Paper Menu >>
Journal Menu >>
 Open Journal of Internal Medicine, 2011, 1, 6-8 OJIM doi:10.4236/ojim.2011.11003 Published Online June 2011 (http://www.SciRP.org/journal/ojim/). Published Online June 2011 in SciRes. http://www.scirp.org/journal/OJIM Staphylococcus haemolyticus superinfection of legionella pneumonia during infliximab therapy Marianna Porzio, Luca Valenti, Daniela Bignamini, Francesca Ricchini, Alessandro Palleschi*, Paolo Tarsia, Silvia Fargion Department of Internal Medicine, Università degli Studi di Milano, Fondazione IRCCS “Ca’ Granda” Ospedale Policlinico; *Thoracic Surgery, Fondazione IRCCS “Ca’ Granda” Ospedale Policlinico; Department of Pulmonary Disease, Fondazione IRCCS “Ca’ Granda” Ospedale Policlinico, Milano. E-mail: marianna.porzio@studenti.unimi.it Received 3 May 2011; revised 9 June 2011, accepted 18 June 2011.. ABSTRACT We present the case of a 42-year-old man affected by psoriasis with Staphylococcus Haemolyticus superin- fection of Legionella pneumonia during infliximab therapy. The introduction of compounds that block TNF-α has yielded great benefits for patients affected by selected autoimmune diseases that fail to respond to classic anti-inflammatory agents, but, on the other hand, has led to an increased susceptibility to infec- tions, in particular of those caused by intracellular pathogens, such as L. Pneumophila. Emerging evi- dence suggests that legionellosis can be complicated by superinfection with other agents, including sap- rophytic microorganisms, among which coagulase- negative staphylococci. To our knowledge, this is the first report of systemic legionellosis with superinfec- tion by S. Haemolyticus, an emerging nosocomial multi-resistant pathogen that commonly causes sep- ticemia, osteomyelitis or endocarditis, but has not so far been associated with necrotizing pneumonia. De- spite the optimal antimicrobial therapy for Staphylo- coccus spp. Pneumonia is still controversial, evidence suggests that in patients with confirmed positivity for methicillin resistant strains, particularly if sensitivity to vancomycin is suboptimal, linezolid should be the first choice therapy, being superior to vancomycin and teicoplanin. Keywords: Infliximab; Legionella Pneumophila; Line- zolid; Necrotizing Pneumon ia; Psoriasis; Staphylococcu s Haemolyticus 1. INTRODUCTION On September 2010, a 42-year-old man presented to the emergency department because of fever, productive cough, and chest pain. He had a history of psoriasis, pre- viously treated with acitretine 25 mg, which was discon- tinued because of dyslipidemia. Nine months before the current admission he required hospitalization for erythrodermic psoriasis. Since then, he was treated with infliximab, and received the eighth infusion one week before presentation. Due to the incomplete control of disease activity, he was concurrently started on meth- otrexate (10 mg s.c.). Five days before admission he reported the abrupt onset of fever which reached 39˚C, dry cough, asthenia, and burning pain in the right sub- scapular region, which were unresponsive to aceta- minophen and amoxicillin-clavulanate. On admission the temperature was 39.5˚C, blood pressure 125⁄80 mmHg, pulse 115, respiratory rate 24, peripheral oxygen saturation 98% while he was breath- ing ambient air. Clinical examination revealed bilateral latero-cervical lymph nodes (soft, non-tender, and mo- bile, < 2 cm), regular heart activity, slight rales at the left lower lobe, and splenomegaly. An electrocardiogram showed sinus tachycardia without alterations of the ST tract; biochemical test are shown in Table 1. Chest Rx showed a right middle lobe infiltrate (Figure 1(a)). Ab- dominal ultrasonography was only notable for spleno- megaly (153 mm). Oxygen supplementation and hydra- tion were provided. Microbiological analyses of blood were repeatedly negative and sputum was negative for M. tuberculosis and other microorganisms; L. Pneumophila serotype 1 antigen was detected in urine samples. Intravenous levofloxacin 750 mg/day was started with improvement in systemic symptoms, and correction within 5 days of the alterations of creatine-phosphokinase levels, and hyponatremia, (Table 1). Because of the persistence of unremitting fever (> 38˚C), and increased inflammatory markers, azithromycin 500 mg/day was added on day 5. Blood cultures remained negative, as well as autoimmu- nity markers.  M. Porzio et al. / Open Journal of Internal Medicine 1 (2011) 6-8 Copyright © 2011 SciRes. OJIM 7 One week after admission, the patient underwent a chest computed tomography (CT) scan showing upper and middle right lobe extensive infiltrate with an aerial bronchogram and patchy ground-glass infiltrates in the upper left lobe, minimal pleural and pericardial effusions and multiple bilateral supraclavicular, paratracheal, un- dercarinal, and axillary lymphoadenopathies (up to 1.5 cm). On day 8 Staphylococcus Haemolyticus was iso- lated from the sputum, but initially interpreted as a con- taminant (supplementary Tab le 1). A bronchoscopy was performed on day 10: the airways appeared normal and cytologic examination of bronchoalveolar-lavage (BAL) specimens of the right lobes revealed acute inflammation, but not malignant cells. Tests were negative for M. Tu- bercolosis, Aspergillus spp, P. Jirovecii, Epstein-Barr Virus, Cytomegalovirus, Respiratory Syncythial virus multiplex PCR, whereas a nested PCR-based assay con- firmed the positivity for L. Pneumophila. Broad-spectrum empirical antimicrobical therapy was started including piperacillin-tazobactam and imipenem (day 10); while clindamycin 600 mg t.i.d. i.v. was added on day 14 according to the S. Haemolyticus antibiogram (supplementary table), without benefit. On day 15 a CT scan was repeated, which showed the progression of the right pulmonary infiltrate with areas of colliquation and excavation (Figure 1(b)). Rifampicin replaced previous empirical therapy on day 17 because of the reported synergic effect with fluoroquinolones, and macrolides in the treatment of resistant legionellosis in immunosuppressed patients [1]. To exclude an immunosuppression related lymphomas, laterocervical lymph node biopsy was performed, which showed a reactive inflammatory pattern. A positron emission tomography CT scan showed inflammation in the right upper and lower pulmonary lobes and in the mediastinal lymph nodes. Several reports highlited an increased risk of infection by atypical pathogens associated with infliximab, in- cluding M. Tubercolosis, Listeria, Pneumococcus, Le- gionella, Salmonella, and Bartonella spp.), fungi, and viruses [2]. Although the patient chest Rx and protein purified derivative intradermoreaction were negative before starting infliximab and BAL was PCR negative for M.Tubercolosis, a quantiferon test for Mycobacte- rium was performed, but it confirmed negativity for M.Tubercolosis. An echocardiogram was carried out on day 21, with no evidence of endocarditis. To exclude a pulmonary superinfection, a CT-guided needle-aspiration of the pulmonary lesion was per- formed, with isolation of rare colonies of S. Haemo- lyticus (supplementary Table 1). Vancomycin (minimal inhibitory concentration (MIC): 1) was started i.v. (day 22) without response. In view of the sub-optimal MIC, incomplete pulmonary diffusion of vancomycin, and the relatively low plasma concentration achieved after treatment titration (12.5 µg/mL), linezolid (600 mg td i.v.) was introduced on day 33, with immediate remis- sion of fever and normalization of inflammatory indi- ces (Table 1). The patient was discharged on day 45. A chest CT scan after 4 weeks demonstrated resolution of the inflammatory infiltrates and a reduction of the ex- cavated areas. This case is notable for several aspects. The introduc- tion into clinical practice of compounds that block TNF-α has yielded great benefits for patients affected by selected autoimmune diseases that fail to respond to classic anti-inflammatory agents, but, on the other hand, has led to an increased su sceptibility to infections [3 ], in particular of those caused by intracellular pathogens [4], and L. Pneumophila pneumonia has been reported as a life-threatening infection that frequently complicates anti-TNF-α therapy [3]. In line with our experience, evidence is accumulating that legionellosis, particularly in patients under immunosuppressive therapy, can be complicated by superinfection with other agents, includ- ing saprophytic microorganisms. Furthermore, positivity for Legionella DNA may persist despite appropriate treatment in immunocompromised patients, and lung colliquative necrosis supports a diagnosis of coinfection with other bacterial agents. To our knowledg e, this is th e first report of systemic legionellosis with superinfection by S. Haemolyticus, an emerging nosocomial multire- sistant pathogen that commonly causes septicemia, os- teomyelitis or endocarditis, but h as not so far been asso- ciated with necrotizing pneumonia [5]. Finally, despite the optimal antimicrobial therapy for Staphylococcus spp. pneumonia is still controversial, evidence suggests th at in cases with confirmed p ositivity (a) (b) Figure 1. (a) Chest X-ray on admission: right middle lobe infiltrate and left mild perihilar infiltrate; (b) Chest CT scan (day 15): upper right lobe extensive infiltrate with areas of colliquation and excavation (arrowheads) and patchy ground- glass infiltrates in the upper right lobe (arrows). 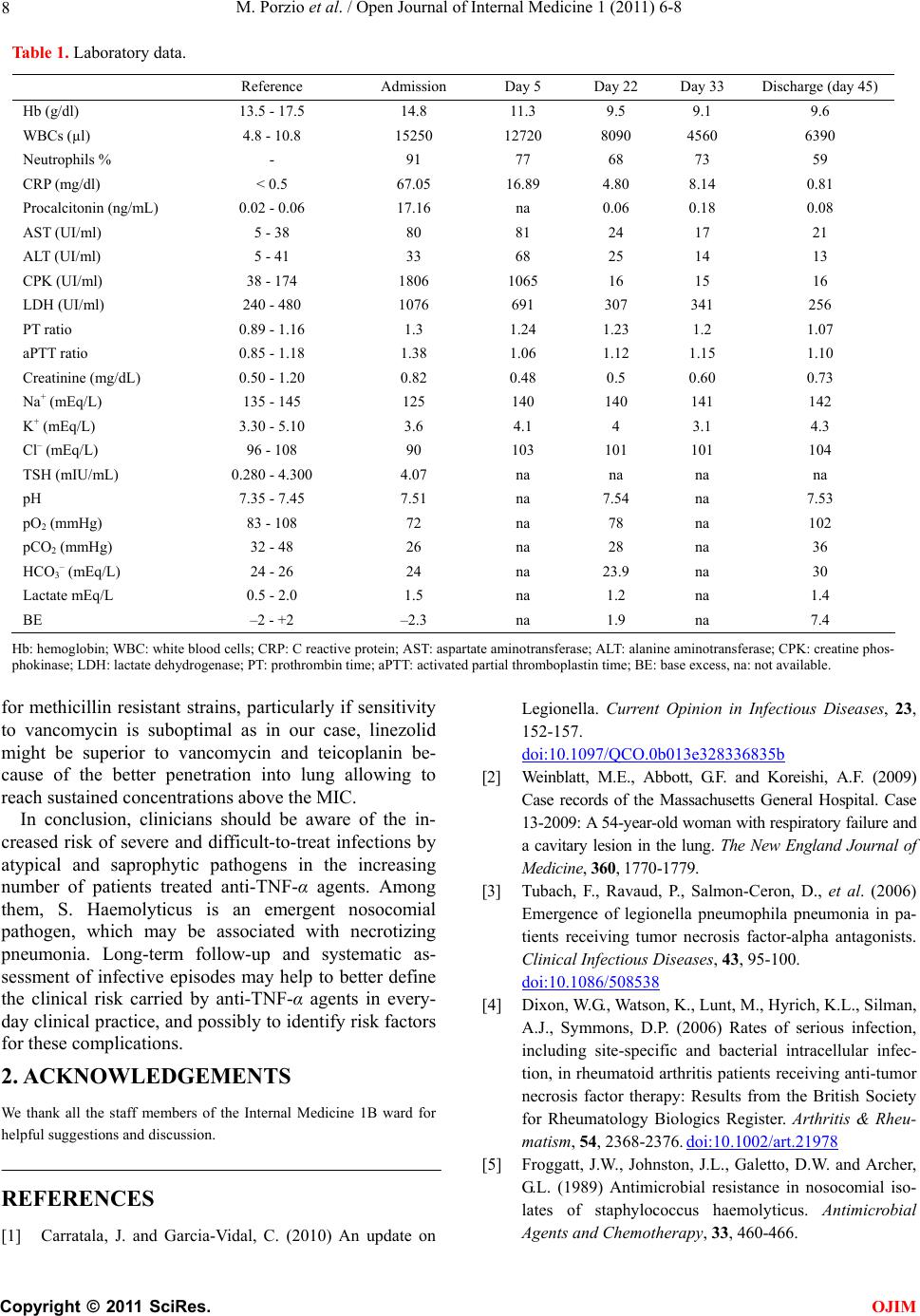 M. Porzio et al. / Open Journal of Internal Medicine 1 (2011) 6-8 Copyright © 2011 SciRes. OJIM 8 Table 1. Laboratory data. Hb: hemoglobin; WBC: white blood cells; CRP: C reactive protein; AST: aspartate aminotransferase; ALT: alanine aminotransferase; CPK: creatine phos- phokinase; LDH: lactate dehydrogenase; PT: prothrombin time; aPTT: activated partial th romboplastin time; BE: base excess, na: n ot available. for methicillin resistant strains, particularly if sensitivity to vancomycin is suboptimal as in our case, linezolid might be superior to vancomycin and teicoplanin be- cause of the better penetration into lung allowing to reach sustained concentrations above the MIC. In conclusion, clinicians should be aware of the in- creased risk of severe and difficult-to-treat infections by atypical and saprophytic pathogens in the increasing number of patients treated anti-TNF-α agents. Among them, S. Haemolyticus is an emergent nosocomial pathogen, which may be associated with necrotizing pneumonia. Long-term follow-up and systematic as- sessment of infective episodes may help to better define the clinical risk carried by anti-TNF-α agents in every- day clinical practice, and possib ly to identify risk factors for these complications. 2. ACKNOWLEDGEMENTS We thank all the staff members of the Internal Medicine 1B ward for helpful suggestions and discussion. REFERENCES [1] Carratala, J. and Garcia-Vidal, C. (2010) An update on Legionella. Current Opinion in Infectious Diseases, 23, 152-157. doi:10.1097/QCO.0b013e328336835b [2] Weinblatt, M.E., Abbott, G.F. and Koreishi, A.F. (2009) Case records of the Massachusetts General Hospital. Case 13-2009: A 54-year-old woman with respirat ory failure and a cavitary lesion in the lung. The New England Journal of Medicine, 360, 1770-1779. [3] Tubach, F., Ravaud, P., Salmon-Ceron, D., et al. (2006) Emergence of legionella pneumophila pneumonia in pa- tients receiving tumor necrosis factor-alpha antagonists. Clinical Infectious Diseases, 43, 95-100. doi:10.1086/508538 [4] Dixon, W.G., Watson, K., Lunt, M., Hyrich, K.L., Silman, A.J., Symmons, D.P. (2006) Rates of serious infection, including site-specific and bacterial intracellular infec- tion, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: Results from the British Society for Rheumatology Biologics Register. Arthritis & Rheu- matism, 54, 2368-2376. doi:10.1002/art.21978 [5] Froggatt, J.W., Johnston, J.L., Galetto, D.W. and Archer, G.L. (1989) Antimicrobial resistance in nosocomial iso- lates of staphylococcus haemolyticus. Antimicrobial Agents and Chemotherapy, 33, 460-466. Reference Admission Day 5 Day 22 Day 33 Discharge (day 45) Hb (g/dl) 13.5 - 17.5 14.8 11.3 9.5 9.1 9.6 WBCs (µl) 4.8 - 10.8 15250 12720 8090 4560 6390 Neutrophils % - 91 77 68 73 59 CRP (mg/dl) < 0.5 67.05 16.89 4.80 8.14 0.81 Procalcitonin (ng/mL) 0.02 - 0.06 17.16 na 0.06 0.18 0.08 AST (UI/ml) 5 - 38 80 81 24 17 21 ALT (UI/ml) 5 - 41 33 68 25 14 13 CPK (UI/ml) 38 - 174 1806 1065 16 15 16 LDH (UI/ml) 240 - 480 1076 691 307 341 256 PT ratio 0.89 - 1.16 1.3 1.24 1.23 1.2 1.07 aPTT ratio 0.85 - 1.18 1.38 1.06 1.12 1.15 1.10 Creatinine (mg/dL) 0.50 - 1.20 0.82 0.48 0.5 0.60 0.73 Na+ (mEq/L) 135 - 145 125 140 140 141 142 K+ (mEq/L) 3.30 - 5.10 3.6 4.1 4 3.1 4.3 Cl– (mEq/L) 96 - 108 90 103 101 101 104 TSH (mIU/mL) 0.280 - 4.300 4.07 na na na na pH 7.35 - 7.45 7.51 na 7.54 na 7.53 pO2 (mmHg) 83 - 108 72 na 78 na 102 pCO2 (mmHg) 32 - 48 26 na 28 na 36 HCO3– (mEq/L) 24 - 26 24 na 23.9 na 30 Lactate mEq/L 0.5 - 2.0 1.5 na 1.2 na 1.4 BE –2 - +2 –2.3 na 1.9 na 7.4 |

