot fresh weight. Dis- ease incidence (DI) of plants was based on the percent- age of plants with foliar symptoms typical of SDS [1]. Symptoms ranged from leaf curling and rugosity, mar- ginal cupping, mottling, chlorotic interveinal spots, in- terveinal chlorosis and necrosis, to leaf drop and stunt- ing. Foliar disease severity (DS) was rated during 5 weeks after planting based on a scale of 1 to 5, where 1 = no symptoms; 2 = light symptom development with mottling and mosaic (1% - 20% foliage affected); 3 = moderate symptom development with interveinal chlorosis and necrosis (21% - 50% foliage affected); 4 = heavy symp- tom development (51% - 80% foliage affected); and 5 = severe symptom development with interveinal chlorosis and necrosis and/or dead plants (81% - 100% foliage affected) [14]. At the end of the experiment, all plants were rated for height and the fresh shoot weight was determined. Data from this experiment were subjected to analysis of vari- ance (ANOVA). Treatment means were compared by Copyright © 2011 SciRes. AJPS 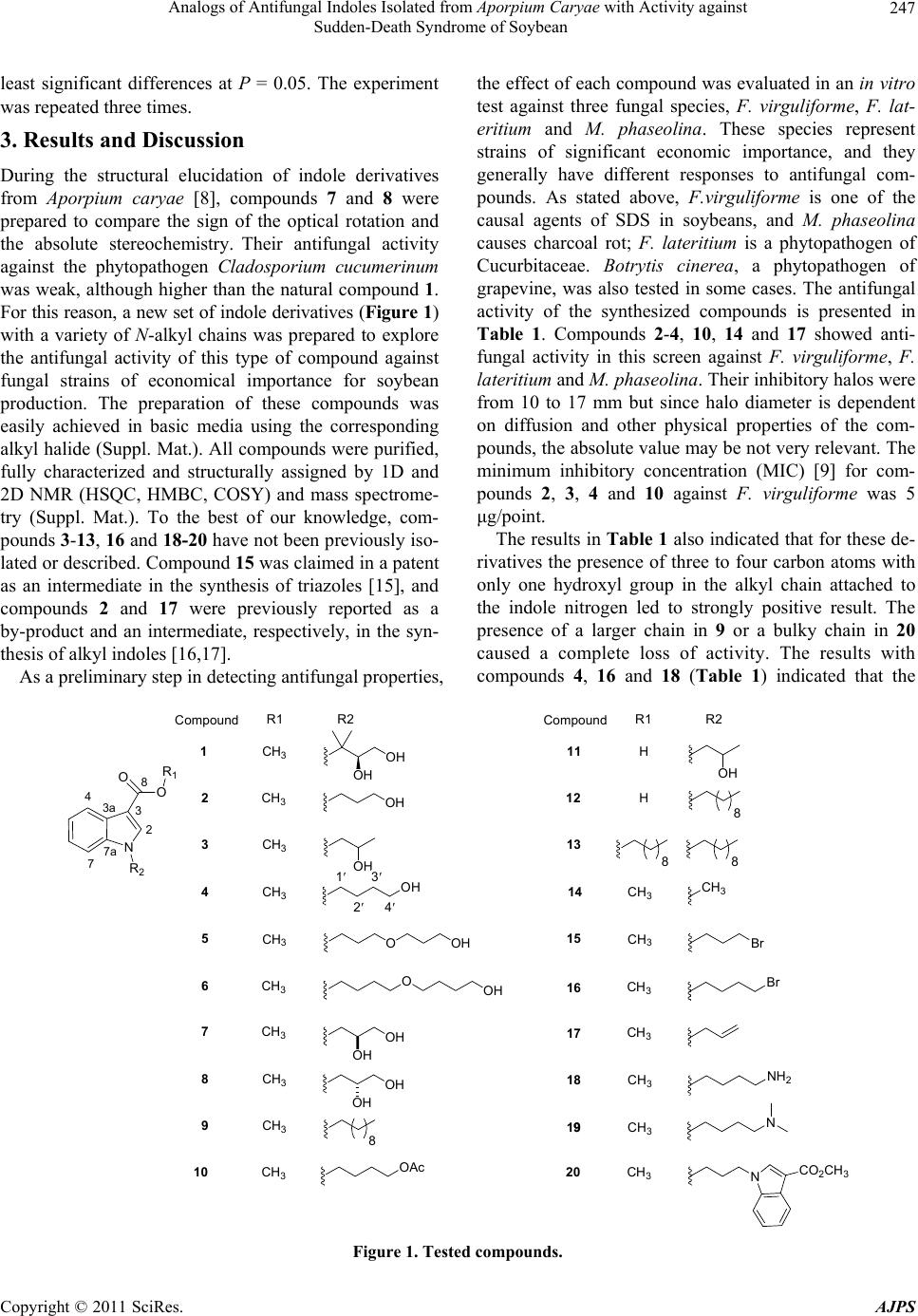 Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against Sudden-Death Syndrome of Soybean Copyright © 2011 SciRes. AJPS 247 least significant differences at P = 0.05. The experiment was repeated three times. 3. Results and Discussion During the structural elucidation of indole derivatives from Aporpium caryae [8], compounds 7 and 8 were prepared to compare the sign of the optical rotation and the absolute stereochemistry. Their antifungal activity against the phytopathogen Cladosporium cucumerinum was weak, although higher than the natural compound 1. For this reason, a new set of indole derivatives (Figure 1) with a variety of N-alkyl chains was prepared to explore the antifungal activity of this type of compound against fungal strains of economical importance for soybean production. The preparation of these compounds was easily achieved in basic media using the corresponding alkyl halide (Suppl. Mat.). All compounds were purified, fully characterized and structurally assigned by 1D and 2D NMR (HSQC, HMBC, COSY) and mass spectrome- try (Suppl. Mat.). To the best of our knowledge, com- pounds 3-13, 16 and 18-20 have not been previously iso- lated or described. Compound 15 was claimed in a patent as an intermediate in the synthesis of triazoles [15], and compounds 2 and 17 were previously reported as a by-product and an intermediate, respectively, in the syn- thesis of alkyl indoles [16,17]. As a preliminary step in detecting antifungal properties, the effect of each compound was evaluated in an in vitro test against three fungal species, F. virguliforme, F. lat- eritium and M. phaseolina. These species represent strains of significant economic importance, and they generally have different responses to antifungal com- pounds. As stated above, F.virguliforme is one of the causal agents of SDS in soybeans, and M. phaseolina causes charcoal rot; F. lateritium is a phytopathogen of Cucurbitaceae. Botrytis cinerea, a phytopathogen of grapevine, was also tested in some cases. The antifungal activity of the synthesized compounds is presented in Table 1. Compounds 2-4, 10, 14 and 17 showed anti- fungal activity in this screen against F. virguliforme, F. lateritium and M. phaseolina. Their inhibitory halos were from 10 to 17 mm but since halo diameter is dependent on diffusion and other physical properties of the com- pounds, the absolute value may be not very relevant. The minimum inhibitory concentration (MIC) [9] for com- pounds 2, 3, 4 and 10 against F. virguliforme was 5 μg/point. The results in Table 1 also indicated that for these de- rivatives the presence of three to four carbon atoms with only one hydroxyl group in the alkyl chain attached to the indole nitrogen led to strongly positive result. The presence of a larger chain in 9 or a bulky chain in 20 caused a complete loss of activity. The results with compounds 4, 16 and 18 (Table 1) indicated that the N OO R1 R2 CH3 Compound OH OH CH3OH CH3 CH3OH OH CH3OOH CH3OOH CH3OH OH CH3OH OH CH38 CH3OAc 1 2 3 4 5 6 7 8 9 10 R1 R2 CH3CH3 CH3Br CH3Br CH3 CH3 CH3 14 15 16 17 18 19 NH2 N CH3 9 20 NCO2CH3 8 8 13 Compound R1 R2 H OH H8 11 12 2 3 43a 77a 8 1´ 2´ 3´ 4´ 1′ 3′ 2′4′ Figure 1. Tested compounds. 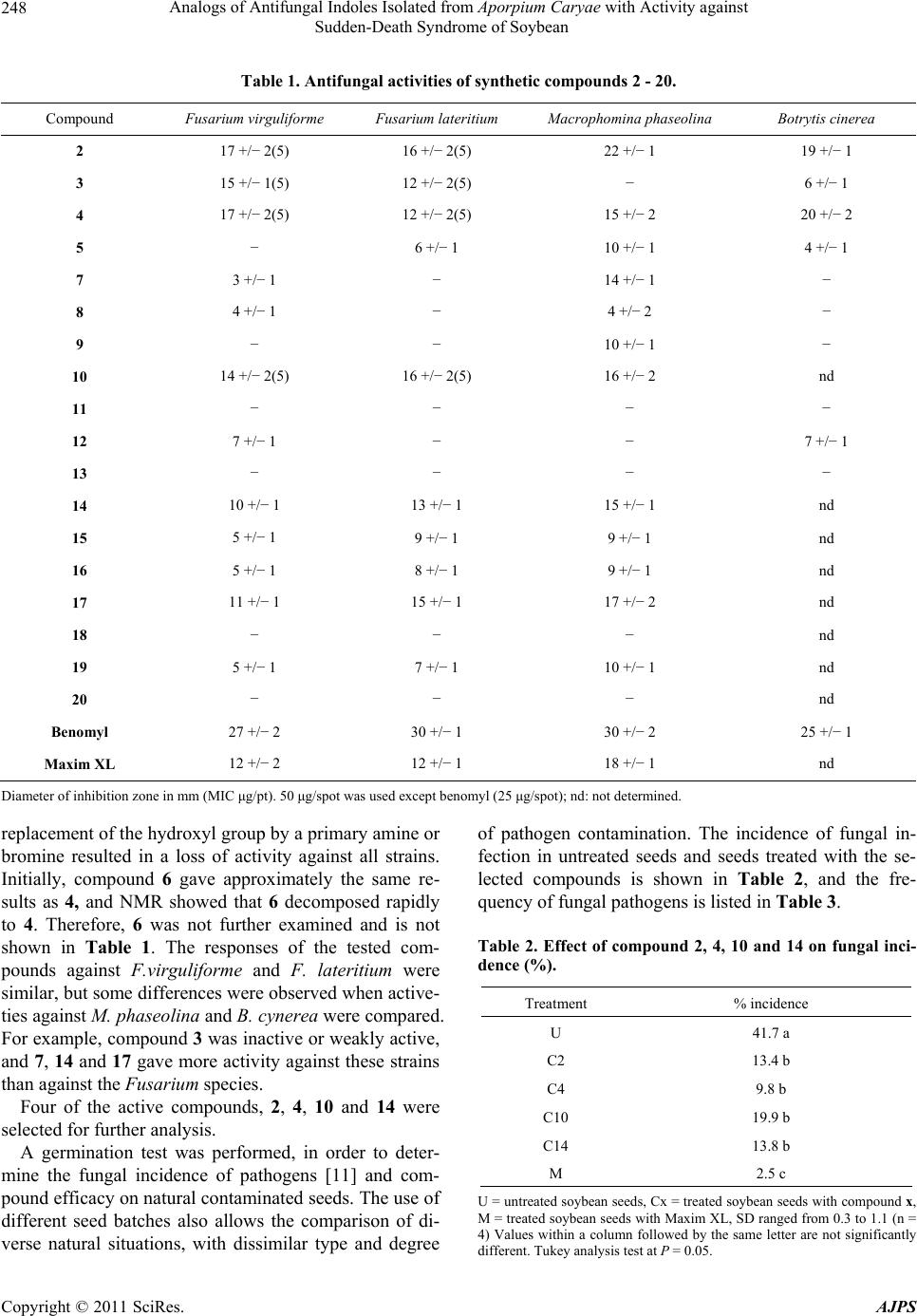 Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against 248 Sudden-Death Syndrome of Soybean Table 1. Antifungal activities of synthetic compounds 2 - 20. Compound Fusarium virguliforme Fusarium lateritium Macrophomina phaseolina Botrytis cinerea 2 17 +/− 2(5) 16 +/− 2(5) 22 +/− 1 19 +/− 1 3 15 +/− 1(5) 12 +/− 2(5) − 6 +/− 1 4 17 +/− 2(5) 12 +/− 2(5) 15 +/− 2 20 +/− 2 5 − 6 +/− 1 10 +/− 1 4 +/− 1 7 3 +/− 1 − 14 +/− 1 − 8 4 +/− 1 − 4 +/− 2 − 9 − − 10 +/− 1 − 10 14 +/− 2(5) 16 +/− 2(5) 16 +/− 2 nd 11 − − − − 12 7 +/− 1 − − 7 +/− 1 13 − − − − 14 10 +/− 1 13 +/− 1 15 +/− 1 nd 15 5 +/− 1 9 +/− 1 9 +/− 1 nd 16 5 +/− 1 8 +/− 1 9 +/− 1 nd 17 11 +/− 1 15 +/− 1 17 +/− 2 nd 18 − − − nd 19 5 +/− 1 7 +/− 1 10 +/− 1 nd 20 − − − nd Benomyl 27 +/− 2 30 +/− 1 30 +/− 2 25 +/− 1 Maxim XL 12 +/− 2 12 +/− 1 18 +/− 1 nd Diameter of inhibition zone in mm (MIC μg/pt). 50 μg/spot was used except benomyl (25 μg/spot); nd: not determined. replacement of the hydroxyl group by a primary amine or bromine resulted in a loss of activity against all strains. Initially, compound 6 gave approximately the same re- sults as 4, and NMR showed that 6 decomposed rapidly to 4. Therefore, 6 was not further examined and is not shown in Table 1. The responses of the tested com- pounds against F.virguliforme and F. lateritium were similar, but some differences were observed when active- ties against M. phaseolina and B. cynerea were compared. For example, compound 3 was inactive or weakly active, and 7, 14 and 17 gave more activity against these strains than against the Fusarium species. Four of the active compounds, 2, 4, 10 and 14 were selected for further analysis. A germination test was performed, in order to deter- mine the fungal incidence of pathogens [11] and com- pound efficacy on natural contaminated seeds. The use of different seed batches also allows the comparison of di- verse natural situations, with dissimilar type and degree of pathogen contamination. The incidence of fungal in- fection in untreated seeds and seeds treated with the se- lected compounds is shown in Table 2, and the fre- quency of fungal pathogens is listed in Table 3 . Table 2. Effect of compound 2, 4, 10 and 14 on fungal inci- dence (%). Treatment % incidence U 41.7 a C2 13.4 b C4 9.8 b C10 19.9 b C14 13.8 b M 2.5 c U = untreated soybean seeds, Cx = treated soybean seeds with compound x, M = treated soybean seeds with Maxim XL, SD ranged from 0.3 to 1.1 (n = 4) Values within a column followed by the same letter are not significantly ifferent. Tukey analysis test at P = 0.05. d Copyright © 2011 SciRes. AJPS  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against 249 Sudden-Death Syndrome of Soybean Table 3. Effect of compounds on frequency (%) of pathogens. Treatment Fusarium spp.i Phomopsis spp.i Fusarium spp.ii Phomopsis spp.ii Cercospora kikuchiiiii U 26.5 a 7.8 a 81.9 a 28.8 a 6.7 a C2 4.9 c 3.5 ab 64.9 b 9.3 d 3.5 a C4 2.7 cd 3.5 ab 71.0 ab 10.9 bcd 6.9 a C10 10.6 b 4.3 a 61.1 b 20.7 a 5.2 a C14 6.9 bc 5.3 a 62.0 b 19.4 ab 6.3 a M 0.0 d 0.9 b 33.9 c 10.0 cd 0.4 b i-iii experiments using different seed batches, U = untreated soybean seeds, Cx = treated soybean seeds with compound x, M = treated soybean seeds with Maxim XL, SD ranged from 0.1 to 1.2 (n = 4), Values within a column followed by the same letter are not significantly different. Tukey analysis test at P = 0.05. Significant differences were observed between the un- treated seeds (treatment U, Table 2), which showed a high percent of fungal incidence (41.7% +/− 0.6%), seeds treated with any of the tested compounds 2, 4, 10 or 14, which showed from 9.8 % to 19.9 % (treatment Cx), and seeds treated with Maxim XL, which showed the small- est percentage of fungal incidence (2.5% +/− 0.4%, treatment M). There were no significant differences be- tween the tested compounds. When the frequency of fungal pathogens was analyzed, some differences were observed, depending on the pathogen. Compounds 2, 4, 10 or 14 showed to be effective in the inhibition of Fusa- rium spp. with different significance between them, be- ing the order 4, 2, 14, 10 from higher to smaller response (Table 3). The tendency was less pronounced against Phomopsis spp. and a lack of activity was observed in the case of the pathogen Cercospora kikuchii. When the seed batch was naturally very contaminated (Table 3, entries ii), the same tendency remains although the percentages of frequency were high. Furthermore, compound 2 was as active as Maxim in this situation. Other fungal pathogens were also present although their frequency was too small to be statistically consid- ered. All these results would indicate that compounds 2, 4, 10 and 14 are acting as fungal growth inhibitors. This outcome is in accordance with other studies which have reported that indoles like indole-3-acetic acid possess fungistatic activity [18]; at the same time, indole also has been shown to act synergistically with other known anti- fungal compounds to enhance their fungistatic properties [19]. A greenhouse experiment was performed with these compounds to see their effect on the incidence of SDS on soybean plants inoculated with Fusarium virguliforme (Suppl. Mat., Table 1). All the tested compounds pre- vented plant infection comparing with plants derived from untreated seeds (95% +/− 2% infected). It is note- worthy that the infected plants had light symptoms of foliar disease severity, with a development of chlorotic mottling as the main infection. In another experiments, which resulted in a more se- vere global infection, carried out only with 2, it was shown that this compound did not affect plant growth, and both height and fresh shoot weight of treated plants were not significantly different from untreated plants (Table 4). A significant difference in height and fresh shoot weights were seen between seeds inoculated with F. virguliforme and left untreated (U + Fv), compared to those treated with compound 2 (C + Fv). The percentage of incidence of SDS for U + Fv was very high (85% ± 2%) from the first 15 dpi (days post-inoculation) to the last measurement at 35 dpi (90% ± 2%), while in the case of C + Fv a 48% ± 4% incidence at 15 dpi and 67% ± 4% incidence at 35 dpi (Table 5) was observed. This differ- ence is especially important when the severity results are included (Figure 2), because the severity factor at 35 dpi was near 5 for U + Fv, and less than 3 for C + Fv. These results are in full agreement with previous tests and sup- port the conclusion that compound 2 has a clear effect on the incidence of F. virguliforme on soybean plants. It is noteworthy that a good correlation between the in vitro antifungal test using bioautography and the other tests was observed in this work, probably because all the compounds belong to the same structural family and have similar physical properties. As there are few data in the literature related to the control of SDS, the results achieved in this work may not be deeply compared with other works. In general, there were no studies showing remarkable performance for preventing SDS in soybean. For example the oligosac- charide chitosan (poly-β-(1,4)-D-glucosamine) capability to prevent SDS on soybean caused by Fusarium solani was evaluated in greenhouse experiments [20]. Chitosan Copyright © 2011 SciRes. AJPS  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against 250 Sudden-Death Syndrome of Soybean Table 4. Effect of compound 2 on height and fresh shoot weight of soybean plants inoculated with Fusarium virguli- forme under greenhouse co nditions. Height and fresh shoot weight Treatment Height (cm) fresh shoot weight (g) U 94 +/− 2 a 11 +/− 1 a C2 90 +/− 2 a 12 +/−2 a U + Fv 22 +/− 2 c 1.4 +/− 0.2 c C2 + Fv 31 +/− 5 b 4 +/− 1 b U = untreated soybean seeds, C2 = treated soybean seeds with compound 2, Fv = inoculation with Fusarium virguliforme, Values within a column fol- lowed by the same letter are not significantly different. Tukey analysis test at P = 0.05. Table 5. Effect of compound 2 on incidence of Sudden-de ath syndrome (SDS) on soybean plants inoculated with Fusa- rium virguliforme under greenhouse conditions. Incidence (%) Treatment 15 dpi 18 dpi 24 dpi 32 dpi 35 dpi U + Fv 85 ± 2 85 ± 2 90 ± 2 90 ± 2 90 ± 2 C + Fv 48 ± 4 63 ± 5 63 ± 5 67 ± 4 67 ± 4 15 20 25 30 35 2 3 4 5 % (FV) % (FV-C) Disease severity Days after inoculation Figure 2. Effect of compound 2 on foliar severity of Sud- den-death syndrome (SDS) on soybean plants inoculated with Fusarium virguliforme under greenhouse conditions. showed certain ability to retard the SDS symptom ex- pression in soybean leaves but it could not absolutely protect the soybean from disease incidence. Strobilurins, which are known fungicides used in crop protection, were examined in field experiments in combination with potassium chloride [21]. These studies revealed that stro- bilurin fungicide treatments showed variable effects on the severity of SDS disease and no significant yield re- sponse to foliar application was displayed. It is worth mentioning that the present work is the first one where the activity of compounds against Fusarium virguliforme is investigated and no similar studies about SDS in soy- bean caused by this phytopathogen were found. 4. Conclusions In summary, nineteen analogs of the natural compound 1 were prepared, characterized and screened for antifungal activity. Six analogs, designated 2-4, 10, 14 and 17, were determined to have antifungal activity against F. virguli- forme in vitro and 2, 4, 10 and 14 also when applied as seed treatments. Their antifungal activity is dependent on the degree of contamination, being more effective in the first period of infection or when low pathogen content is present, and is more selective against Fusarium spp. These substances represent new leads for the treatment of SDS, for which no specific treatment has been reported, and for which commercial fungicides have only limited effects [22] and eventually would be useful for the de- velopment of a combination treatment with known fun- gicides, as a strategy to reduce their widespread use. 5. Acknowledgements We thank Universidad de Buenos Aires (X029), ANPCYT and CONICET for financial support and CONICET for the fellowship to BVB. REFERENCES [1] A. Takayuki, K. O’Donnell and M. M. Scandiani, “Sud- den-Death Syndrome of Soybean in South America is Caused by Four Species of Fusarium: Fusarium Brasil- iense Sp. Nov., F. Cuneirostrum Sp. Nov., F. Tucuma- niae, and F. Virguliforme,” Mycoscience, Vol. 46, No. 3, 2005, pp. 162-183. doi:10.1007/s10267-005-0235-y [2] M. Bjørling-Poulsen, H. R. Andersen and P. Grandjean, “Potential Developmental Neurotoxicity of Pesticides Used in Europe,” Environmental Health, Vol. 7, No. 1, 2008, pp. 1-22. [3] E. Hodgson and R. Rose, “Metabolic Interactions of Ag- rochemicals in Humans,” Pest Management Science, Vol. 64, No. 6, 2008, pp. 617-621. doi:10.1002/ps.1563 [4] M. F. Simoniello, E. C. Kleinsorge, J. A. Scagnetti, R. A. Grigolato, G. L. Poletta and M. A. Carballo, “DNA Damage in Workers Occupationally Exposed to Pesticide Mixtures,” Journal of Applied Toxicology, Vol. 28, 2008, pp. 957-965. [5] Y. Tanaka, “Antifungal Agents,” In: S. Omura, Ed., The Search for Bioactive Compounds from Microorganisms, Springer-Verlag Inc., New York, 1992, pp. 31-44. [6] D. M. Gibson and S. B. Krasnoff, “Exploring the Poten- tial of Biologically Active Compounds from Plants and Copyright © 2011 SciRes. AJPS  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against Sudden-Death Syndrome of Soybean Copyright © 2011 SciRes. AJPS 251 Fungi,” In: H. G. Cutler and S. J. Cutler, Eds., Biologi- cally Active Natural Products: Agrochemicals, CRC Press, Boca Raton, 1999, pp. 231-242. doi:10.1201/9781420048629.ch20 [7] H. L. Ypema and R. E. Gold, “Kresoxim-Methyl, Modi- fication of a Naturally Occurring Compound to Produce a New Fungicide,” Plant Disease, Vol. 83, No. 1, 1999, pp. 4-19. doi:10.1094/PDIS.1999.83.1.4 [8] L. M. Levy, G. M. Cabrera, J. E. Wright and A. M. Seldes, “Indole Alkaloids from the Culture of the Fungus Aporpium Caryae,” Phytochem, Vol. 54, No. 8, 2000, pp. 941-943. doi:10.1016/S0031-9422(00)00127-8 [9] F. Hadacek and H. Greger, “Testing of Antifungal Natu- ral Products: Methodologies, Comparability of Results and Assay Choice,” Phytochemical Analysis, Vol. 11, No. 3, 2000, pp. 137-147. [10] A. L. Homans and A. Fuchs, “Direct Bioautography on Thin-Layer Chromatography as a Method for Detecting Fungitoxic Substances,” Journal of Chromatography, Vol. 51, No. 2, 1970, pp. 325-328. doi:10.1002/(SICI)1099-1565(200005/06)11:3<137::AID -PCA514>3.0.CO;2-I [11] D. Sharma-Poudyal, E. Duveiller and R. C. Sharma, “Ef- fects of Seed Treatment and Foliar Fungicides on Hel- minthosporium Leaf Blight and on the Performance of Wheat in Warmer Growing Conditions,” Journal of Phy- topathology, Vol. 153, No. 7-8, 2005, pp. 401-408. doi:10.1111/j.1439-0434.2005.00992.x [12] C. Leach, 1963. “The Qualitative and Quantitative Rela- tionship of Monochromatic Radiation to Sexual and Asexual Reproduction of Pleospora herbarum,” Mycologia, Vol. 55, No. 2, 1963, pp. 151-163. doi:10.2307/3756286 [13] M. M. Scandiani and A. G. Luque, “Identificación de Patógenos en Semilla de Soja,” In: G. Rolando, Ed., Su- plemento Especial N2, Análisis de Semillas, Estudio Rolando, Rosario, 2009, pp. 1-148. [14] Y. H. Huang and G. L. Hartman, “Reaction of Selected Soybean Genotypes to Isolates of Fusarium Solani F. Sp. Glycines and Their Culture Filtrates,” Plant Disease, Vol. 82, No. 9, 1998, pp. 999-1002. doi:10.1094/PDIS.1998.82.9.999 [15] K. Karabelas, M. Lepisto and P. Sjo, “Preparation of New Triazoles as Pharmaceutically Active Compounds Activ- ity as Kinase Inhibitors,” Appl. US 2000, 646972. [16] M. Ishikura, W. Ida and K. Yanada, “A One-Pot Access to Cycloalkano [1,2-A]Indoles through an Intramolecular Alkyl Migration Reaction in Indolylborates,” Tetrahe- dron, Vol. 62, No. 5, 2006, pp. 1015-1024. doi:10.1016/j.tet.2005.10.045 [17] F. Ziegler and L. O. Jeroncic, “A New Route to 9,9a- Dihydro-3H-Pyrrolo[1,2-A]Indoles via Radical Cycliza- tion,” Journal of Organic Chemistry, Vol. 56, No. 42, 1991, pp. 3479-3486. doi:10.1021/jo00011a008 [18] Q. Yue and C. J. Miller, J. F. White and M. Richardson, “Isolation and Characterization of Fungal Inhibitors from Epichloë Festucae,” Journal of Agricultural and Food Chemistry, Vol. 48, No. 10, 2000, pp. 4687-4692. doi:10.1021/jf990685q [19] M. Himejima and I. Kubo, “Fungicidal Activity of Poly- godial in Combination with Anethole and Indole against Candida Albicans,” Journal of Agricultural and Food Chemistry, Vol. 41, No. 10, 1993, pp. 1776-1779. doi:10.1021/jf00034a048 [20] B. Prapagdee, K. Kotchadat, A. Kumsopa and N. Visara- thanonth, “The Role of Chitosan in Protection of Soybean from Sudden Death Syndrome Caused by Fusarium So- lani F. sp. Glycines,” Bioresource Technology, Vol. 98, No. 7, 2007, pp. 1353-1358. doi:10.1016/j.biortech.2006.05.029 [21] K. A. Nelson, P. P. Motavalli, W. E. Stevens, D. Dunn and C. G. Meinhardt, “Soybean Response to Preplant and Foliar-Applied Potassium Chloride with Strobilurin Fun- gicides,” Agronomy Journal, Vol. 102, No. 6, 2010, pp. 1657-1663. doi:10.2134/agronj2010.0065 [22] G. E. Shaner, D. H. Scott and T. S. Abney, “Sudden- Death Syndrome in Soybeans,” 2004. http://www.ces.purdue.edu/extmedia/BP/BP-58.pdf.  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against 252 Sudden-Death Syndrome of Soybean APPENDIX 1. Preparation of the N-Alkylindoles The N-alkylindoles 2-4, 7-10, 15, 16 and 20 were pre- pared by treatment of methyl 3-indole-carboxylate with NaH in THF or DMSO, and subsequent substitution of the corresponding alkyl halides. Typical conditions were as follows: to a solution of methyl 3-indole-carboxylate (200 mg, 1.14 mmol) in dry DMSO (2ml), 2 eq. NaH were added and the mixture was stirred for about 20 min- utes until it turned green-colored. The alkyl chloride (1.2eq) was then added drop wise to the resulting solution. The mixture was stirred overnight at room temperature. Methanol was used to remove the excess of hydride, wa- ter was added and the products were extracted with ethyl acetate (3 × 5 ml). After concentration in vacuo, the resi- due was purified by HPLC RP-18. Yields obtained were between 40 to 95%. Compounds 5, 6, 11, 12-13 and 17 were obtained as minor by-products, in the preparation of 2, 4, 3, 9 and 15 respectively, and were separated by HPLC (YMC C18, 5 m, 22.5 × 2.5 cm, MeOH-H2O 6:4 (5, 11), MeOH-H2O 7:3 (6, 17) or preparative TLC (silicagel, cyclohexane- CH2Cl2 1:1)(12, 13). Compounds 18 (and 19) were pre- pared by treatment of 16 with NH3 (or dimethyl amine). 2. Spectroscopical Data of Previously Undescribed Compounds Compound 3. 1-(2-Hydroxy-p ropyl)-1H-indole-3-carboxylic acid methyl ester Oil. IR (KBr, cm–1) max: 3453 (OH), 2937 (CH), 1677 (C=O). 1H NMR (CDCl3): d 8.04 (m, 1H, H-4); 7.75 (s, 1H, H-2); 7.27 (m, 1H, H-7); 7.16 (m, 2H, H-5,6); 4.10 (m, 1H, H-2’); 4.04 (dd, J = 14.4 and 3.9 Hz, 1H, H-1’); 3.92 (dd, J = 14.4 and 7.7 Hz, 1H, H-1’); 3.69 (s, 3H, CH3O); 2.40 (brs, 1H, OH); 1.16 (d, J = 6.2 Hz, 3H, H-3’). 13C NMR (CDCl3): d 165.5 (C-8); 136.8 (C-7a); 135.2 (C-2); 126.5 (C-3a); 122.7, 121.9 (C-5, C-6); 121.7 (C-4); 110.0 (C-7); 107.1 (C-3); 66.6 (C-2’); 54.0 (C-1’); 50.9 (CH3O); 20.6 (C-3’). HR ESI-MS m/z: 234.1119 [M+H]+ (calcd for C13H16NO3, 234.1125, D 2.4 ppm). EIMS (70 eV): m/z (%) 233 [M]+·(52), 202 (15), 188 (100), 130 (43). Compound 4. 1-(4-Hydroxy-butyl)-1H-indole-3-carboxy- lic acid methyl ester Oil. IR (KBr, cm–1) vma x : 3408 (OH), 2951 (CH), 2876 (CH), 1696 (C=O). 1H NMR (CDCl3): d 8.16 (m, 1H, H-4); 7.81 (s, 1H, H-2); 7.34 (m, 1H, H-7); 7.25 (m, 2H, H-5, 6); 4.13 (t, J = 7.1 Hz, 2H, H-1’); 3.88 (s, 3H, CH3O); 3.60 (t, J = 6.2 Hz, 2H, H-4’); 1.96 (m, 2H, H-2’); 1.63 (brs, 1H, OH); 1.55 (m, 2H, H-3’). 13C NMR (CDCl3): d 165.6 (C-8); 136.4 (C-7a); 134.2 (C-2); 126.6 (C-3a); 122.6, 121.7, 121.6 (C-4, C-5, C-6); 109.9 (C-7); 106.7 (C-3); 61.8 (C-4’); 50.7 (CH3O); 46.6 (C-1’); 29.6 (C-3’); 26.3 (C-2’). HR ESI-MS m/z: 248.1207 [M+H]+ (calcd for C14H18NO3, 248.1281, D 2.9 ppm). EIMS (70 eV): m/z (%) 247 [M]+·(100), 216 (27), 188 (86), 144 (50). Compound 5. 1-[3-(3-Hydroxy-propoxy)-propyl]- 1H- indole-3-carboxylic acid methyl ester Oil. IR (KBr, cm–1) vmax: 3417 (OH), 2928 (CH), 1696 (C=O), 1105 (C-O-C). 1H NMR (CDCl3): d 8.17 (m, 1H, H-4); 7.84 (s, 1H, H-2); 7.38 (m, 1H, H-7); 7.27 (m, 2H, H-5, 6); 4.29 (t, J = 6.6 Hz, 2H, H-1’); 3.91 (s, 3H, CH3O); 3.81 (dd, J = 6.1, 5.0 Hz, 2H, H-7’); 3.56 (t, J = 5.70 Hz, 2H, H-3’ ); 3.33 (t, J = 5.70 Hz, 2H, H-5’); 2.15 (brs, 1H, OH); 2.11 (m, 2H, H-2’); 1.86 (m, 2H, H-6’). 13C NMR (CDCl3): d 165.6 (C-8); 136.5 (C-7a); 134.6 (C-2); 126.7 (C-3a); 122.7, 121.9, 121.8 (C-4, C-5, C-6); 109.9 (C-7); 107.1 (C-3); 69.7, 67.1 (C-5’, C-7’); 61.4 (C-3’); 50.9 (CH3O); 43.5 (C-1’); 32.2 (C-2’); 29.8 (C-6’). EIMS (70 eV): m/z (%) 291 [M]+· (60), 260 (7), 189 (76), 188 (39), 130 (100). Compound 6. 1-[4-(4-Hydroxy-butoxy)-butyl]-1H-indole- 3-carboxylic acid methyl ester Oil. IR (KBr, cm–1) vma x : 3431 (OH), 2945 (CH), 2862 (CH), 1699 (C=O). 1H NMR (CDCl3): d 8.18 (m, 1H, H-4); 7.85 (s, 1H, H-2); 7.37 (m, 1H, H-7); 7.27 (m, 2H, H-5, 6); 4.19 (t, J = 7.1 Hz, 2H, H-1’); 3.91 (s, 3H, CH3O); 3.64 (dd, J = 11.0 Hz and 5.6 Hz,, 2H, H-9’); 3.43 (m, 4H, H-4’ y 6’); 2.20 (brs, 1H, OH); 1.97 (m, 2H, H-2’); 1.66 (m, 4H, H-7’, 8’); 1.60 (m, 2H, H-3’). 13C NMR (CDCl3): d 165.6 (C-8); 136.4 (C-7a); 134.2 (C-2); 126.6 (C-3a); 122.6, 121.7, 121.6 (C-4, C-5, C-6); 109.9 (C-7); 106.7 (C-3); 71.0 (C-6’); 70.3 (C-4’); 62.8 (C-9’); 62.8 (C-9’); 50.9 (CH3O); 46.6 (C-1’); 30.3 (C-7’); 26.9 (C-2’, C-3’); 26.7 (C-8’). HR ESI-MS m/z: 320.1866 [M+H]+ (calcd for C18H26NO4, 320.1856, D -3.0 ppm). EIMS (70 eV): m/z (%) 319 [M]+·(39), 216 (37), 188 (100), 172 (73), 130 (83). Compound 7. (S) 1-(2,3-Dihydroxy-propyl)-1H-indole -3-carboxylic acid methyl ester Mp 108˚C - 110˚C. [a]D 25= -20 (c 0.4, MeOH). IR (KBr, cm-1) max: 3459 (OH), 2900 (CH), 1674 (C=O). 1H NMR (CDCl3): d 8.02 (m, 1H, H-4); 7.79 (s, 1H, H-2); 7.29 (m, 1H, H-7); 7.16 (m, 2H, H-5, 6); 4.15 (dd, J = 14.4 and 4.8 Hz, 1H, H-1’); 4.03 (dd, J = 14.4 and 7.3 Hz, 1H, H-1’); 3.94 (m, 1H, H-2’); 3.72 (s, 3H, CH3O); 3.53 (dd, J = 11.4 and 3.9 Hz, 1H, H-3’); 3.38 (dd, J = 11.4 and 5.7 Hz, 1H, H-3’); 3.05 (brs, 1H, OH). 13C NMR (CDCl3): d 165.6 (C-8); 136.8 (C-7a); 135.2 (C-2); 126.6 (C-3a); 123.0, 122.1, 121.8 (C-4, C-5, C-6); 109.9 (C-7); 107.5 (C-3); 70.6 (C-2’); 63.7 (C-3’); 51.0 (CH3O); 49.1 (C-1’). HR ESI-MS m/z: 250.1061 [M+H]+ (calcd for Copyright © 2011 SciRes. AJPS  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against 253 Sudden-Death Syndrome of Soybean C13H16NO4, 250.1074, D 5.2 ppm). EIMS (70 eV): m/z (%) 249 [M]+·(63), 218 (20), 189 (22), 188 (100), 130 (24). Compound 8. (R) 1-(2,3-Dihydroxy-propyl)-1H-indole -3-carboxylic acid methyl ester Mp 109˚C - 110˚C. [a]D 25= 16 (c 0.4, MeOH). IR (KBr, cm-1) max: 3459 (OH), 2910 (CH), 1674 (C=O). 1H NMR (CDCl3): d 8.06 (m, 1H, H-4); 7.86 (s, 1H, H-2); 7.37 (m, 1H, H-7); 7.21 (m, 2H, H-5, 6); 4.25 (dd, J = 14.4 and 5.0 Hz, 1H, H-1’); 4.10 (dd, J = 14.4 and 7.3 Hz, 1H, H-1’); 3.97 (m, 2H, H-2’); 3.83 (s, 3H, CH3O); 3.55 (dd, J = 11.4 and 4.3 Hz, 1H, H-3’); 3.43 (dd, J = 11.4, 5.5 Hz, 1H, H-3’); 3.05 (brs, 1H, OH). 13C NMR (CDCl3): d 166.0 (C-8); 136.8 (C-7a); 135.5 (C-2); 126.4 (C-3a); 122.7, 121.8, 121.4 (C-4, C-5, C-6); 110.0 (C-7); 106.8 (C-3); 70.3 (C-2’); 63.4 (C-3’); 50.9 (CH3O); 49.0 (C-1’). HR ESI-MS m/z: 250.1078 [M+H]+ (calcd for C13H16NO4, 250.1074, D -1.7 ppm). EIMS (70 eV): m/z (%) 249 [M]+· (52), 218 (12), 189 (18), 188 (100), 130 (13). Compound 9. 1-Decyl-1H-indole-3-carboxylic acid methyl ester Oil. IR (KBr, cm–1) vmax: 2920 (CH), 2851 (CH), 1702 (C=O). 1H NMR (CDCl3): d 8.10 (m, 1H, H-4); 7.74 (s, 1H, H-2); 7.28 (m, 1H, H-7); 7.19 (m, 2H, H-5, 6); 4.04 (t, J = 7.3 Hz, 2H, H-1’); 3.83 (s, 3H, CH3O); 1.78 (qi, J = 7.1 Hz, 2H, H-2’); 1.15-1.23 (m, 14H, H-3’ to 9’); 0.80 (t, J = 6.8 Hz, 3H, H-10’). 13C NMR (CDCl3): d 165.5 (C-8); 136.6 (C-7a); 134.2 (C-2); 126.7 (C-3a); 122.6, 121.7 (C-4, C-5, C-6); 109.9 (C-7); 106.9 (C-3); 50.9 (CH3O); 47.0 (C-1’); 31.8 (C-2’); 29.8, 29.5, 29.4, 29.2, 29.1, 26.8, 22.6 (C-3’ to C-9’); 14.1 (C-10’). HR ESI-MS m/z: 316.2261 [M+H]+ (calcd for C20H30NO2, 316.2271, D 3.1 ppm). EIMS (70 eV): m/z (%) 315 [M]+·(100), 284 (18), 188 (64), 130 (42). Compound 10. 1-(4-Acetoxy-butyl)-1H-indole-3- car- boxylic acid methyl ester Oil. IR (KBr, cm–1) vmax: 2954 (CH), 1735 (C=O), 1696 (C=O), 1241 (C-O). 1H NMR (CDCl3): d 8.20 (t, 1H, H-4); 7.82 (s, 1H, H-2); 7.34 (t, 1H, H-7); 7.29 (m, 2H, H-5, 6); 4.19 (t, J = 7.0 Hz, 2H, H-1’); 4.06 (t, J = 6.4 Hz, 2H, H-4’); 3.92 (s, 3H, CH3O); 2.03 (s, 3H, CH3CO); 1.92 (qi, J = 7.0 Hz, 2H, H-2’); 1.63 (m, 2H, H-3’). 13C NMR (CDCl3): d 170.9 (CH3CO); 165.3 (C-8); 136.4 (C-7a); 134.2 (C-2); 126.7 (C-3a); 122.7, 121.8, 121.7 (C-4, C-5, C-6); 109.9 (C-7); 106.9 (C-3); 63.7 (C-4’); 51.1 (CH3O); 46.6 (C-1’); 26.7, 26.1 (C-2’, C-3’); 20.9 (CH3CO). HR ESI-MS m/z: 290.1403 [M+H]+ (calcd for C16H20NO4, 290.1387, D -4.53 ppm). EIMS (70 eV): m/z (%) 289[M]+· (99), 258 (27), 188 (100), 144 (22). Compound 11. 1-(2-Hydroxy-propyl)-1H-indole -3- carboxylic acid Mp 150˚C - 151˚C. IR (KBr, cm–1) vmax : 3342 (OH), 2923 (CH), 1549.1 (C=O). 1H NMR (CDCl3-CD3OD 5%): d 8.20 (m, 1H, H-4); 7.73 (s, 1H, H-2); 7.36 (m, 1H, H-7); 7.12 (m, 2H, H-5, 6); 4.13 (m, 2H, H-2’); 4.10 (m, 2H, H-1’); 1.16 (d, J = 6.0 Hz, 3H, H-3’). 13C NMR (CDCl3-CD3OD 5%): d 174.1 (C-8); 138.1 (C-7a); 134.6 (C-2); 128.6 (C-3a); 122.7, 122.4, 121.2 (C-4, C-5, C-6); 114.1 (C-3); 110.5 (C-7); 67.3 (C-2’); 54.4 (C-1’); 20.9 (C-3’). HR ESI-MS m/z: 220.0979 [M+H]+ (calcd for C12H14NO3, 220.0968, D -4.8 ppm). EIMS (70 eV): m/z (%) 219 [M]+·(27), 175(35), 174 (39), 130 (100). Compound 12. 1-Decyl-1H-indole-3-carboxylic acid Mp 87˚C - 88˚C. IR (KBr, cm–1) vmax: 3239 (OH), 2917 (CH), 1663 (C=O). 1H NMR (CDCl3): d 8.13 (m, 1H, H-4); 7.80 (s, 1H, H-2); 7.27 (m, 1H, H-7); 7.18 (m, 2H, H-5, 6); 4.02 (t, J = 7.1 Hz, 2H, H-1’); 1.76 (qi, J = 7.1 Hz, 2H, H-2’), 1.15-1.23 (m, 14H, H-3’ to H-9’); 0.78 (t, J = 6.8 Hz, 3H, H-10’). 13C NMR (CDCl3): d 168.9 (C-8); 136.6 (C-7a); 135.1 (C-2); 126.9 (C-3a); 122.6, 121.8, 121.7 (C-4, C-5, C-6); 109.9 (C-7); 106.3 (C-3); 46.9 (C-1’); 31.7 (C-2’); 29.7, 29.3, 29.3, 29.1, 29.0, 26.7, 22.5 (C-3’ to C-9’); 13.9 (C-10’). HR ESI-MS m/z: 302.2128 [M+H]+ (calcd for C19H28NO2, 302.2115, D -4.6 ppm). EIMS (70 eV): m/z (%) 301 [M]+·(100), 256 (14), 174 (80), 130 (56). Compound 13. 1-Decyl-1H-indole-3-carboxylic acid decyl ester Oil. IR (KBr, cm–1) vmax: 2929 (CH), 1707 (C=O). 1H NMR (CDCl3): d 8.17 (m, 1H, H-4); 7.82 (s, 1H, H-2); 7.36 (m, 1H, H-7); 7.26 (m, 2H, H-5, 6); 4.32 (t, J = 6.6 Hz, 2H, H-1’’); 4.13 (t, J = 7.3 Hz, 2H, H-1’); 1.80-1.87 (m, 4H, H-2’, 2’’); 1.25-1.47 (m, 28H, H-3’ to H-9’, H-3’’ to H-9’’); 0.88 (t, J = 7.3 Hz, 3H, H-10’*); 0.87 (t, J = 7.1 Hz, 3H, H-10’’*). 13C NMR (CDCl3): d 165.4 (C-8); 136.6 (C-7a); 134.2 (C-2); 126.7 (C-3a); 122.5, 121.8, 121.7 (C-4, C-5, C-6); 109.9 (C-7); 107.3 (C-3); 63.9 (C-4’); 47.0 (C-1’); 31.9, 29.9, 29.5, 29.3, 29.2, 29.0, 26.9, 22.7 (C-3’ to C-9’, C-3’’ to C-9’’); 14.1 (C-10’, C-10’’). HR ESI-MS m/z: 442.3689 [M+H]+ (calcd for C29H48NO2, 442.3680, D -2.1 ppm). EIMS (70 eV): m/z (%) 441 [M]+·(100), 301 (17), 284 (23), 174 (15), 130 (13).* may be interchanged. Compound 16. 1-(4-Bromo-butyl)-1H-indole-3-carboxy- lic acid methyl ester Oil. IR (KBr, cm–1) vmax: 2942 (CH), 1691 (C=O), 747 (CBr). 1H NMR (CDCl3): d 8.18 (m, 1H, H-4); 7.82 (s, 1H, H-2); 7.37 (m, 1H, H-7); 7.29 (m, 2H, H-5, 6); 4.20 (t, J = 6.9 Hz, 2H, H-1’); 3.91 (s, 3H, CH3O); 3.39 (t, J = 6.5 Hz, 2H, H-4’); 2.06 (m, 2H, H-2’); 1.88 (m, 2H, H-3’). 13C NMR (CDCl3): d 165.6 (C-8); 136.6 (C-7a); 134.1 (C-2); 126.9 (C-3a); 123.0, 122.1 (C-5, C-6); 122.0 (C-4); 109.9 (C-7); 107.4 (C-3); 51.2 (CH3O); 46.3 Copyright © 2011 SciRes. AJPS  Analogs of Antifungal Indoles Isolated from Aporpium Caryae with Activity against Sudden-Death Syndrome of Soybean Copyright © 2011 SciRes. AJPS 254 (C-1’); 32.8 (C-4’); 29.9 (C-3’); 28.6 (C-2’). HR ESI-MS m/z: 310.0440 [M+H]+ (calcd for C14H17BrNO2, 310.0437, D -0.9 ppm). EIMS (70 eV): m/z (%) 311 [M+2]+·(48), 309 [M]+·(44), 280 (13), 278 (13), 188 (100), 55 (49). Compound 18. 1-(4-Amino-butyl)-1H-indole-3-carboxy- lic acid methyl ester Oil. IR (KBr, cm–1) vmax: 3459, 3423 (NH2), 2917 (CH), 1680 (C=O). 1H NMR (CDCl3-CD3OD 5%): d 8.14 (m, 1H, H-4); 7.88 (s, 1H, H-2); 7.39 (m, 1H, H-7); 7.29 (m, 2H, H-5, 6); 4.24 (t, J = 6.8 Hz, 2H, H-1’); 3.91 (s, 3H, CH3O); 2.90 (t, J = 7.6 Hz, 2H, H-4’); 1.97 (m, 2H, H-2’); 1.68 (m, 2H, H-3’). 13C NMR (CDCl3-CD3OD 5%): d 165.9 (C-8); 136.2 (C-7a); 134.4 (C-2); 126.4 (C-3a); 123.1, 122.2 (C-5, C-6); 121.9 (C-4); 110.0 (C-7); 106.9 (C-3); 51.2 (CH3O); 46.2 (C-1’); 39.4 (C-4’); 26.8 (C-2’); 25.2 (C-3’). HR ESI-MS m/z: 247.1449 [M+H]+ (calcd for C14H19N2O2, 247.1441, D -3.0 ppm). EIMS (70 eV): m/z (%) 246 [M]+·(15), 214 (16), 188 (21), 144 (36), 43 (100). Compound 19. 1-(4-Dimethylamino-butyl)-1H-indole- 3-carboxylic acid methyl ester Oil. IR (KBr, cm–1) vmax: 2942 (CH), 1702 (C=O). 1H NMR (CDCl3): d 8.18 (m, 1H, H-4); 7.83 (s, 1H, H-2); 7.37 (m, 1H, H-7); 7.28 (m, 2H, H-5, 6); 4.18 (t, J = 7.1 Hz, 2H, H-1’); 3.91 (s, 3H, CH3O); 2.40 (t, J = 7.5 Hz, 2H, H-4’); 2.27 (s, 6H, NCH3); 1.91 (m, 2H, H-2’); 1.55 (m, 2H, H-3’). 13C NMR (CDCl3): d 165.6 (C-8); 136.6 (C-7a); 134.3 (C-2); 126.9 (C-3a); 122.9, 122.0 (C-5, C-6); 121.9 (C-4); 110.0 (C-7); 107.2 (C-3); 51.1 (CH3O); 58.3 (C-4’); 46.9 (C-1’); 44.7 (NCH3); 27.7 (C-2’); 24.3 (C-3’). HR ESI-MS m/z: 275.1741 [M+H]+ (calcd for C16H23N2O2, 275.1754, D 4.7 ppm). EIMS (70 eV): m/z (%) 274 [M]+· (4), 188 (2), 58 (100). Compound 20. 1,3-di-[(3’-methoxycarbonyl)-1H -indol- 1-yl] propane Mp 176˚C - 177˚C. IR (KBr, cm–1) max: 2920 (CH), 1691 (C=O). 1H NMR (CDCl3): d 8.20 (m, 2H, H-4); 7.76 (s, 2H, H-2); 7.29 (m, 2H, H-5); 7.26 (m, 2H, H-6); 7.19 (m, 2H, H-7); 4.16 (t, J = 6.9 Hz, 4H, H-1’); 3.91 (s, 6H, CH3O); 2.50 (qi, J = 6.9 Hz, 2H, H-2’). 13C NMR (CDCl3): d 165.4 (C-8); 136.4 (C-7a); 133.8 (C-2); 126.9 (C-3a); 123.3 (C-6); 122.4 (C-5); 122.2 (C-4); 109.8 (C-7); 107.9 (C-3); 51.2 (CH3O); 43.9 (C-1’); 29.8 (C-2’). HR ESI-MS m/z: 391.1669 [M+H]+ (calcd for C23H23N2O4, 391.1652, D -4.2 ppm). EIMS (70 eV): m/z (%) 390 [M]+·(24), 359 (6), 189 (50), 188 (15), 130 (100).
|